Abstract
Chronic hepatitis B (CHB) remains a relatively major public health problem. Simultaneously, an unhealthy lifestyle causes a series of metabolic abnormalities, the most critical of which are metabolic syndrome (MS) and nonalcoholic fatty liver disease (NAFLD). Therefore, it is increasingly common for MS and NAFLD to coexist with CHB. MS is a cluster of metabolic disorders, while NAFLD is always considered as the manifestation of MS in the liver. The aim of this article is to review recent advances to explain the complex relationship among MS, NAFLD, and hepatitis B virus (HBV) infection. MS and NAFLD both have obesity and insulin resistance as central factors and both can lead to adverse hepatic and extrahepatic outcomes. However, there is insufficient evidence to associate NAFLD with all components of MS, and genetically related NAFLD has little association with MS. Incidences of MS and NAFLD are inversely associated with HBV infection. However, the effect of HBV infection on the risk of insulin resistance and dyslipidemia is not well understood. Evidence from both clinical studies and animal experiments suggested that hepatic steatosis inhibits HBV replication. MS and NAFLD may have adverse effects on CHB disease progression and prognosis. Furthermore, in related studies of CHB with normal alanine aminotransferase (ALT), the roles of MS and NAFLD should also be emphasized. In conclusion, there are complicated interactions that are not yet fully defined among MS, NAFLD, and CHB. To control chronic liver disease effectively, the relationship among the three must be clarified.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
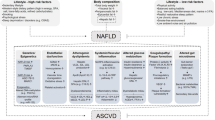
Metabolic syndrome and nonalcoholic fatty liver disease both have obesity and insulin resistance as their key factors, but they are not completely equivalent. |
Chronic hepatitis B complicated with metabolic abnormalities or fatty liver has become a common clinical condition. |
Metabolic syndrome and nonalcoholic fatty liver disease may promote the progression of chronic hepatitis B disease, while the effect of CHB on glucose and lipid metabolism is not yet clear. |
The relationships among these three diseases in patients with normal ALT are worth exploring. |
Introduction
Chronic hepatitis B (CHB) is a chronic inflammatory disease of the liver caused by persistent chronic hepatitis B virus (HBV) infection and it is one of the main causes of liver cirrhosis, liver failure, and hepatocellular carcinoma (HCC) [1]. There are approximately 248 million people with chronic HBV infection in the world, and more than 680,000 patients die of HBV infection-related diseases every year, of which 46.0% and 44.0% die of liver cirrhosis and HCC, respectively [2, 3]. However, only 10% of all patients with CHB are diagnosed, and 94 million of them are indicated for anti-HBV treatment, but only 5% of them actually receive antiviral therapy [4]. CHB is still a major public health problem worldwide.
It has been reported that 40–70% of patients with CHB have persistently normal alanine aminotransferase (ALT) levels [5, 6]. Although most of these patients do not meet the antiviral treatment standards recommended by current guidelines, the disease may still progress insidiously and adverse outcome events may occur. For example, a recent study from China found that among 327 patients with hepatitis B envelope antigen (HBeAg)-negative CHB, 37.3% had liver biopsy-proven necroinflammation, and 53.2% had liver fibrosis [7]. Similarly, another multicenter study also suggested that approximately half of patients with CHB and normal ALT had liver inflammation and/or fibrosis [8]. Untreated patients with normal ALT levels have a significantly increased risk of HCC, liver transplantation, or death, regardless of HBeAg status, compared with patients with CHB receiving antiviral therapy with ALT greater than two times the upper limit of normal [9, 10].
With the rapid development of modern society and the economy, metabolic diseases related to poor eating habits and lifestyles have become increasingly prominent, leading to the rising incidence of obesity, diabetes mellitus, hypertension, and cardiovascular diseases. Metabolic syndrome (MS) comprises any three of five following conditions (elevated waist circumference, elevated serum triglyceride, reduced high-density lipoprotein, elevated blood pressure, and elevated fasting glucose) [11]. According to estimates by the International Diabetes Federation, approximately 25% of the world’s population suffers from MS, and the prevalence in China has reached 20–35%, and it is still increasing each year [12]. The prevalence of nonalcoholic fatty liver disease (NAFLD), another public health problem related to the aforementioned metabolic disorders, has also risen sharply to approximately 30% and has become the most common chronic liver disease in China [13, 14]. One study predicted that the total number of NAFLD cases will increase by approximately 30% from 2016 to 2030. As a result of the impact of urbanization, China will have the fastest growth, and the disease burden associated with NAFLD will also increase [15]. Recently, the terminology of metabolic dysfunction-associated fatty liver disease (MAFLD) has been proposed and gradually widely accepted [16]. However, most of the current studies still use the definition of NAFLD. So, this review mainly discussed NAFLD.
In clinical practice, it is not uncommon for MS or NAFLD to occur in patients with CHB. As the prevention and control of CHB disease enter a new era, CHB with normal ALT has become a focused issue, and the influence of MS and NAFLD is becoming increasingly difficult to neglect. As a result of the intricate relationships among the three diseases and their increasing incidence, the health of the public is seriously threatened and the economic burden on society is greatly increased. In recent years, researchers have conducted more in-depth studies on the interactions of MS, NAFLD, and HBV infection and their interactions with multiple organs in their epidemiology, pathogenesis, clinical manifestations, disease prevention, and therapy. This article will review the relationships among metabolic syndrome, hepatic steatosis, and HBV infection. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Methods
This review is based on a targeted search of the literature databases including PubMed, Embase, Medline, Web of Science, the Cochrane Central Register of Controlled trials, and the Cochrane Database of Systematic Reviews, using search terms combining chronic hepatitis B, hepatitis B virus, hepatic steatosis, nonalcoholic fatty liver disease, metabolic syndrome, and alanine aminotransferase. Other relevant articles identified from the included article reference lists were also included. Studies were included if they described any association among CHB, metabolic factors, and NAFLD. Non-English language articles were excluded. A narrative review was undertaken because of the heterogeneity of the identified papers. This review followed the SANRA reporting recommendations for high-quality narrative reviews [17]. Statistical analyses were performed with R version 4.1.3 using the meta and metafor packages.
Relationship between MS and NAFLD
Obesity and Insulin Resistance in NAFLD
Metabolic syndrome is a cluster of metabolic disorders, while nonalcoholic fatty liver disease is a pathological state in which excess fat accumulates in the liver as a result of nonalcoholic causes, including simple fatty liver, non-alcoholic steatohepatitis (NASH), and fibrosis. Two key components of MS, glucose and triglycerides, are overproduced in fatty liver. Therefore, the liver is a key determinant of metabolic abnormalities, and NAFLD is considered one of the manifestations of metabolic syndrome [18]. Obesity and insulin resistance are not only the key components of MS but also the initiating factors for NAFLD. Abdominal obesity and insulin resistance cause an imbalance in free fatty acid (FFA) transport between the periphery and the liver, and an imbalance among FFA synthesis, output, and catabolism, leading to hepatic steatosis. In the context of increased hepatic FFA, hepatocyte injury is triggered by immune mechanisms, leading to the activation of hepatic stellate cells and the deposition of collagen and eventually developing into NASH and liver fibrosis [19]. The increasing prevalence of abdominal obesity and NAFLD is parallel, and several conjectures about their similar underlying mechanisms have been raised. Visceral fat has a higher rate of lipolysis than subcutaneous fat depots. Visceral fat can release large amounts of nonesterified fatty acids to the portal vein, leading to hepatic steatosis and directly impacting liver metabolism, which is the so-called portal theory. In addition, visceral fat may also release more inflammatory cytokines than subcutaneous adipose tissue, resulting in a persistent low-grade inflammatory state that promotes disease progression [20, 21].
Dyslipidemia and Hypertension in NAFLD
Patients with NAFLD often have marked dyslipidemia. A large cohort study in Wuhan showed that among 3709 patients with NAFLD, only 41.8% had normal blood lipids, and among their multiple dyslipidemia phenotypes, 17.7% had metabolic syndrome dyslipidemia. Dyslipidemia is strongly associated with an increased risk of NAFLD, and this association is independent of sex, BMI, blood pressure, fasting glucose, and uric acid status [22]. In recent years, a number of mechanisms have been proposed linking NAFLD to hypertension, including increased stimulation of the sympathetic nervous system by insulin resistance or hyperinsulinemia, enhanced renal sodium reabsorption by hyperinsulinemia, impaired vasodilation stimulated by insulin, etc. [18]. However, hypertension does not always coexist with metabolic abnormalities such as obesity, insulin resistance, and dyslipidemia. Therefore, whether insulin resistance plays a leading role in the pathological process of blood pressure elevation, or whether hypertension is a downstream condition due to arteriosclerosis caused by MS and NAFLD is not yet known.
Genetic Susceptibility of NAFLD
Both disorders can be attributed, in part, to certain acquired causes, including excessive intake of a high-calorie diet and physical inactivity. Each component of MS may be related to hepatic fat accumulation. A large number of studies have shown that MS and NAFLD are risk factors for liver and extrahepatic diseases, especially by increasing the risk of type 2 diabetes and cardiovascular and cerebrovascular diseases [18]. Therefore, NAFLD is considered to be the hepatic manifestation of MS. However, some types of NAFLD are dominated by genetic susceptibility, such as genetic variants of patin-like phospholipase domain-containing protein 3 (PNPLA3), transmembrane 6 superfamily member 2 (TM6SF2), membrane bound O-acyltransferase domain containing 7 (MBOAT7), glucokinase regulatory protein (GCKR), and hydroxysteroid 17-beta dehydrogenase 13 (HSD17B13), which are often not associated with any other features of metabolic abnormalities [23,24,25]. Genetic variants affect NAFLD by interacting with environmental factors and other gene variants. For example, the effect of the PNPLA3-I148M variant was potentiated by obesity [26], and loss of Tm6sf2 was associated with reduced expression of PNPLA3 [27].
All forms of NAFLD, whether related to lifestyle or genetics, increase the risk of NASH, cirrhosis, and hepatocellular carcinoma. There are also known genes associated with obesity and MS, but genetic susceptibility is considered a secondary cause in these conditions with a rapidly increasing incidence [28]. Overall, MS and NAFLD are causally bidirectionally correlated, but not exactly equivalent. Therefore, in clinical practice, careful screening for NAFLD and related extrahepatic diseases is necessary for patients with MS, and vice versa.
Relationship between MS and CHB
Glucose Metabolism in CHB
According to recent studies, the prevalence of MS in patients with CHB is approximately 12–22%, and there is a negative correlation between MS and HBV infection. That is, the prevalence of MS in patients with chronic HBV infection is lower than that in patients without HBV infection [29,30,31]. Patients with chronic HBV infection have been in a state of chronic inflammation and immune disorder for a long time. The progression of CHB and aggravation of liver damage may have adverse effects on the body’s blood glucose and lipid metabolism. Some studies suggested that HBV infection was closely related to diabetes in Asian Americans. The prevalence of diabetes in HBsAg-positive patients was 58.9%, which was significantly higher than that in HBsAg-negative patients (33.3%). After adjustment for potential confounding variables, Asian Americans with HBV infection had a 3.17-fold higher risk of developing diabetes than those without [32]. In both patients with and without diabetes, a higher self-monitored blood glucose was associated with a higher HBsAg positivity rate and increased risk of HBV infection in patients with diabetes [33]. Male sex, age > 55 years, and BMI ≥ 24 kg/m2 were risk factors for HBV infection in patients with type 2 diabetes mellitus [34].
Relevant mechanistic exploration found that HBV x (HBx) protein could directly damage the liver insulin signaling pathway. Furthermore, inflammatory factors released by chronic HBV infection mediate intracellular signal transduction, resulting in phosphorylation of insulin receptor substrate-1 serine in insulin-sensitive cells, such as hepatocytes, adipocytes, and muscle cells, or inhibition of its tyrosine phosphorylation, which blocks insulin signal transduction and thereby induces insulin resistance [35]. Genetic factors may also play a role. For example, type 2 diabetes mellitus was significantly associated with genotype C HBV major hydrophilic region (MHR) mutation [34]. However, a study proposed that HBV itself does not promote diabetes, and this study found that the risk of diabetes did not increase in asymptomatic chronic HBV-infected patients compared with non-CHB control groups through a 10-year community follow-up [36]. In view of the currently insufficient research evidence and the lack of relevant large-scale prospective clinical cohort studies and systematic mechanism studies, a causal relationship between HBV infection and insulin resistance and diabetes cannot be concluded.
Lipid Metabolism in CHB
The liver is an important organ for lipid synthesis, metabolism, and transportation. Hepatocytes are responsible for regulating various components of blood lipids in the human body and keeping them relatively constant. When liver damage occurs in HBV-infected patients, the gene expression of the individual changes, especially the pathway of lipid biosynthesis enzymes, and blood lipid levels lose their balance accordingly [37]. For example, patients with CHB have lower levels of triglycerides, total cholesterol, and LDL cholesterol, and higher levels of HDL cholesterol than healthy controls. Lower apolipoprotein B-100 was also found in the CHB group [18, 29]. Several observations and hypotheses exist regarding the possible proatherosclerotic effects of HBV. A study from Turkey showed that HBsAg carriers had a greater mean platelet volume, which was considered to be a new risk factor for atherothrombosis. Therefore, the researchers proposed that inactive HBsAg carriers had relatively increased risk of platelet activation and atherothrombosis [38]. However, the clinical relevance of this observation is unclear. A study from Japan exploring whether HBV infection affects the severity of arteriosclerosis in subjects found that there were no significant differences in systolic blood pressure, bilateral ankle-brachial index, heart-ankle pulse wave velocity, or heart-carotid pulse wave velocity between patients with CHB and healthy controls [39]. Chronic HBV infection did not have a statistical effect on the evaluation of carotid intima-media thickness, maximum common carotid intima-media thickness, and extracranial carotid atherosclerosis score [40]. In a 17-year follow-up study, HBsAg seropositivity was not associated with an increased risk of atherosclerosis-related death or cardiovascular disease-related death [41]. Therefore, patients with CHB do not appear to be at increased risk of developing atherosclerosis, and HBV infection may not be a predictor of atherosclerosis-related death or cardiovascular disease-related death.
Impact of MS on CHB Progression
The impact of metabolic factors on the progression and prognosis of CHB disease is unclear. MS seems to be an independent determinant of a poor prognosis in patients with CHB treated with oral nucleoside analogues. CHB combined with MS resulted in significantly higher cumulative incidences of virological breakthrough, genotypic resistance, HCC, disease progression, and overall adverse outcomes in patients with CHB. The overall survival time of the combined MS group was also significantly shorter than that of the control group [42]. Wong et al. found that MS was an independent risk factor for cirrhosis in patients with CHB, and the odds ratio increased with the increase in MS components [43]. This team conducted a follow-up study and found that co-occurrence of MS increased the progression risk of liver fibrosis in patients with CHB, and this association was independent of viral loads and hepatitis activity [44]. However, Khalili et al. reported that MS did not directly increase the risk of liver fibrosis in patients with CHB [31]. In addition to its effect on liver fibrosis, metabolic abnormalities including diabetes, obesity, and central fat deposition are also risk factors for HCC [45]. These factors are associated with HBV-related HCC progression and decreased survival rates. Oxidative stress and lipid peroxidation may be involved in the pathogenesis and acceleration of liver damage [46].
In conclusion, HBV may be related to blood glucose and blood lipid metabolism. MS may have adverse effects on the progression and prognosis of CHB disease. However, the existing data cannot fully reveal the relationship between HBV and the risks of MS, insulin resistance, and arteriosclerosis. This needs to be confirmed by further research. MS is also strongly associated with elevated ALT levels. Thus, metabolic factors may be one of the key links in studies on patients with ALT-normal CHB.
Relationship between NAFLD and CHB
Impact of NAFLD on CHB Progression
According to recent studies, the incidence of hepatic steatosis in patients with CHB ranges from 14% to 60% [47,48,49]. A series of clinical studies suggested that HBV infection was independently associated with a lower risk of fatty liver, possibly due to altered lipid metabolism by HBV [48, 50, 51]. Yuen et al. reported that steatosis was associated with a lower HBV DNA load, while severe steatosis was associated with liver fibrosis in patients with CHB [52]. Their subsequent prospective study also found that hepatic steatosis increased HBsAg seroclearance by threefold, but at the same time increased the risk of fibrosis progression [47]. Even though hepatic steatosis could advance HBsAg seroclearance by approximately 5 years compared with non-NAFLD HBsAg carriers, the benefits of HBsAg seroclearance did not necessarily outweigh the harms of steatosis such as hepatic fibrosis and cirrhosis [53]. NAFLD also increased the risk of HCC in patients with CHB in whom HBV was effectively suppressed by antiviral therapy [54]. Conversely, HBV infection was found to be associated with a higher risk of HCC in patients with NAFLD [55].
There are also studies that presented inconsistent views. Li et al. suggested that fatty liver was significantly associated with a reduced risk of cirrhosis and HCC in patients with CHB [56]. A Texas study showed that there was no significant difference in the risk of cirrhosis and HCC between NAFLD and non-NAFLD subjects with persistently normal ALT [57]. In general, although the conclusion that the harms of combined steatosis outweigh the benefits still needs to be supported by further research data, with the gradual deepening of clinical awareness of the harms of steatosis, advocating the screening and prevention of NAFLD in patients with CHB will be an important part of the hepatitis B control strategy.
Mechanism of NAFLD Inhibiting HBV Replication
Animal experiments further verified the clinical conclusion that NAFLD inhibited the replication of HBV. Zhang et al. established a rodent model of NAFLD complicated with chronic HBV infection by using a high-fat diet and transgenic manipulation, which showed that steatosis suppressed HBV virological factors [58]. Similarly, another team, using a high-fat diet and high-pressure tail vein injection of HBV plasmids, established an immunocompetent mouse model of HBV infection combined with NAFLD, in which the coexistence of NAFLD reduced HBV DNA load and HBV antigens, but HBV replication did not alter lipid metabolism in mice [59]. The underlying mechanism of the interaction between NAFLD and CHB may be that metabolic alterations of NAFLD directly inhibit HBV replication or indirectly enhance antiviral responses by activating innate immunity, and NAFLD-mediated apoptosis may destroy HBV-infected host cells, thereby inhibiting CHB progression. Genetic susceptibility is also a part of the reason. For example, in comorbid patients, PNPLA3 gene polymorphisms are associated with a lower HBV DNA load [60]. HBx protein is associated with the effect of HBV on NAFLD. HBx alters the expression and activity of multiple metabolism-related transcription factors and inhibits VLDL secretion, ultimately leading to lipid accumulation in hepatocytes. HBx also promotes the expression of adiponectin, which may improve insulin sensitivity. Another viral protein, the Pre-S1 domain, may also upregulate endogenous cholesterol synthesis, disrupting normal bile acid uptake [61]. In addition, patients with CHB may pay more attention to healthy lifestyles and eating habits [62].
Impact of NAFLD on Anti-HBV Therapy
The effect of fatty liver on anti-HBV therapy with nucleoside analogues and its mechanism are still inconclusive. A prospective nested case–control study, with a mean follow-up of 79.3 weeks, found that hepatic steatosis was closely associated with entecavir treatment failure, and metabolic factors were independent risk factors for hepatic steatosis. The possible explanation was that the accumulation of fat in hepatocytes reduced the contact area between the drug and the hepatocytes, resulting in a decrease in the bioavailability of entecavir. On the other hand, a decrease in the activity of hepatic cytochrome hindered drug metabolism. Moreover, the coexistence of insulin resistance, obesity, and hepatic steatosis might lead to the impairment of cellular immunity function [63]. Some studies showed that NAFLD does not affect the long-term total virological response rate or HBeAg seroconversion rate in entecavir-treated patients with CHB, but it does reduce the long-term biochemical response rate, which is positively correlated with the steatosis severity and insulin resistance index [34]. The conclusion that fatty liver does not affect the antiviral effect of pegylated interferon is more consistent. Two studies from Turkey reported that steatosis was not associated with viral load or the effect of interferon antiviral therapy [64, 65]. Shi et al. believed that hepatic steatosis might affect the biochemical response of patients with CHB but not their virological response to interferon therapy [66]. Current clinical studies and mechanism explorations cannot reveal the specific effect of NAFLD on antiviral therapy in patients with CHB, and few studies have reported whether timely control of hepatic steatosis and metabolic abnormalities was conducive to improving the clinical prognosis of patients with CHB.
The relationship between chronic HBV infection and NAFLD is complex and clinically important, including many aspects such as the effect of HBV on blood lipid metabolism, the effect of steatosis on HBV replication, the effect of steatosis on the progression of CHB disease, and the effect of steatosis on the effect of anti-HBV therapy. As mentioned above, research in recent years has made great progress. The problem that needs to be solved in future studies is effectively preventing and controlling fatty liver to improve the clinical outcomes and prognoses of patients with CHB.
MS and NAFLD in ALT-normal CHB
Abnormal Liver Histology in ALT-Normal CHB
Recent studies have found that patients with CHB and normal ALT often have abnormal liver histology. A meta-analysis including 2271 treatment-naïve CHB participants found that the pooled proportions of significant inflammation, fibrosis, and cirrhosis were 35%, 30%, and 3%, respectively [67]. A multicenter study showed that half of patients with CHB and normal ALT had liver histological abnormalities, including 36.4% with moderate to severe inflammation and 34.0% with significant fibrosis [8]. Cheng et al. also found marked necroinflammation and fibrosis in 36.5% and 15.5% of patients with CHB with persistently normal ALT, respectively [68]. As a result of differences in sample size, study population, diagnostic methods, and viral factors, the proportion of histological abnormalities obtained in each study was not the same. We summarized the results from recent studies in Fig. 1 [7, 8, 67,68,69,70,71,72,73,74].
The aforementioned ten studies were then retrieved for meta-analysis to determine the prevalence of liver inflammation and fibrosis in patients with CHB and normal ALT levels. The results showed that the proportions of liver inflammation and fibrosis were 28.0% (95% CI 0.20–0.35 by random-effects model; heterogeneity: I2 = 97%) and 26.0% (95% CI 0.18–0.34 by random-effects model; heterogeneity: I2 = 96%), respectively (Figs. 2 and 3).
Status Quo of ALT-Normal CHB with MS or NAFLD
Combined MS and NAFLD is less of a concern to clinicians in the presence of normal ALT in patients with CHB, even though many current academic studies have excluded patients with these disorders [6, 7, 68]. The prevalence of MS and NAFLD and their impact on liver disease progression, including hepatic fibrosis, have also been poorly studied in the few studies that did not overtly exclude these two abnormalities. A recent study tentatively explored this area and showed that MS affected 23.1% and NAFLD affected 37.2% of treatment-naïve CHB participants with normal ALT levels. It also found the increased metabolic components aggravated steatosis, which increased the risk of significant fibrosis [75]. However, these conclusions need to be validated by carrying out prospective studies. Moreover, there are still research gaps in the effects of CHB on insulin resistance, glucose metabolism, and lipid metabolism when ALT appears normal.
A multicenter study from China found that 50.2% of the study patients with normal ALT levels fulfilled the histological indications for antiviral therapy. Some of these subjects received anti-HBV treatment and it had a similar efficacy as for patients with elevated ALT [8]. Another multicenter study found that lower BMI and weight reduction affect NASH resolution in patients with CHB, and proposed that weight management in patients with CHB during antiviral treatment deserves further attention [76]. It can be speculated from the aforementioned findings that patients with CHB and normal ALT levels can benefit from timely antiviral therapy or weight control. However, no study has been conducted to investigate whether synchronous antiviral therapy and control of MS and NAFLD could be additive to this benefit. In addition, researchers should carefully consider whether to include MS and NAFLD as an indication for early antiviral therapy in the future, and whether to stratify patients with CHB according to the presence or absence of metabolic problems during the management of chronic liver disease.
Whether these questions are answered in the same way in the subgroup of patients with CHB and normal ALT levels as in the overall CHB population is unknown. Whether lowering the antiviral threshold and simultaneously intervening in metabolic disorders and steatosis can improve CHB outcomes is still inconclusive. Therefore, there is an urgent need for well-designed randomized controlled trials. There is also a lack of evidence for any effect of CHB on various metabolic processes, and few studies have explored whether this effect is related to ALT levels. We have to obtain more data before drawing solid conclusions.
Conclusion
NAFLD is associated with several components of MS and is a hepatic manifestation of metabolic abnormalities. Although HBV affects blood glucose and blood lipid metabolism to a certain extent and NAFLD inhibits HBV replication, MS and NAFLD are mutually complementary and reciprocally causal, which will ultimately adversely affect the progression and prognosis of CHB. The effects of metabolic factors and fatty liver on antiviral therapy are unclear. Therefore, the interactions of metabolic factors, fatty liver, and HBV infection may jointly promote the progression of liver disease, but the mechanism is still inconclusive (Fig. 4). Large-scale prospective clinical studies are needed for further verification, and more mechanistic studies are needed for further exploration. In addition, to overcome CHB, researchers have turned their attention to patients with CHB and normal ALT levels. The current issues are whether such patients are at risk of disease progression and whether they need antiviral therapy, but the roles of metabolic factors and steatosis are rarely explored. With the increasing prevalence of MS and NAFLD, both will receive more attention and discussion in future studies about patients with CHB and normal ALT.
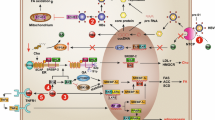
The relationships among metabolic syndrome, steatosis, and HBV infection. Two key components of MS, obesity and insulin resistance, serve as initiating factors of liver steatosis. Steatosis is usually combined with dyslipidemia, another important component of MS. However, the relationship between steatosis and hypertension remains unclear. Hepatic steatosis could inhibit HBV replication but increase the risk of liver fibrosis. HBV infection appears to reduce the MS and NAFLD risk and affect glucose and lipid metabolism by HBx protein, PreS1 protein, and possibly strict diet-control. The effect of MS and NAFLD on antiviral therapy is still controversial. However, it is clear that these three diseases can synergistically result in the progression of chronic liver disease. Whether the above relationship exists in patients with CHB with normal ALT remains to be explored. NAFLD nonalcoholic fatty liver disease, HBV hepatitis B virus, HBx hepatitis B virus x protein, PreS1 hepatitis B virus PreS1 protein, ALT alanine aminotransferase, CHB chronic hepatitis B, MS metabolic syndrome, HCC hepatocellular carcinoma
References
Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560–99.
Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546–55.
Mohsen N, Haidong W, Rafael L, et al. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–71.
Devin RS, Ivane G, Mindie HN, et al. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3:383–403.
Tseng TC, Liu CJ, Hsu CY, et al. High level of hepatitis B core-related antigen associated with increased risk of hepatocellular carcinoma in patients with chronic HBV infection of intermediate viral load. Gastroenterology. 2019;157:1518–1529.e1513.
Tan YW, Zhou XB, Ye Y, He C, Ge GH. Diagnostic value of FIB-4, aspartate aminotransferase-to-platelet ratio index and liver stiffness measurement in hepatitis B virus-infected patients with persistently normal alanine aminotransferase. World J Gastroenterol. 2017;23:5746–54.
Duan M, Chi X, Xiao H, Liu X, Zhuang H. High-normal alanine aminotransferase is an indicator for liver histopathology in HBeAg-negative chronic hepatitis B. Hepatol Int. 2021;15:318–27.
Wu Z, Ma AL, Xie Q, et al. Significant histological changes and satisfying antiviral efficacy in chronic hepatitis B virus infection patients with normal alanine aminotransferase. Antiviral therapy decision in chronic HBV patients with normal ALT. Clin Res Hepatol Gastroenterol. 2021;45:101463.
Choi GH, Kim GA, Choi J, Han S, Lim YS. High risk of clinical events in untreated HBeAg-negative chronic hepatitis B patients with high viral load and no significant ALT elevation. Aliment Pharmacol Ther. 2019;50:215–26.
Kim GA, Lim YS, Han S, et al. High risk of hepatocellular carcinoma and death in patients with immune-tolerant-phase chronic hepatitis B. Gut. 2018;67:945–52.
Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5.
Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20:12.
Wang FS, Fan JG, Zhang Z, Gao B, Wang HY. The global burden of liver disease: the major impact of China. Hepatology. 2014;60:2099–108.
Zhou F, Zhou J, Wang W, et al. Unexpected rapid increase in the burden of NAFLD in China from 2008 to 2018: a systematic review and meta-analysis. Hepatology. 2019;70:1119–33.
Estes C, Anstee QM, Arias-Loste MT, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J Hepatol. 2018;69:896–904.
Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73:202–9.
Baethge C, Goldbeck-Wood S, Mertens S. SANRA—a scale for the quality assessment of narrative review articles. Res Integr Peer Rev. 2019;4:5.
Yki-Järvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014;2:901–10.
Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10:330–44.
Frayn KN. Visceral fat and insulin resistance—causative or correlative? Br J Nutr. 2000;83(Suppl 1):S71-77.
Tchernof A, Després JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93:359–404.
Du T, Sun X, Yuan G, et al. Lipid phenotypes in patients with nonalcoholic fatty liver disease. Metabolism. 2016;65:1391–8.
Speliotes EK, Butler JL, Palmer CD, Voight BF, Hirschhorn JN. PNPLA3 variants specifically confer increased risk for histologic nonalcoholic fatty liver disease but not metabolic disease. Hepatology. 2010;52:904–12.
Martin K, Hatab A, Athwal VS, Jokl E, Piper HK. Genetic contribution to non-alcoholic fatty liver disease and prognostic implications. Curr Diab Rep. 2021;21:8.
Jonas W, Schürmann A. Genetic and epigenetic factors determining NAFLD risk. Mol Metab. 2021;50: 101111.
Stender S, Kozlitina J, Nordestgaard BG, Tybjærg-Hansen A, Hobbs HH, Cohen JC. Adiposity amplifies the genetic risk of fatty liver disease conferred by multiple loci. Nat Genet. 2017;49:842–7.
Smagris E, Gilyard S, BasuRay S, Cohen JC, Hobbs HH. Inactivation of Tm6sf2, a gene defective in fatty liver disease, impairs lipidation but not secretion of very low density lipoproteins. J Biol Chem. 2016;291:10659–76.
Locke AE, Kahali B, Berndt SI, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197–206.
Huang CY, Lu CW, Liu YL, Chiang CH, Lee LT, Huang KC. Relationship between chronic hepatitis B and metabolic syndrome: a structural equation modeling approach. Obesity (Silver Spring). 2016;24:483–9.
Yan LB, Liao J, Han N, et al. Association between hepatitis B virus infection and metabolic syndrome in Southwest China: a cross-sectional study. Sci Rep. 2020;10:6738.
Khalili M, Shuhart MC, Lombardero M, et al. Relationship between metabolic syndrome, alanine aminotransferase levels, and liver disease severity in a multiethnic North American cohort with chronic hepatitis B. Diabetes Care. 2018;41:1251–9.
Li-Ng M, Tropp S, Danoff A, Bini EJ. Association between chronic hepatitis B virus infection and diabetes among Asian Americans and Pacific Islanders. Dig Liver Dis. 2007;39:549–56.
Han B, Liu W, Yang S, et al. Association between self-monitoring of blood glucose and hepatitis B virus infection among people with diabetes mellitus: a cross-sectional study in Gansu Province, China. BMJ Open. 2021;11: e048463.
Zhu H, Wang Y, Yu L, et al. Serological and molecular analysis on the relationships between type 2 diabetes mellitus and hepatitis B virus infection. J Infect Dev Ctries. 2016;10:837–44.
Kim K, Kim KH, Cheong J. Hepatitis B virus X protein impairs hepatic insulin signaling through degradation of IRS1 and induction of SOCS3. PLoS ONE. 2010;5: e8649.
Huang ZS, Huang TS, Wu TH, Chen MF, Hsu CS, Kao JH. Asymptomatic chronic hepatitis B virus infection does not increase the risk of diabetes mellitus: a ten-year observation. J Gastroenterol Hepatol. 2010;25:1420–5.
Hajjou M, Norel R, Carver R, et al. cDNA microarray analysis of HBV transgenic mouse liver identifies genes in lipid biosynthetic and growth control pathways affected by HBV. J Med Virol. 2005;77:57–65.
Turhan O, Coban E, Inan D, Yalcin AN. Increased mean platelet volume in chronic hepatitis B patients with inactive disease. Med Sci Monit. 2010;16:Cr202–205.
Moritani M, Adachi K, Arima N, et al. A study of arteriosclerosis in healthy subjects with HBV and HCV infection. J Gastroenterol. 2005;40:1049–53.
Yang KC, Chen MF, Su TC, et al. Hepatitis B virus seropositivity is not associated with increased risk of carotid atherosclerosis in Taiwanese. Atherosclerosis. 2007;195:392–7.
Wang CH, Chen CJ, Lee MH, Yang HI, Hsiao CK. Chronic hepatitis B infection and risk of atherosclerosis-related mortality: a 17-year follow-up study based on 22,472 residents in Taiwan. Atherosclerosis. 2010;211:624–9.
Kim NH, Cho YK, Kim BI, Kim HJ. Effect of metabolic syndrome on the clinical outcomes of chronic hepatitis B patients with nucleos(t)ide analogues treatment. Dig Dis Sci. 2018;63:2792–9.
Wong VW, Wong GL, Choi PC, et al. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut. 2010;59:969–74.
Wong GL, Chan HL, Yu Z, et al. Coincidental metabolic syndrome increases the risk of liver fibrosis progression in patients with chronic hepatitis B–a prospective cohort study with paired transient elastography examinations. Aliment Pharmacol Ther. 2014;39:883–93.
Yip TC, Lee HW, Chan WK, Wong GL, Wong VW. Asian perspective on NAFLD-associated HCC. J Hepatol. 2022;76:726–34.
Zhao J, Zhao Y, Wang H, Gu X, Ji J, Gao C. Association between metabolic abnormalities and HBV related hepatocelluar carcinoma in Chinese: a cross-sectional study. Nutr J. 2011;10:49.
Mak LY, Hui RW, Fung J, et al. Diverse effects of hepatic steatosis on fibrosis progression and functional cure in virologically quiescent chronic hepatitis B. J Hepatol. 2020;73:800–6.
Wong VW, Wong GL, Chu WC, et al. Hepatitis B virus infection and fatty liver in the general population. J Hepatol. 2012;56:533–40.
Tsochatzis E, Papatheodoridis GV, Manesis EK, Chrysanthos N, Kafiri G, Archimandritis AJ. Hepatic steatosis in chronic hepatitis B develops due to host metabolic factors: a comparative approach with genotype 1 chronic hepatitis C. Dig Liver Dis. 2007;39:936–42.
Cheng YL, Wang YJ, Kao WY, et al. Inverse association between hepatitis B virus infection and fatty liver disease: a large-scale study in populations seeking for check-up. PLoS ONE. 2013;8: e72049.
Wang MM, Wang GS, Shen F, Chen GY, Pan Q, Fan JG. Hepatic steatosis is highly prevalent in hepatitis B patients and negatively associated with virological factors. Dig Dis Sci. 2014;59:2571–9.
Hui RWH, Seto WK, Cheung KS, et al. Inverse relationship between hepatic steatosis and hepatitis B viremia: results of a large case-control study. J Viral Hepat. 2018;25:97–104.
Chu CM, Lin DY, Liaw YF. Clinical and virological characteristics post HBsAg seroclearance in hepatitis B virus carriers with hepatic steatosis versus those without. Dig Dis Sci. 2013;58:275–81.
Cho H, Chang Y, Lee JH, et al. Radiologic nonalcoholic fatty liver disease increases the risk of hepatocellular carcinoma in patients with suppressed chronic hepatitis B. J Clin Gastroenterol. 2020;54:633–41.
Chan TT, Chan WK, Wong GL, et al. Positive Hepatitis B core antibody is associated with cirrhosis and hepatocellular carcinoma in nonalcoholic fatty liver disease. Am J Gastroenterol. 2020;115:867–75.
Li J, Yang HI, Yeh ML, et al. Association between fatty liver and cirrhosis, hepatocellular carcinoma, and hepatitis B surface antigen seroclearance in chronic hepatitis B. J Infect Dis. 2021;224:294–302.
Natarajan Y, Kramer JR, Yu X, et al. Risk of cirrhosis and hepatocellular cancer in patients with NAFLD and normal liver enzymes. Hepatology. 2020;72:1242–52.
Zhang Z, Pan Q, Duan XY, et al. Fatty liver reduces hepatitis B virus replication in a genotype B hepatitis B virus transgenic mice model. J Gastroenterol Hepatol. 2012;27:1858–64.
Hu D, Wang H, Wang H, et al. Non-alcoholic hepatic steatosis attenuates hepatitis B virus replication in an HBV-immunocompetent mouse model. Hepatol Int. 2018;12:438–46.
Pan Q, Zhang RN, Wang YQ, et al. Linked PNPLA3 polymorphisms confer susceptibility to nonalcoholic steatohepatitis and decreased viral load in chronic hepatitis B. World J Gastroenterol. 2015;21:8605–14.
Zhang J, Lin S, Jiang D, et al. Chronic hepatitis B and non-alcoholic fatty liver disease: Conspirators or competitors? Liver Int. 2020;40:496–508.
Kim CH, Kallman JB, Bai C, et al. Nutritional assessments of patients with non-alcoholic fatty liver disease. Obes Surg. 2010;20:154–60.
Jin X, Chen YP, Yang YD, Li YM, Zheng L, Xu CQ. Association between hepatic steatosis and entecavir treatment failure in Chinese patients with chronic hepatitis B. PLoS ONE. 2012;7: e34198.
Cindoruk M, Karakan T, Unal S. Hepatic steatosis has no impact on the outcome of treatment in patients with chronic hepatitis B infection. J Clin Gastroenterol. 2007;41:513–7.
Ateş F, Yalnız M, Alan S. Impact of liver steatosis on response to pegylated interferon therapy in patients with chronic hepatitis B. World J Gastroenterol. 2011;17:4517–22.
Shi JP, Lu L, Qian JC, et al. Impact of liver steatosis on antiviral effects of pegylated interferon-alpha in patients with chronic hepatitis B. Zhonghua Gan Zang Bing Za Zhi. 2012;20:285–8.
Zhang C, Li JW, Wu Z, Zhao H, Wang GQ. Significant histologic changes are not rare in treatment-naive hepatitis B patients with normal alanine aminotransferase level: a meta-analysis. J Clin Transl Hepatol. 2021;9:615–25.
Cheng JL, Wang XL, Yang SG, Zhao H, Wu JJ, Li LJ. Non-ALT biomarkers for markedly abnormal liver histology among Chinese persistently normal alanine aminotransferase-chronic hepatitis B patients. World J Gastroenterol. 2017;23:2802–10.
Lai M, Hyatt BJ, Nasser I, Curry M, Afdhal NH. The clinical significance of persistently normal ALT in chronic hepatitis B infection. J Hepatol. 2007;47:760–7.
Gui HL, Wang H, Yang YH, et al. Significant histopathology in Chinese chronic hepatitis B patients with persistently high-normal alanine aminotransferase. J Viral Hepat. 2010;17(Suppl 1):44–50.
Göbel T, Erhardt A, Herwig M, et al. High prevalence of significant liver fibrosis and cirrhosis in chronic hepatitis B patients with normal ALT in central Europe. J Med Virol. 2011;83:968–73.
Wang H, Ru GQ, Yan R, Zhou Y, Wang MS, Cheng MJ. Histologic disease in chinese chronic hepatitis B patients with low viral loads and persistently normal alanine aminotransferase levels. J Clin Gastroenterol. 2016;50:790–6.
Li Q, Lu C, Li W, Huang Y, Chen L. The independent predictors of significant liver histological changes in chronic hepatitis B virus infection patients with persistently high-normal or low-normal alanine transaminase levels. Discov Med. 2017;23:19–25.
Xing YF, Zhou DQ, He JS, et al. Clinical and histopathological features of chronic hepatitis B virus infected patients with high HBV-DNA viral load and normal alanine aminotransferase level: a multicentre-based study in China. PLoS ONE. 2018;13:e0203220.
Diao Y, Hu D, Hu X, et al. The role of metabolic factors and steatosis in treatment-naïve patients with chronic hepatitis B and normal alanine aminotransferase. Infect Dis Ther. 2022;11:1133.
Chang XJ, Shi YW, Wang J, et al. Influence of weight management on the prognosis of steatohepatitis in chronic hepatitis B patients during antiviral treatment. Hepatobiliary Pancreat Dis Int. 2021;20:416–25.
Acknowledgements
Funding
This study was funded by the Key Science and Technology Plan Projects of Zigong (2020ZC06). The authors funded the journal’s Rapid Service Fee.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authors’ Contributions
Chunfang You and Yuting Diao had the idea for the article. Xuerong Wang and Yuting Diao performed the literature search and data analysis. Yuting Diao drafted the paper. Chunfang You, Juan Tang, Wei Deng and Jing Tang critically revised the work.
Disclosures
The authors Yuting Diao, Juan Tang, Xuerong Wang, Wei Deng, Jing Tang and Chunfang You declare that they have no conflicts of interest.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data availability
All data generated or analyzed during this study are included in this published article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Diao, Y., Tang, J., Wang, X. et al. Metabolic Syndrome, Nonalcoholic Fatty Liver Disease, and Chronic Hepatitis B: A Narrative Review. Infect Dis Ther 12, 53–66 (2023). https://doi.org/10.1007/s40121-022-00725-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-022-00725-6