Abstract
Introduction
There is no consensus regarding optimal duration of antibiotic therapy for Pseudomonas aeruginosa bacteremia. We aimed to evaluate the impact of short antibiotic course.
Methods
We present a retrospective multicenter study including patients with P. aeruginosa bacteremia during 2009–2015. We evaluated outcomes of patients treated with short (6–10 days) versus long (11–15 days) antibiotic courses. The primary outcome was a composite of 30-day mortality or bacteremia recurrence and/or persistence. Univariate and inverse probability treatment-weighted (IPTW) adjusted multivariate analysis for the primary outcome was performed. To avoid immortal time bias, the landmark method was used.
Results
We included 657 patients; 273 received a short antibiotic course and 384 a long course. There was no significant difference in baseline characteristics of patients. The composite primary outcome occurred in 61/384 patients in the long-treatment group (16%) versus 32/273 in the short-treatment group (12%) (p = 0.131). Mortality accounted for 41/384 (11%) versus 25/273 (9%) of cases, respectively. Length of hospital stay was significantly shorter in the short group [median 13 days, interquartile range (IQR) 9–21 days, versus median 15 days, IQR 11–26 days, p = 0.002]. Ten patients in the long group discontinued antibiotic therapy owing to adverse events, compared with none in the short group. On univariate and multivariate analyses, duration of therapy was not associated with the primary outcome.
Conclusions
In this retrospective study, 6–10 days of antibiotic course for P. aeruginosa bacteremia were as effective as longer courses in terms of survival and recurrence. Shorter therapy was associated with reduced length of stay and less drug discontinuation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Pseudomonas aeruginosa bacteremia is a severe infection, often treated with long-course (~ 14 days) antibiotics. |
We aimed to assess whether 6–10 days of antibiotics would be as effective as 11–15 days for this infection. |
Data from 657 patients with P. aeruginosa bacteremia collected retrospectively demonstrated no association between duration of therapy and mortality or bacteremia recurrence. |
Short therapy was associated with less drug discontinuation and shorter length of stay. |
Short course of antibiotics (6–10 days) may be considered for P. aeruginosa bacteremia. |
Introduction
In recent years, evidence has accumulated to support short duration of antibiotic therapy for Gram-negative bacteremia in general. Several nonrandomized studies have demonstrated no difference in mortality or other outcomes between short (6–10 days) and longer therapy for Gram-negative bacteremia [1,2,3,4,5,6]. Three randomized controlled trials have also demonstrated noninferiority of 7 days antibiotic treatment compared with 14 days. The vast majority of these studies, however, included Enterobacterales [7,8,9]
Patients with Pseudomonas aeruginosa bacteremia are more likely to be neutropenic or have another immunosuppressive condition, and more likely to have hospital-acquired bacteremia compared with those with Enterobacterales bacteremia. The most common sources of bacteremia are pulmonary, central line-associated, urinary tract, and unknown source, with pulmonary source carrying worse prognosis [10,11,12]. Intrinsic resistance to some antibiotics, enhanced by tremendous ability to acquire resistance to others, in addition to paucity of available oral agents, makes this pathogen difficult to treat [13]. Hence, some recommend to use prolonged (≥ 14 days) antibiotic course to treat P. aeruginosa bacteremia [14]. Few data are available to guide the duration of antibiotic treatment for P. aeruginosa bacteremia. Two previous retrospective studies including ~ 540 patients in total demonstrated no difference in mortality or recurrence using 7–11 days of antibiotics compared with longer treatment of 12–21 days [11, 15].
We aimed to further investigate whether short-duration antibiotics is non-inferior to longer duration in cases of P. aeruginosa bacteremia.
Methods
Data Collection and Patient Inclusion
We used a large database of P. aeruginosa bacteremia collected during 2009–2015 from nine countries, 25 centers [16]. The collection of data was approved by the medical ethical committees of each participating center. STROBE guidelines for reporting in epidemiological studies were followed for the reporting of the current study.
We included consecutive adult patients (age ≥ 18 years) hospitalized with P. aeruginosa bacteremia. Further details on patients’ identification and data collection are published elsewhere [12, 16, 17]. For the current analysis, and considering previous studies [11, 15], we included patients who received at least 6 days of antibiotics and less than 16 days, assuming that patients with prolonged treatments probably had a complicated course, with confirmed/suspected metastatic foci of infection, and endovascular or osteoarticular infection. Patients receiving either monotherapy or combination therapy were included, excluding monotherapy with aminoglycosides, polymyxins, antipseudomonal penicillin, or initial oral fluoroquinolone treatment. Patients with step-down to oral fluoroquinolones following any duration of initial intravenous therapy were also included. We dichotomized the comparison to short (6–10 days) and longer therapy (11–15 days), considering any in vitro antibiotic courses (as above), administered in hospital and post-discharge.
We documented baseline patients characteristics (demographics, comorbidities), infection-related parameters (source, severity, susceptibility data), and management data—the latter included documentation of appropriateness of empirical treatment. Appropriate was defined as in vitro antibiotic courses administered withing 24 or 48 h (both were documented). Type of main definitive antibiotic therapy (i.e., administered according to susceptibility testing) and duration were documented as well. Outcomes were also collected, as below.
Outcomes
The primary outcome was a composite of mortality or recurrence/persistence of bloodstream infection within 30 days of positive blood culture, defined as: recurrence—positive blood cultures for Pseudomonas after negative ones; and persistence—positive blood cultures for Pseudomonas after more than 72 h of treatment, as documented in patient records. Recurrence/persistence was considered an outcome if occurring upon completion of antibiotic treatment.
Secondary outcomes included individual components of the primary outcome: length of hospital stay, Clostridioides difficile-associated diarrhea, and discontinuation of antibiotics due to adverse events. Detailed definitions of these outcomes were previously described [16].
Statistical Analysis
Categorical data are expressed as numbers and percentages. Continuous data are expressed as means with standard deviation (SD) or medians with interquartile range (IQR), as appropriate. The t-test was used for comparison of normally distributed variables, and Mann–Whitney test for abnormally distributed variables. Comparison of categorical variables was conducted using χ2 test or Fisher’s exact test, as appropriate. A propensity score for duration of therapy was calculated for each patient by adjusting for all pretreatment variables potentially confounding the duration of treatment. Variables used to estimate the propensity score are detailed in Supplementary Material. Assuming baseline differences between groups, we used inverse probability weighting to control for these differences. Standardized mean difference was used to check whether balance between the baseline characteristics was achieved; less than 10% difference was considered a reasonable balance. Weighted subjects were entered in the generalized estimating equation (GEE) binary logistics. To avoid immortal time bias, the landmark method was used. Landmark was set at day 11 to ensure no deaths occurred in the short treatment group (6–10 days). Missing values of covariates were handled by multiple imputation. The center was introduced as a random effect variable using GEE models. For model selection, we used the quasi-likelihood under the independence model criterion (QIC). Six models with varied subset of covariates were tested, and the model with the lowest QIC was selected.
Variables in the analyses were considered statistically significant if they had a p value < 0.05. All statistical analyses were conducted using SPSS program.
Results
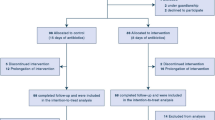
In the original database, 2396 patients were included. Of these, 657 patients were treated for 6–15 days with an in vitro course of antipseudomonal therapy that fulfilled our inclusion criteria above (see Supplemental Fig. 1 for flow chart of patient selection). Median age was 67 years [interquartile range (IQR) 55–77 years], and 234 (35.8%) were females. Of these patients, 273 received antipseudomonal regimen for 6–10 days (short course), and 384 received 11–15 days (long course). Overall, median treatment duration in the entire cohort was 11 days (IQR 9–14 days), with the short-arm median duration being 8 days (7–10 days) and the long arm 13 days (12–14 days, p < 0.001). Baseline characteristics of patients by duration of therapy are presented in Table 1. Variables included in the propensity score are detailed in Supplementary Material, with standardized mean difference of the original and weighted cohort.
No significant differences were documented in either demographic data, infection characteristics, or treatment between patients receiving short or long therapy. Sources of bacteremia were also similar between groups (Table 1). A trend for older age and more frequent use of corticosteroids was observed in the short-duration group, though nonsignificant [median age 68 years, interquartile range (IQR) 57.5–79 years versus 67 years (54–76 years), p = 0.075; corticosteroids 69/272, 25.4% versus 75/384, 19.5%, p = 0.094]. Multidrug-resistant (MDR) P. aeruginosa rates were similar between groups (short 20 (7.3%) versus long 39 (10.2%), p = 0.211) (Table 1).
The primary outcome of mortality or recurrence/persistence occurred overall in 93 patients, 61/384 in the long-duration group (15.9%) and 32/273 in the short-duration group (11.7%) (p = 0.131). Components of the primary outcome were without significant difference between groups (Table 2). All-cause mortality at 30 days was 41 (10.7%) among those treated with longer duration, compared with 25 (9.2%) among patients treated with short antibiotic course (p = 0.523). Repeated blood cultures were obtained from 105 patients, 69/384 (18%) in the long treatment group and 36/273 (13.2%) in the short arm (p = 0.11). Positive repeated cultures, indicating recurrence/persistence, were demonstrated among 21/375 (5.6%) long-arm patients versus 8/264 (3%) short-arm patients (p = 0.124). Length of hospital stay was significantly shorter in the short-duration group (median 13 days, IQR 9–21 days, versus 15 days, IQR 11–26 days, p = 0.002). Drug discontinuation due to adverse events was significantly more common in the long-duration group, with ten cases compared with none in the short treatment group (p = 0.006). These ten cases included five cases of discontinuation of β-lactams and five quinolones. Similar rates of C. difficile were observed between the groups (Table 2).
Univariate analysis for risk factors for the composite outcome of mortality or recurrence is described in Table 3. Arrival to hospital from another institution (nursing home or another hospital); metastatic malignancy; previous hospitalization within 90 days; nasogastric tube or urinary catheter at bacteremia onset; higher sequential organ failure assessment (SOFA) score; and lower systolic blood pressure were significantly associated with the primary outcome. MDR P. aeruginosa rates, early appropriate empirical antibiotic therapy, and combination therapy rates were nonsignificantly different. Duration of therapy (short versus long) was not associated with this outcome (Table 3). Multivariate analysis demonstrated baseline metastatic malignancy [odds ratio (OR) 2.5, 95% confidence interval (CI) 1.36–4.6]; higher SOFA score (OR 1.1, 95% CI 1.01–1.2); and presence of nasogastric tube (OR 2.11, 95% CI 1.18–3.74) as predictors of mortality or recurrence. Duration of treatment was not significantly associated with the primary outcome in this analysis (OR 1.66, 95% CI 0.94–2.95) [Table 4; odds ratio > 1 represents higher risk for the composite outcome with long-duration therapy (not statistically significant)].
Discussion
In this multinational, multicenter study including 657 patients with P. aeruginosa bacteremia, we did not find a strong association between duration of therapy and the composite outcome of mortality or recurrence/persistence of infection among patients treated with 6–10 days antibiotic course compared with a longer course of 11–15 days. Mortality at 30 days was similar (9.2% with short antibiotic course and 10.7% with longer duration, p = 0.523). Duration of hospital stay was significantly shorter in the short-duration group, and discontinuation of antibiotics due to adverse events was significantly more common in the long-duration group. No significant differences were observed for C. difficile-associated diarrhea rates. Predictors of the composite outcome of mortality or recurrence in multivariate analysis included baseline metastatic malignancy, and a more severe clinical presentation of infection, represented by a higher SOFA score, and probably more severe baseline condition, represented by a nasogastric tube. Duration of treatment (short or long) was not a significant predictor.
These findings are in accordance with previous results of two smaller, retrospective, single-country studies from the USA and South Korea. Fabre et al. included 249 patients with P. aeruginosa bacteremia, 69 of whom received short-duration therapy of 7–11 days and 180 of whom received longer regimens of 12–21 days. No difference in rates of death or recurrent infection within 30 days were demonstrated between short and long therapy in this study. Length of hospital stay was significantly shorter in the short-duration group, similar to our findings. [11] Similarly, Bae et al. evaluated 290 patients with P. aeruginosa bacteremia, 97 of whom received short-course therapy (7–11 days) and 193 of whom received 12–21 days of therapy. This study also found no significant difference in mortality or recurrence between groups.
In recent years, shortening the duration of antibiotic therapy has become an important backbone of antibiotic stewardship. Potential benefits are reduction in adverse events, cost, superinfections including fungal infection and C. difficile, and prevention of resistance development. Shorter antibiotic courses have been demonstrated effective in randomized controlled trials for the treatment of various respiratory infections, urinary tract infections, intraabdominal infections, and others [18]. Specifically for uncomplicated Gram-negative bacteremia, three recent randomized controlled trials have demonstrated noninferiority of 7 days antibiotic course compared with 14 days [7,8,9]. However, only one of these trials included nonfermenters, with limited number of P. aeruginosa cases [7]; and immunocompromised patients were excluded from one trial [8] and constituted less than one-fourth of patients in the other trials [7, 9]. P. aeruginosa bacteremia is frequently hospital acquired, and occurs in patients who are immunocompromised, critically ill, or with chronic underlying medical conditions. Antibiotic resistance is common, and mortality is high [19]. Consequently, traditional duration of therapy is considered by some to be 14 days [11, 15]. Our results, accompanied by previous studies, support the use of shorter duration of therapy.
Rac et al. proposed using a combination of several vital signs and laboratory values at 72–96 h after Gram-negative bacteremia onset as early clinical failure criteria. These were suggested as a tool to guide treatment duration in a personalized approach [20]. Considering this, and our results, supporting the safety of shorter therapy, further studies may test this approach for P. aeruginosa bacteremia.
Limitations of our study are its retrospective design, with data collected until 2015. Though propensity score weighting was used to adjust for differences between groups, residual bias cannot be excluded. In addition, patients treated for more than 15 days were excluded, assuming a complicated infection, without detailed documentation of these complications, owing to the retrospective nature of this study. The comparison of duration ranges (6–10 days versus 11–15 days), rather than a fixed duration (e.g., 7 or 14 days), limits our ability to provide a specific recommended duration that is safe and effective. It also selects for a specific population, possibly limiting the generalizability of our results for any patient with P. aeruginosa bacteremia. Since the study was retrospective, the outcome of recurrent and/or persistent bacteremia was not based on a preplanned culture collection schedule, rather the practice used in each center according to the patient’s clinical condition. In addition, data regarding source control are lacking. Since we excluded patients treated more than 15 days, we assume that most cases without appropriate source control were not included in this analysis, though this could not be verified. Another limitation lies in the use of the landmark method, which disregards patients who died/were censored before the landmark and restricts implementation of results to included patients. This inclusion only of patients who survived at least 11 days may produce a bias favoring the short therapy. The propensity score analysis for this study on duration may have better used variables of days 6–7, trying to mimic a randomized controlled trial recruiting at that point. However, these variables were missing for a considerable portion of patients as well as, when available, probably reflecting sicker patients still hospitalized and tested. Considering these limitations, randomized controlled trials are needed to test our findings, and are the optimal design to solve this clinical question.
Conclusions
In this retrospective study, for patients with P. aeruginosa bacteremia, we did not demonstrate a difference in clinical outcomes between patients treated with 6–10 days antibiotics compared with longer courses. Duration of hospital stay was shorter and adverse events leading to drug discontinuation were less common among patients treated with a shorter course. Prospective studies, ideally randomized controlled trials, should be designed to test shorter antibiotic therapy for P. aeruginosa bacteremia, and define subgroups of patients who would benefit most from this approach.
References
Chotiprasitsakul D, Han JH, Cosgrove SE, Harris AD, Lautenbach E, Conley AT, et al. Comparing the outcomes of adults with Enterobacteriaceae bacteremia receiving short-course versus prolonged-course antibiotic therapy in a multicentre, propensity score-matched cohort. Clin Infect Dis. 2018;66:172–7. https://doi.org/10.1093/cid/cix767.
Sousa A, Pérez-Rodríguez MT, Suárez M, Val N, Martínez-Lamas L, Nodar A, et al. Short- versus long-course therapy in Gram-negative bacilli bloodstream infections. Eur J Clin Microbiol Infect Dis. 2019;38:851–7. https://doi.org/10.1007/s10096-019-03467-5.
Giannella M, Pascale R, Toschi A, Ferraro G, Graziano E, Furii F, et al. Treatment duration for Escherichia coli bloodstream infection and outcomes: retrospective single-centre study. Clin Microbiol Infect. 2018;24:1077–83. https://doi.org/10.1016/j.cmi.2018.01.013.
Doi A, Morimoto T, Iwata K. Shorter duration of antibiotic treatment for acute bacteraemic cholangitis with successful biliary drainage: a retrospective cohort study. Clin Microbiol Infect. 2018;24:1184–9. https://doi.org/10.1016/j.cmi.2018.01.021.
Uno S, Hase R, Kobayashi M, Shiratori T, Nakaji S, Hirata N, et al. Short-course antimicrobial treatment for acute cholangitis with Gram-negative bacillary bacteremia. Int J Infect Dis. 2017;55:81–5. https://doi.org/10.1016/j.ijid.2016.12.018.
Nelson AN, Justo JA, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. Optimal duration of antimicrobial therapy for uncomplicated Gram-negative bloodstream infections. Infection. 2017;45:613–20. https://doi.org/10.1007/s15010-017-1020-5.
Yahav D, Franceschini E, Koppel F, Turjeman A, Babich T, Bitterman R, et al. Seven versus fourteen days of antibiotic therapy for uncomplicated Gram-negative bacteremia: a non-inferiority randomized controlled trial. Clin Infect Dis. 2018. https://doi.org/10.1093/cid/ciy1054.
von Dach E, Albrich WC, Brunel A-S, Prendki V, Cuvelier C, Flury D, et al. Effect of C-reactive protein-guided antibiotic treatment duration, 7-day treatment, or 14-day treatment on 30-day clinical failure rate in patients with uncomplicated Gram-negative bacteremia: a randomized clinical trial. JAMA. 2020;323:2160–9. https://doi.org/10.1001/jama.2020.6348.
Molina J, Montero-Mateos E, Praena-Segovia J, León-Jiménez E, Natera C, López-Cortés LE, et al. Seven-versus 14-day course of antibiotics for the treatment of bloodstream infections by Enterobacterales: a randomized, controlled trial. Clin Microbiol Infect. 2021. https://doi.org/10.1016/j.cmi.2021.09.001.
Choi Y, Paik JH, Kim JH, Han SB, Durey A. Clinical predictors of Pseudomonas aeruginosa bacteremia in emergency department. Emerg Med Int. 2018;2018:7581036. https://doi.org/10.1155/2018/7581036.
Fabre V, Amoah J, Cosgrove SE, Tamma PD. Antibiotic therapy for Pseudomonas aeruginosa bloodstream infections: how long is long enough? Clin Infect Dis. 2019. https://doi.org/10.1093/cid/ciz223.
Babich T, Naucler P, Valik JK, Giske CG, Benito N, Cardona R, et al. Risk factors for mortality among patients with Pseudomonas aeruginosa bacteraemia: a retrospective multicentre study. Int J Antimicrob Agents. 2019. https://doi.org/10.1016/j.ijantimicag.2019.11.004.
Herrera S, Bodro M, Soriano A. Predictors of multidrug resistant Pseudomonas aeruginosa involvement in bloodstream infections. Curr Opin Infect Dis. 2021;34:686–92. https://doi.org/10.1097/QCO.0000000000000768.
Kanj S, Sexston D. Pseudomonas aeruginosa bacteremia and endocarditis. Waltham: Up To Date; 2021.
Bae M, Jeong Y, Bae S, Kim MJ, Chong YP, Kim S-H, et al. Short versus prolonged courses of antimicrobial therapy for patients with uncomplicated Pseudomonas aeruginosa bloodstream infection: a retrospective study. J Antimicrob Chemother. 2021. https://doi.org/10.1093/jac/dkab358.
Babich T, Naucler P, Valik JK, Giske CG, Benito N, Cardona R, et al. Ceftazidime, carbapenems, or piperacillin–tazobactam as single definitive therapy for Pseudomonas aeruginosa bloodstream infection—a multi-site retrospective study. Clin Infect Dis. 2019. https://doi.org/10.1093/cid/ciz668.
Babich T, Naucler P, Valik JK, Giske CG, Benito N, Cardona R, et al. Combination versus monotherapy as definitive treatment for Pseudomonas aeruginosa bacteraemia: a multicentre retrospective observational cohort study. J Antimicrob Chemother. 2021;76:2172–81. https://doi.org/10.1093/jac/dkab134.
Spellberg B, Rice LB. Duration of antibiotic therapy: shorter is better. Ann Intern Med. 2019;171:210–1. https://doi.org/10.7326/M19-1509.
Bassetti M, Vena A, Croxatto A, Righi E, Guery B. How to manage Pseudomonas aeruginosa infections. Drugs Context. 2018;7: 212527. https://doi.org/10.7573/dic.212527.
Rac H, Gould AP, Bookstaver PB, Justo JA, Kohn J, Al-Hasan MN. Evaluation of early clinical failure criteria for Gram-negative bloodstream infections. Clin Microbiol Infect. 2020;26:73–7. https://doi.org/10.1016/j.cmi.2019.05.017.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Dafna Yahav, Tanya Babich, and Leonard Leibovici contributed to the study conception and all authors participated in the study’s protocol writing and study design. Material preparation and data collection were performed by all authors. Dafna Yahav, Tanya Babich, and Leonard Leibovici performed the analysis and wrote the first draft of the manuscript. All authors commented on previous versions of the manuscript, and read and approved the final manuscript.
Disclosures
Tanya Babich, Pontus Naucler, John Karlsson Valik, Christian G. Giske, Natividad Benito, Ruben Cardona, Alba Rivera, Celine Pulcini, Manal Abdel Fattah, Justine Haquin, Alasdair Macgowan, Sally Grier, Bibiana Chazan, Anna Yanovskay, Ronen Ben Ami, Michal Landes, Lior Nesher, Adi Zaidman-Shimshovitz, Kate McCarthy, David L Paterson, Evelina Tacconelli, Michael Buhl, Susanna Mauer, Jesús Rodríguez-Baño, Marina de Cueto, Antonio Oliver, Enrique Ruiz de Gopegui, Angela Cano, Isabel Machuca, Monica Gozalo-Marguello, Luis Martinez-Martinez, Eva M Gonzalez-Barbera, Iris Gomez Alfaro, Miguel Salavert, Bojana Beovic, Andreja Saje, Manica Mueller–Premru, Leonardo Pagani, Virginie Vitrat, Diamantis Kofteridis, Maria Zacharioudaki, Sofia Maraki, Yulia Weissman, Mical Paul, Yaakov Dickstein, Leonard Leibovici and Dafna Yahav all have nothing to disclose.
Compliance with Ethics Guidelines
The study was approved by the local ethical committees of each participating center, which waved informed consent due to the retrospective nature of the study.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request, and with agreement of all contributing authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Babich, T., Naucler, P., Valik, J.K. et al. Duration of Treatment for Pseudomonas aeruginosa Bacteremia: a Retrospective Study. Infect Dis Ther 11, 1505–1519 (2022). https://doi.org/10.1007/s40121-022-00657-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-022-00657-1