Abstract
Severe fever with thrombocytopenia syndrome virus (SFTSV) is a tick-borne virus that produces severe fever with thrombocytopenia syndrome (SFTS). It is widespread in Japan, South Korea, and Central and Eastern China. The epidemic has developed rapidly through China in recent years. SFTS cases have been reported in 25 provinces in China, mainly distributed in rural areas in mountainous and hilly areas. The infection has a high case fatality rate and no specific treatments or vaccinations. Therefore, early diagnosis and treatment of SFTS infection is important to survival and disease control. In this article, we provide an overview on different aspects of SFTS with an emphasis on management, to explore the current treatment and prophylactic measures further.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Severe fever with thrombocytopenia syndrome (SFTS) is an acute, systemic viral infection caused by Dabie bandavirus in the family Phenuiviridae, transmitted mainly by Haemaphysalis longicornis. |
It is reported that the mortality rates of SFTS in Japan, South Korea, and China are 27%, 23.3%, and 6.18%, respectively. |
The susceptibility of B cells to SFTSV and the mechanism of virus transmission remain to be explored. |
There is no clinical trial to prove the beneficial effect of ribavirin in the treatment of hospitalized patients with SFTS. |
Studies have shown that favipiravir might be more effective than ribavirin in the treatment of patients with SFTS. |
At present, there is no specific antiviral treatment effective for this disease, and the recovery mainly depends on supportive treatment. |
Introduction
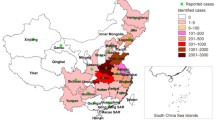
Severe fever with thrombocytopenia syndrome (SFTS) is a newly recognized viral infectious disease that was initially identified in China in 2009 [1] and has since spread to several regions such as South Korea [2], Japan, and the USA [3, 4]. The epidemic has developed rapidly through China in recent years. SFTS cases have been reported in 25 provinces in China, mainly distributed in rural areas in mountainous and hilly areas. The cases are highly sporadic, but relatively concentrated in regional distribution, mainly in Henan, Shandong, Anhui, Hubei, Liaoning, Zhejiang, and Jiangsu (Fig. 1). SFTS virus (SFTSV) is a tick-borne virus and formally named as Dabie bandavirus. It is a member of the genus bandavirus, which belongs to the Phenuiviridae family and Bunyavirales order [5].
SFTSV particles are spherical with surface spines and are mainly distributed in the infected cells’ microsomes. SFTSV genome consists of three (large, medium, and small) single-stranded RNA negative chains (Fig. 2), coding RNA polymerase, virus membrane protein, and nonstructural protein, respectively [6].
At present, SFTSV poses a threat to public health, and there is no vaccine or drug available to prevent SFTS. At the same time, the pathogenic mechanisms are largely unknown. The study showed that the spleen is the main target organ of SFTSV [7]. SFTSV could directly infect macrophages and continue to lurk in splenic macrophages (Fig. 3). Park et al. showed that the B cell lineage, especially plasma cells, is linked to lethality in SFTSV infections [8]. SFTSV could target RIG-I or other RIG-I-like receptors to activate type I interferon (IFN) response, while IFN-stimulated genes were overexpressed across plasmablast after SFTSV infection [9].
Progression to cytokine storm. Innate immune response: SFTSV is recognized by macrophages, dendritic cells, and natural killer cells. T cells secrete various cytokines that provoke an inflammatory storm. B cells differentiate into plasma cells, which secrete anti-SFTSV antibodies. SFTSV severe fever with thrombocytopenia syndrome virus, APC antigen-presenting cells. Created with BioRender.com
The clinical manifestations of SFTSV infection are fever, muscle soreness, nausea and vomiting, rapid decrease of peripheral blood platelet in most infected cases, and damage of the liver, kidney, and other organs [10]. The clinical progression of SFTS encompasses four distinct stages: incubation period, fever period, multiple organ dysfunction syndrome (MODS) period, and convalescence period.
- Stage 1:
-
The incubation period of the disease ranges from 5 to 14 days after tick bite, and is affected by virus dose.
- Stage 2:
-
This period lasts for 5–11 days, and is characterized by high viral load, which is a measure for diagnosis. Fatigue, anorexia, muscle soreness, diarrhea, nausea, and lymphadenopathy occur in more than half of patients, while abdominal pain, vomiting, cough, and oral bleeding occur in less than 50%. Laboratory findings include leukopenia, thrombocytopenia, elevated transaminases, and lactate dehydrogenase in patients.
- Stage 3:
-
A small number of patients with SFTS will reach stage 3, which is associated with higher mortality. In this stage, a minority of patients experience disturbance of consciousness, along with organ damage (e.g., liver and cardiac) and derangements of coagulation biomarkers.
- Stage 4:
-
The patient’s temperature returned to normal, symptoms improved, and organ functions and laboratory indicators gradually returned to normal.
The therapy options for SFTSV infection remain limited, and the primary clinical treatment is symptomatic care. In order to provide available evidence to select antiviral therapy to treat SFTS, the present state of work and future directions in developing antiviral therapies for SFTSV are discussed in detail in this article.
Methods
This review discusses the different treatment and prevention measures found in the recent literature. A comprehensive literature search was conducted on March 1, 2021, using the Chinese National Knowledge Infrastructure (CNKI), Web of Science, and PubMed databases. An updated search was performed weekly until March 2022. Search terms were predefined and consisted of the combination of the following: “SFTSV”, “dabie bandavirus”, “antiviral drug”, “treatments”, and “bunyavirus”. On the basis of the literature search results, the most common therapies were favipiravir, ribavirin, interferons, antibody, plasma exchange, and combinations thereof.
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
RNA-Dependent RNA Polymerase (RdRp) Inhibitors
Ribavirin
Direct and indirect mechanisms can explain the antiviral properties of ribavirin. The former phase includes lethal mutagenesis, polymerase inhibition, and interference with RNA capping, while the latter stage contains immune regulation and inosine monophosphate dehydrogenase inhibition [11]. Ribavirin is an antiviral drug that works against a variety of RNA viruses. It belongs to the class of medications known as nucleoside antimetabolites, which prevent viral genetic material from being duplicated. Ribavirin has important activities against Hantaan, flaviviruses, and Crimean–Congo hemorrhagic fever virus (CCHF). It is reported that ribavirin effectively treats infection caused by hemorrhagic fever viruses, such as the SFTS virus [12]. Although ribavirin reduces viral activity in vitro, it does not affect platelet counts or viral loads in patients with fatal or non-fatal disease during the hospital stay, suggesting that it may have limited therapeutic value in treating SFTSV infection [13]. Upon looking through the literature (Table 1), it becomes clear that research on ribavirin’s usefulness in patients with SFTS is conflicting [16]. A small observational study in 2019 found no significant difference in the mortality rate and recovery of some laboratory indexes between those taking ribavirin and those not [17].
An in vitro test showed that ribavirin had inhibitory effect before virus inoculation. Shimojima et al. suggested that ribavirin should be used as a preventive drug for SFTS [13]. Bone marrow suppression can cause reversible dose-dependent hemolytic anemia and increase plasma uric acid, iron, and bilirubin levels [18]. They are not recommended for usage during pregnancy because of their teratogenic and mutagenic properties [19].
Favipiravir
Toyama Chemical (Japan) developed favipiravir (T-705, Avigan), a broad-spectrum antiviral drug that effectively suppresses RNA virus’s RdRp. Favipiravir was licensed in Japan in March 2014 to treat new or recurring influenza virus infections [20]. It is active against influenza viruses, West Nile virus, yellow fever virus, flavivirus, arenavirus, bunyaviruses, alphavirus, enteroviruses, and Rift Valley fever virus. Favipiravir is a potential therapeutic target for treating influenza and other RNA viral infections [21]. Several studies utilizing small animals have shown that favipiravir has potency against SFTSV and other bunyaviruses [22, 23]. Two days following favipiravir treatment, the serum virus load in mice infected with SFTSV was undetectable [24]. There was no mortality in the favipiravir-treated bunyavirus-infected mice in another investigation. On the other hand, the ribavirin-treated group experienced a high death rate [25], showing favipiravir’s possible protective impact against the SFTSV.
Clinical trials of the drug have been carried out in many countries worldwide, including standard randomized controlled trials (RCTs), small randomized trials, case series trials, and observational trials. There are differences in the research reports; almost all suggest the effectiveness of favipiravir.
According to one study, two individuals with SFTS were treated with favipiravir (1600 mg twice orally on day 1, then 600 mg orally twice daily for 4 days). Favipiravir was given to the patients between day 4 and day 11 after onset. As a result, the patients recovered completely [26]. In a randomly assigned, open-label, single-blind clinical study of patients with SFTS (n = 145), favipiravir (1800 mg twice orally on day 1, then 1000 mg twice daily for 5 days) performed significantly better than the control group (standard supportive care) in terms of viral clearance and disease progression [27]. In an uncontrolled single-arm, non-randomized, prospective, open-label, multicenter clinical study that included patients with SFTS (n = 23), favipiravir (1800 mg orally twice daily on day 1, then 800 mg orally twice daily for 7–14 days) had a greater 2-day recovery rate than reported earlier research from Japan [29].
Favipiravir is safe to use in humans because it has no inhibitory effect on mammalian DNA or RNA synthesis and is non-toxic to mammalian cells. Favipiravir appears to have more favorable effectiveness and safety than ribavirin.
Fludarabine
Fludarabine is a synthetic adenine nucleoside analogue mainly used in the first-line and second-line treatment of various lymphoproliferative malignancies [30]. In 1969 it was initially synthesized by Hewson and Montgomery [31]. The results revealed that the fludarabine suppressed SFTSV replication in human microglia HMC3 cells (IC50 = 0.42 ± 0.01 μM) and astrocyte U251 MG cells (IC50 = 0.28 ± 0.17 μM) in a dose-dependent manner. By detecting the replication and proliferation of virus and the expression of virus protein in cells, the researchers stated that fludarabine had a good antiviral effect on SFTSV in U251 and HMC3 nerve cells. According to a similar study, fludarabine exhibits antiviral effectiveness against positive-stranded RNA viruses, Zika virus (ZIKV), and Enterovirus A71 [32].
However, fludarabine has certain cytotoxicity and clinical side effects. Myelosuppression, lymphocytopenia, and secondary infection are the most common side effects of fludarabine treatment. Its toxicity includes gastrointestinal side effects such as increased liver enzymes, vomiting, and nausea. It should be noted that patients with SFTSV infection often have liver and kidney function injury and multiple organ failure [33]. When fludarabine is used in patients with SFTSV, the condition may be further aggravated (Table 1).
Calcium Channel Inhibitors
Ca2+ is a ubiquitous and multifunctional intracellular messenger with several functions that modulate various cellular pathways [34]. Calcium may affect viral infection by regulating the calcium-dependent cytokines required for viral infection [35]. Antiviral activity of calcium channel blockers (CCBs) against various fatal viruses such as Japanese encephalitis, West Nile virus (WNV), Junin, Marburg, and Ebola has been progressively documented. It was observed that CCBs reduced Ebola viral entrance and Junin virus fusion and access [36,37,38]. Moreover, while the stage of viral infection harmed by CCBs has been identified, the molecular basis by which CCBs block these viruses is unknown. It may represent the intricate regulation and efficiency of intracellular Ca2+ ions, which are involved in various cellular processes, including intracellular membrane fusion, endocytosis, and transcription, by regulating the features of Ca2+-dependent cellular proteins.
Several investigations have shown that nifedipine can significantly reduce SFTSV infection. The US Food and Drug Administration (FDA)-approved drug library was searched, and it was discovered that benidipine hydrochloride and nifedipine suppressed SFTSV replication in vitro [39]. A retrospective clinical analysis on a cohort of 2087 patients with SFTS revealed that nifedipine treatment improved virus clearance, relieved severe symptoms, and significantly decreased the mortality rate of patients with SFTS by more than fivefold. Thus, CCBs are strong contenders for broad-spectrum antiviral therapies.
Caffeic Acid
Caffeic acid (CA) and its derivatives are abundant in nature and widely used in several types of biologically active molecules [40]. They are natural active ingredients with broad application prospects. The structures of CA and its derivatives may be modified by adding or replacing different groups and converting them into other esters, amides, and polymer derivatives with diverse biological activities.
CA, chemical name 3,4-dihydroxycinnamic acid, belongs to the group of phenolic compounds, which have a high content in fruits and vegetables. CA has been proven to have numerous beneficial effects in vitro and in vivo, including anti-inflammatory, antiproliferative, anxiolytic, neuroprotective, antimicrobial, immunomodulatory, and antioxidant activities [41,42,43]. The o-dihydroxybenzene backbone of 3,4-dihydroxybenzoic acid, 3,4-dihydroxyphenylacetic acid, methyl CA, and CA phenethyl ester has been examined for anti-SFTSV properties. In our current investigation, all suppressed SFTSV propagation in a dose-dependent manner [42, 43]. The o-dihydroxybenzene backbone of quercetin and catechin reduced SFTSV infection at the virus attachment stage. CA had the greatest selectivity index (SI) value among the o-dihydroxybenzene-based compounds examined, with varying CC50 and IC50 values.
Quinoline Analogues
Amodiaquine
Amodiaquine (AQ) is a 4-aminoquinoline antimalarial similar to chloroquine and is used extensively to treat and prevent malaria. Amodiaquine is a drug that has been approved for the treatment of malaria in clinics. Simultaneously, it was active against various human pathogens, including viruses such as bunyaviruses, alphaviruses, coronaviruses, and flaviviruses [44, 45].
Baba et al. demonstrated that the antimalarial drug amodiaquine is a potent inhibitor of SFTSV proliferation [46]. Amodiaquine’s anti-SFTSV activity was nearly identical to that of favipiravir. All derivatives exhibited anti-SFTSV action when the chlorine of amodiaquine was substituted by another halogen, such as iodine, bromine, or fluorine.
Chloroquine/Hydroxychloroquine
Chloroquine and hydroxychloroquine are also quinoline analogues that are primarily used to treat malaria. However, their application range has gradually expanded to the treatment of serious infectious diseases, rheumatic immune diseases, tumors, etc. owing to continuous research on the drugs’ mechanism of action [47]. In-depth study results show that chloroquine and hydroxychloroquine have antiviral effects against coronavirus [48], influenza virus, and CCHF [49], and they have significant clinical efficacy in treating rheumatoid diseases such as systemic lupus erythematosus and rheumatoid arthritis. The main adverse reaction of hydroxychloroquine is eye damage, especially retinopathy. Patients treated with this drug should undergo regular eye examinations.
Statins
Statins reversibly block 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase, a rate-limiting enzyme in cholesterol production. The FDA has approved statins for lowering cholesterol levels and preventing primary and secondary cardiovascular disorders. Statins block a variety of biological mechanisms, including cholesterol formation. Cholesterol is involved in the reproduction cycle of many viruses, particularly members of the Flaviviridae family [50]. Lovastatin has been shown to decrease dengue virus (DENV) multiplication in vitro across numerous cell types. It has been demonstrated that it acts in the late phases of the DENV replication cycle (maturation and egress), reducing the development of infectious viral progeny [51].
Urata et al. found that several chemical compounds restraining fatty acid and cholesterol synthesis (fenofibrate and lovastatin) inhibited SFTSV replication and propagation [52]. Thus, the efficacy of statins against SFTSV infection needs to be further assessed clinically.
Plasma Exchange
Plasma exchange is a method to remove various metabolic toxins and pathogenic factors by blood purification under cardiopulmonary bypass. Studies have shown that plasma exchange can restore homeostasis of the internal environment and improve the coagulation state of the body. Plasma exchange has been used in the treatment of patients with severe sepsis, H7N9, and Ebola virus infection [53]. In a previous study, the symptoms of a critically ill patient were not improved after plasma exchange. Nevertheless, when ribavirin was combined with plasma exchange, two patients with quickly developing SFTS had clinical improvements [54]. For patients with SFTS at the MODS stage, a combination of plasma exchange and ribavirin could be employed as a viable salvage treatment.
A recent study in South Korea showed that because the serum viral load of patients with SFTS decreased significantly and the clinical and laboratory parameters improved rapidly after therapeutic plasma exchange (TPE), the authors believed that TPE may help alleviate the deterioration of patients with rapidly progressive SFTS [55]. However, in one case report, the patient’s viral load did not decrease after plasma exchange [56]. The aforementioned studies of TPE are limited as they only show a relatively small number of clinical samples without strict comparative control. Physicians still need to observe the characteristic clinical manifestations of the patients and to use TPE with caution.
Additionally, we also need to understand the side effects of TPE. In the process of plasma exchange, a small portion of blood will circulate in vitro, and repeated entry into the human body will greatly impact cardiopulmonary function. Secondly, the use of anticoagulants in the replacement process leads to the loss of some coagulation factors, which affects the coagulation function of patients. In severely ill and critically ill patients, plasma exchange is usually used in the early and middle stages of cytokine storms. At the same time, some of these patients have multiple organ injuries. Therefore, the correct selection of appropriate patients for plasma exchange requires the joint judgment of clinicians in combination with clinical indications and laboratory and imaging data.
Targeted Therapy Strategy
Arginine
In 2018, researchers first revealed the disorder of arginine metabolism caused by SFTSV infection by the metabonomics method. They then found that the decrease of platelet number and T lymphocyte dysfunction is related to the abnormality of arginine metabolism [57]. That clinical trial proved that arginine supplementation can help patients recover faster. The researchers explored the molecular process of the disease from the perspective of metabolism and immunology, so as to provide a theoretical basis for the unique treatment of the disease. Firstly, 242 clinical cases were analyzed through metabonomics. It was found that the metabolism of the cases infected with SFTSV changed significantly. Subsequent pathway enrichment analysis of differential metabolites showed that the change of the arginine metabolic pathway was the most significant, and arginine was significantly downregulated in SFTSV-infected cases. The subsequent results showed that when arginine was downregulated, platelet nitric oxide (Plt-NO) content decreased, and the degree of platelet activation increased, resulting in platelet overactivation and apoptosis. These findings imply that hypoargininemia may be a contributing factor in coagulation complications.
Antibody Therapy
MAb 4–5 is a newly identified human neutralizing antibody that recognizes a substantially cross-reactive, surface-exposed epitope on the SFTSV Gn glycoprotein’s N-terminal region [58]. MAb 4–5 binds to the SFTSV Gn glycoprotein’s domain III. MAb 4–5’s neutralizing effect has been demonstrated only in vitro, and its efficacy in vivo remains unknown. Ab10 is a monoclonal antibody that has shown therapeutic effects in a mice model of SFTSV infection [59]. The neutralizing efficacy of Ab10, on the other hand, was only examined in Vero cells using the Gangwon/Korea/2012 strain.
The extracellular domain of SFTSV Gn (sGn) expressed in mammalian cells immunized a camel. The immunized camel’s peripheral blood mononuclear cells (PBMCs) were used to create a variable heavy chain domain (VHH) antibody phage library. After multiple rounds of enrichment against sGn, 23 nanobodies with potent neutralizing activities were identified SNB02 (VHH-huFc antibody, named SNB), a high-affinity antibody with a human Fc1, potently neutralized SFTSV in vitro [60].
Ab-based therapies will likely continue to make incremental advances in the repertoire of anti-infective strategies in the near future.
Chinese Medicine
According to the epidemic and clinical characteristics of SFTS, the disease belongs to the traditional Chinese medicine (TCM) category of “spring-warm syndrome,” “damp-warm syndrome,” or “summer-warm syndrome.” There are more than ten TCMs suggested to prevent and treat SFTS, according to an expert agreement on diagnosis and treatment of SFTS using TCM and Western medicine in China: Shengmai injection, Shenfu injection, Qingkailing injection, Xuebijing injection, Xiyanping injection, Reducing injection, Lanqin oral liquid, and Lianhua Qingwen capsule are among these TCMs. Lianhua Qingwen capsule contains 13 kinds of Chinese herbal medicine [61] and has the clinical indications of clearing heat, dispersing lungs, and detoxifying. Sterculia lychnophora, Phellodendri Chinensis cordex, Gardeniae fructus, Scutellariae radix, and Isatidis radix are all ingredients in oral liquid, a Chinese patent medicine. It is well known for its pharmacological effect on upper respiratory tract infections and its ability to reduce pharyngeal edema [62]. Because of its various effects on neutralization of cytotoxins, anticoagulation, microcirculation improvement, immunoregulation, and cytokine reduction, Xuebijing injection, a five-herbal injection medicine with a clinical indication of dissolving stasis and detoxifying, was certified to treat MODS, SIRS, coagulopathy, and sepsis [63].
Three Chinese injection treatments have heart-protective effects: Shenmai injection, Shengmai injection, and Shenfu injection [64]. Nevertheless, few studies have been published on Chinese medicine products in the treatment of SFTS, and even fewer are high-quality investigations. The therapeutic benefits of TCMs must be confirmed in more prospective, rigorous population investigations. Their antiviral mechanism has to be elucidated more thoroughly.
In the face of this new infectious disease with acute onset, serious condition, and multiple syndromes, it is difficult to achieve a perfect treatment outcome by adopting any single treatment mode between TCM and Western medicine. Therefore, it is particularly necessary to exploit the respective advantages of TCM and Western medicine, organically combine disease differentiation and discrimination, and learn from each other to complement each other.
Other Reports in the Literature
It is reported that mycophenolate mofetil, methotrexate, loperamide, and bleomycin may inhibit SFTSV RNA synthesis [65], and hexachlorophene interfered with SFTSV entry and virus-induced cell fusion [66]. As the first clinically approved proteasome inhibitor, bortezomib can inhibit SFTSV replication by affecting the IFN system and apoptosis pathways [67]. In addition, it was found that tilorone had positive anti-SFT activity [68]. Further verification revealed that tilorone could inhibit SFTSV infection by activating a natural immune response in vitro and in vivo and showed a good preventive effect on SFTSV infection (Table 2).
Discussion
The suggested therapy options are based on the most recent SFTSV study findings. The broad-spectrum antivirals have the potential to be the first line of defense to prevent the deterioration of the disease. Among them, the most promising treatment is fapiravir. Fapiravir has potent in vitro activity against SFTSV and is currently undergoing randomized clinical trials. We believe that therapeutic medicines that directly target SFTSV will be the most successful among those alternatives. Future prospects of the antiviral method could depend mainly on the development of targeted therapies like monoclonal antibodies and prevention through vaccination. Strong preclinical and clinical studies are also required to determine a safe and effective treatment for SFTS.
Limitations
This article presents a current overview of SFTS antiviral therapy and further explores the future research direction of therapies. There are also some limitations. First, the literature was limited to studies published in Chinese and English. Second, the dose and duration of therapy were not standardized in the included studies. Third, only a few therapies have been studied through clinical trials.
Conclusion
At present, no standard and effective etiotropic treatment for SFTS is available, and further studies for effective pharmacotherapies and vaccines are necessary.
References
Yu XJ, Liang MF, Zhang SY, et al. Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med. 2011;364(16):1523–32.
Yun SM, Lee WG, Ryou J, et al. Severe fever with thrombocytopenia syndrome virus in ticks collected from humans, South Korea, 2013. Emerg Infect Dis. 2014;20(8):1358–61.
Takahashi T, Maeda K, Suzuki T, et al. The first identification and retrospective study of severe fever with thrombocytopenia syndrome in Japan. J Infect Dis. 2014;209(6):816–27.
McMullan LK, Folk SM, Kelly AJ, et al. A new phlebovirus associated with severe febrile illness in Missouri. N Engl J Med. 2012;367(9):834–41.
Hughes HR, Adkins S, Alkhovskiy S, et al. ICTV virus taxonomy profile: peribunyaviridae. J Gen Virol. 2020;101(1):1–2.
Wang P, Liu L, Liu A, et al. Structure of severe fever with thrombocytopenia syndrome virus L protein elucidates the mechanisms of viral transcription initiation. Nat Microbiol. 2020;5(6):864–71.
Jin C, Liang M, Ning J, et al. Pathogenesis of emerging severe fever with thrombocytopenia syndrome virus in C57/BL6 mouse model. Proc Natl Acad Sci U S A. 2012;109(25):10053–8.
Park A, Park SJ, Jung KL, et al. Molecular signatures of inflammatory profile and B cell function in patients with severe fever with thrombocytopenia syndrome. mBio. 2021;12(1):e02583-20.
Li H, Li X, Lv S, et al. Single-cell landscape of peripheral immune responses to fatal SFTS. Cell Rep. 2021;37(8): 110039.
Seo JW, Kim D, Yun N, Kim DM. Clinical update of severe fever with thrombocytopenia syndrome. Viruses. 2021;13(7):1213.
Mayor J, Engler O, Rothenberger S. Antiviral efficacy of ribavirin and favipiravir against Hantaan virus. Microorganisms. 2021;9(6):1306.
Arab-Bafrani Z, Jabbari A, Mostakhdem Hashemi M, Arabzadeh AM, Gilanipour A, Mousavi E. Identification of the crucial parameters regarding the efficacy of ribavirin therapy in Crimean-Congo haemorrhagic fever (CCHF) patients: a systematic review and meta-analysis. J Antimicrob Chemother. 2019;74(12):3432–9.
Shimojima M, Fukushi S, Tani H, et al. Effects of ribavirin on severe fever with thrombocytopenia syndrome virus in vitro. Jpn J Infect Dis. 2014;67(6):423–7.
Shimojima M, Fukushi S, Tani H, Taniguchi S, Fukuma A, Saijo M. Combination effects of ribavirin and interferons on severe fever with thrombocytopenia syndrome virus infection. Virol J. 2015;2(12):181.
Lee MJ, Kim KH, Yi J, et al. In vitro antiviral activity of ribavirin against severe fever with thrombocytopenia syndrome virus. Korean J Intern Med. 2017;32(4):731–7.
Liu W, Lu QB, Cui N, et al. Case-fatality ratio and effectiveness of ribavirin therapy among hospitalized patients in China who had severe fever with thrombocytopenia syndrome. Clin Infect Dis. 2013;57(9):1292–9.
Zhang Y, Miao W, Xu Y, Huang Y. Severe fever with thrombocytopenia syndrome in Hefei: clinical features, risk factors, and ribavirin therapeutic efficacy. J Med Virol. 2021;93(6):3516–23.
Kowdley KV. Hematologic side effects of interferon and ribavirin therapy. J Clin Gastroenterol. 2005;39(1 Suppl):S3-8.
Sakran R, Frisch A, Elias A, Sliman H, Ammuri H, Kurnik D. Acute and severe ribavirin-associated hyperuricemia and acute kidney injury: an underrecognized adverse effect. Am J Health Syst Pharm. 2021;78(9):794–9.
Furuta Y, Takahashi K, Fukuda Y, et al. In vitro and in vivo activities of anti-influenza virus compound T-705. Antimicrob Agents Chemother. 2002;46(4):977–81.
Furuta Y, Takahashi K, Shiraki K, et al. T-705 (favipiravir) and related compounds: novel broad-spectrum inhibitors of RNA viral infections. Antiviral Res. 2009;82(3):95–102.
Sun J, Min YQ, Li Y, et al. Animal model of severe fever with thrombocytopenia syndrome virus infection. Front Microbiol. 2022;11(12):797189.
Tani H, Komeno T, Fukuma A, et al. Therapeutic effects of favipiravir against severe fever with thrombocytopenia syndrome virus infection in a lethal mouse model: dose-efficacy studies upon oral administration. PLoS ONE. 2018;13(10):e0206416.
Tani H, Fukuma A, Fukushi S, et al. Efficacy of T-705 (favipiravir) in the treatment of infections with lethal severe fever with thrombocytopenia syndrome virus. mSphere. 2016;1(1):e00061-15.
Gowen BB, Westover JB, Miao J, et al. Modeling severe fever with thrombocytopenia syndrome virus infection in golden Syrian hamsters: importance of STAT2 in preventing disease and effective treatment with favipiravir. J Virol. 2017;91(3):e01942-16.
Song R, Chen Z, Li W. Severe fever with thrombocytopenia syndrome (SFTS) treated with a novel antiviral medication, favipiravir (T-705). Infection. 2020;48(2):295–8.
Li H, Jiang XM, Cui N, et al. Clinical effect and antiviral mechanism of T-705 in treating severe fever with thrombocytopenia syndrome. Signal Transduct Target Ther. 2021;6(1):145.
Yuan Y, Lu QB, Yao WS, et al. Clinical efficacy and safety evaluation of favipiravir in treating patients with severe fever with thrombocytopenia syndrome. EBioMedicine. 2021;72:103591.
Suemori K, Saijo M, Yamanaka A, et al. A multicenter non-randomized, uncontrolled single arm trial for evaluation of the efficacy and the safety of the treatment with favipiravir for patients with severe fever with thrombocytopenia syndrome. PLoS Negl Trop Dis. 2021;15(2):e0009103.
Leporrier M. Role of fludarabine as monotherapy in the treatment of chronic lymphocytic leukemia. Hematol J. 2004;5(Suppl 1):S10–9.
Montgomery JA, Hewson K. Nucleosides of 2-fluoroadenine. J Med Chem. 1969;12(3):498–504.
Gao C, Wen C, Li Z, et al. Fludarabine inhibits infection of Zika virus, SFTS phlebovirus, and Enterovirus A71. Viruses. 2021;13(5):774.
Ding X, Herzlich AA, Bishop R, Tuo J, Chan CC. Ocular toxicity of fludarabine: a purine analog. Expert Rev Ophthalmol. 2008;3(1):97–109.
Bagur R, Hajnóczky G. Intracellular Ca2+ sensing: its role in calcium homeostasis and signaling. Mol Cell. 2017;66(6):780–8.
Zhou Y, Frey TK, Yang JJ. Viral calciomics: interplays between Ca2+ and virus. Cell Calcium. 2009;46(1):1–17.
Lavanya M, Cuevas CD, Thomas M, Cherry S, Ross SR. siRNA screen for genes that affect Junín virus entry uncovers voltage-gated calcium channels as a therapeutic target. Sci Transl Med. 2013;5(204):204ra131.
Gehring G, Rohrmann K, Atenchong N, et al. The clinically approved drugs amiodarone, dronedarone and verapamil inhibit filovirus cell entry. J Antimicrob Chemother. 2014;69(8):2123–31.
Sakurai Y, Kolokoltsov AA, Chen CC, et al. Ebola virus. Two-pore channels control Ebola virus host cell entry and are drug targets for disease treatment. Science. 2015;347(6225):995–8.
Li H, Zhang LK, Li SF, et al. Calcium channel blockers reduce severe fever with thrombocytopenia syndrome virus (SFTSV) related fatality. Cell Res. 2019;29(9):739–53.
Muhammad-Abdul-Kadar NN, Ahmad F, Teoh SL, Yahaya MF. Caffeic acid on metabolic syndrome: a review. Molecules. 2021;26(18):5490.
Zielińska D, Zieliński H, Laparra-Llopis JM, Szawara-Nowak D, Honke J, Giménez-Bastida JA. Caffeic acid modulates processes associated with intestinal inflammation. Nutrients. 2021;13(2):554.
Ogawa M, Shirasago Y, Ando S, Shimojima M, Saijo M, Fukasawa M. Caffeic acid, a coffee-related organic acid, inhibits infection by severe fever with thrombocytopenia syndrome virus in vitro. J Infect Chemother. 2018;24(8):597–601.
Ogawa M, Shirasago Y, Tanida I, et al. Structural basis of antiviral activity of caffeic acid against severe fever with thrombocytopenia syndrome virus. J Infect Chemother. 2021;27(2):397–400.
Boonyasuppayakorn S, Reichert ED, Manzano M, Nagarajan K, Padmanabhan R. Amodiaquine, an antimalarial drug, inhibits dengue virus type 2 replication and infectivity. Antiviral Res. 2014;106:125–34.
Gignoux E, Azman AS, de Smet M, et al. Effect of artesunate-amodiaquine on mortality related to Ebola virus disease. N Engl J Med. 2016;374(1):23–32.
Baba M, Toyama M, Sakakibara N, Okamoto M, Arima N, Saijo M. Establishment of an antiviral assay system and identification of severe fever with thrombocytopenia syndrome virus inhibitors. Antivir Chem Chemother. 2017;25(3):83–9.
Rainsford KD, Parke AL, Clifford-Rashotte M, Kean WF. Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseases. Inflammopharmacology. 2015;23(5):231–69.
Persoons L, Vanderlinden E, Vangeel L, et al. Broad spectrum anti-coronavirus activity of a series of anti-malaria quinoline analogues. Antiviral Res. 2021;193:105127.
Ferraris O, Moroso M, Pernet O, et al. Evaluation of Crimean–Congo hemorrhagic fever virus in vitro inhibition by chloroquine and chlorpromazine, two FDA approved molecules. Antiviral Res. 2015;118:75–81.
Jasińska M, Owczarek J, Orszulak-Michalak D. Statins: a new insight into their mechanisms of action and consequent pleiotropic effects. Pharmacol Rep. 2007;59(5):483–99.
Rothwell C, Lebreton A, Young Ng C, et al. Cholesterol biosynthesis modulation regulates dengue viral replication. Virology. 2009;389(1–2):8–19.
Urata S, Uno Y, Kurosaki Y, Yasuda J. The cholesterol, fatty acid and triglyceride synthesis pathways regulated by site 1 protease (S1P) are required for efficient replication of severe fever with thrombocytopenia syndrome virus. Biochem Biophys Res Commun. 2018;503(2):631–6.
Liu X, Zhang Y, Xu X, et al. Evaluation of plasma exchange and continuous veno-venous hemofiltration for the treatment of severe avian influenza A (H7N9): a cohort study. Ther Apher Dial. 2015;19(2):178–84.
Oh WS, Heo ST, Kim SH, Choi WJ, Han MG, Kim JY. Plasma exchange and ribavirin for rapidly progressive severe fever with thrombocytopenia syndrome. Int J Infect Dis. 2014;18:84–6.
Yoo JR, Kim SH, Kim YR, Lee KH, Oh WS, Heo ST. Application of therapeutic plasma exchange in patients having severe fever with thrombocytopenia syndrome. Korean J Intern Med. 2019;34(4):902–9.
Utsunomiya Nishimizu R, Shiota S, Ishii T, et al. Plasma exchange did not reduce viral load in a recovered case of severe fever with thrombocytopenia syndrome. Intern Med. 2022;61(2):253–6.
Li XK, Lu QB, Chen WW, et al. Arginine deficiency is involved in thrombocytopenia and immunosuppression in severe fever with thrombocytopenia syndrome. Sci Transl Med. 2018;10(459):e4162.
Wu Y, Zhu Y, Gao F, et al. Structures of phlebovirus glycoprotein Gn and identification of a neutralizing antibody epitope. Proc Natl Acad Sci U S A. 2017;114(36):E7564–73.
Kim KH, Kim J, Ko M, et al. An anti-Gn glycoprotein antibody from a convalescent patient potently inhibits the infection of severe fever with thrombocytopenia syndrome virus. PLoS Pathog. 2019;15(2): e1007375.
Wu X, Li Y, Huang B, et al. A single-domain antibody inhibits SFTSV and mitigates virus-induced pathogenesis in vivo. JCI Insight. 2020;5(13):e136855.
Yang R, Yang H, Wei J, et al. Mechanisms underlying the effects of lianhua qingwen on sepsis-induced acute lung injury: a network pharmacology approach. Front Pharmacol. 2021;14(12): 717652.
Zheng M, Tian L, Huang H-L, et al. Cost-effectiveness analysis of traditional Chinese medicine for the treatment of upper respiratory tract infections: Yuxingcao Qinlan mixture versus LanQin oral liquid—a prospective study. Eur J Integr Med. 2017;9:97–102.
Luo Z, Chen W, Xiang M, et al. The preventive effect of Xuebijing injection against cytokine storm for severe patients with COVID-19: a prospective randomized controlled trial. Eur J Integr Med. 2021;42:101305.
Lin S, Shi Q, Ge Z, et al. Efficacy and safety of traditional Chinese medicine injections for heart failure with reduced ejection fraction: a Bayesian network meta-analysis of randomized controlled trials. Front Pharmacol. 2021;30(12):659707.
Yamada H, Taniguchi S, Shimojima M, et al. M segment-based minigenome system of severe fever with thrombocytopenia syndrome virus as a tool for antiviral drug screening. Viruses. 2021;13(6):1061.
Yuan S, Chan JF, Ye Z, et al. Screening of an FDA-approved drug library with a two-tier system identifies an entry inhibitor of severe fever with thrombocytopenia syndrome virus. Viruses. 2019;11:385.
Liu S, Liu H, Zhang K, et al. Proteasome inhibitor PS-341 effectively blocks infection by the severe fever with thrombocytopenia syndrome virus. Virol Sin. 2019;34(5):572–82.
Yang J, Yan Y, Dai Q, et al. Tilorone confers robust in vitro and in vivo antiviral effects against severe fever with thrombocytopenia syndrome virus. Virol Sin. 2022;37:145–8.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Disclosures
Yin Zhang, Ying Huang and Yuanhong Xu have no conflict of interests to report.
Author Contributions
All authors contributed to the conception and design of the article, interpreting the relevant data, drafting of the manuscript, and/or critically revising the manuscript for intellectual contribution.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Zhang, Y., Huang, Y. & Xu, Y. Antiviral Treatment Options for Severe Fever with Thrombocytopenia Syndrome Infections. Infect Dis Ther 11, 1805–1819 (2022). https://doi.org/10.1007/s40121-022-00693-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-022-00693-x