Abstract
Introduction
TETRAXIM™ (Sanofi), a combined diphtheria, tetanus, acellular pertussis, and inactivated poliovirus (DTaP-IPV) vaccine, has been licensed in South Korea since 2009. In accordance with the Ministry of Food and Drug Safety regulations, this post-marketing surveillance (PMS) study evaluated the safety of the DTaP-IPV vaccine in real-world clinical practice in infants and children who received it as either a part of the three-dose primary series dose at 2, 4, and 6 months or school entry booster between 4 and 6 years of age.
Methods
This multicenter, observational, PMS study was conducted in real-world practice in South Korea for 6 years (2009–2015) in participants aged between 2 months and 6 years. The study outcomes included solicited reactions, unsolicited adverse events (AEs)/adverse drug reactions (ADRs), unexpected AEs/ADRs, and serious AEs (SAEs)/ADRs.
Results
Data from 647 participants was included in the safety analysis. Overall, 268 AEs were reported by 181 (28%) participants: 47 (17.5%) solicited reactions, 220 (82.1%) unsolicited AEs, and 1 (0.4%) unsolicited ADR. A total of 48 AEs (including 47 solicited reactions) were reported to have a causal relationship with the DTaP-IPV vaccine and were reported by 36 (5.6%) participants. A total of 212 unexpected AEs were reported by 152 (23.5%) participants, none of which had a causal relationship with the DTaP-IPV vaccine. Neither immediate AEs nor SAEs were reported during the study. Among the participants who reported AEs, 220 (34%) were on concomitant medications. Most AEs were of mild intensity, and all participants recovered.
Conclusion
No safety concerns related to the DTaP-IPV vaccine in a real-world setting were raised in participants aged 2–6 months for the primary series and 4–6 years for the school-entry booster dose in the Korean population. The DTaP-IPV vaccine was well tolerated and can be continued as part of routine immunization programs in infants and children.
Trial Registration: NCT01437423.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Post-marketing surveillance of the combined diphtheria, tetanus, acellular pertussis, and inactivated poliovirus (DTaP-IPV) vaccine provides the safety profile of this vaccine in a real-life setting in South Korea. |
No safety concerns related to the DTaP-IPV vaccine in real-world use were raised in participants aged 2–6 months for the primary series and 4–6 years for the school-entry booster dose in the Korean population. |
A combined diphtheria, tetanus, acellular pertussis, and inactivated poliovirus vaccine was well tolerated and can be given as part of routine immunization programs for protection against diphtheria, tetanus, pertussis, and poliomyelitis in infants and children. |
Introduction
Diphtheria, tetanus, and pertussis are potentially severe bacterial diseases. Polio, or poliomyelitis, is a disabling and life-threatening disease caused by the poliovirus. Diphtheria causes a thick pseudomembrane covering the back of the throat, which can lead to breathing issues, paralysis, or heart failure, and tetanus causes painful tightening of the muscles, usually all over the body, and can be fatal [1]. Pertussis, also known as whooping cough, is a highly contagious bacterial infection caused by a Gram-negative coccobacillus, Bordetella pertussis (B. pertussis). It is an upper respiratory tract infection leading to severe paroxysmal coughing and post-tussive emesis [2, 3]. Infants who are too young to be vaccinated and born from pertussis-unvaccinated or incompletely vaccinated mothers are at risk of developing severe forms of pertussis. The most common sources of transmission to infants are siblings, parents, and other family members who are in close contact with them [4, 5]. Additionally, infants bear the highest disease burden due to an elevated risk for pertussis-related complications and mortality [6, 7]. Severe cases that require hospitalization and intensive care admission are most frequently observed in infants < 3 months of age [8]. Maintaining high immunity against diphtheria, tetanus, pertussis, and polio is important across the life span [9,10,11,12]. Although immunization strategies have been successfully implemented, resurgence of pertussis has been reported globally and most likely resulted from the combination of multiple factors [13, 14].
In South Korea, the disease burden of pertussis is still high, with an annual incidence rate that peaked at 24.7 per 100,000 infants per 2018 data [15]. The highest pertussis detection rates are reported in infants (< 6 months of age) and young children, followed by adolescents and adults [15, 16]. During 2010–2011, 33.8% of patients with pertussis were < 3 months old, and 29% were adolescents and adults ≥ 15 years old [17]. Despite the substantial number of cases, it may still be highly underestimated or underreported due to diagnostic challenges and lack of awareness [16].
Vaccination has been the primary preventive strategy for pertussis control for several decades [18]. In 2009, the vaccination schedule recommended by the South Korean National Immunization Program (NIP) included administration of the diphtheria-tetanus-acellular pertussis (DTaP) vaccine as a three-dose primary vaccination series in infants aged 2, 4, and 6 months, with a fourth (booster) dose given at 15–18 months and a fifth (school-entry booster) dose at 4–6 years of age. At that time, the primary vaccination for polio recommended vaccinations at 2, 4, and 6 months of age, with a subsequent fourth (booster) dose at 4–6 years [19, 20]. National vaccination data for South Korea indicate successful implementation of the childhood immunization program with an estimated vaccine coverage rate of 98% for DTaP3 in 2019 [21]. Several studies reported variable vaccine coverage rates for booster immunization [16, 22, 23]. It was 95.4% (January–June 2019) and 93.5% (January–June 2020) for the fifth dose of DTaP, and 96.3% and 94.4%, respectively, for the fourth dose of inactivated poliovirus vaccine (IPV), when calculated based on the resident registration population data from the Korean Ministry of Public Administration and Security [24]. Another study that compared vaccination rates between NIP vaccines and non-NIP vaccines in Korea reported vaccination coverage rates of 56.6% for the fifth dose of DTaP and 73% for the fourth dose of IPV at school-entry age [25]. Additionally, in order to protect the infants while they are too young to be fully vaccinated, pertussis vaccination during pregnancy protects mothers from pertussis infection and disease, and reduces the likelihood of transmission to their newborn by passive transfer of maternal anti-pertussis antibodies that protect infants. Recently, vaccination against pertussis during pregnancy has been recommended as an additional strategy for protection of young infants in South Korea [26, 27].
Several tetravalent, pentavalent, and mainly hexavalent DTaP- and DTwP (diphtheria, tetanus, and whole-cell pertussis)-containing vaccines are licensed globally. The reported waning of immunity post-vaccination has been considered a possible reason for the re-emergence of some vaccine-preventable diseases [28]. Regardless of the type of pertussis vaccine or the primary series immunization schedule, they do not confer lifelong immunity against pertussis disease [29]. To increase the duration of protection after primary vaccination, the World Health Organization (WHO) recommended a pertussis booster dose for children [30]. Completion of the primary series and the first booster is expected to ensure protection against pertussis for around 4–6 years [31,32,33,34,35,36].
Multivalent vaccines that are administered as a single injection increase compliance and vaccination timeliness, improve adherence to vaccination programs, and reduce the time for vaccine preparation and the overall cost for vaccinees and national vaccination programs [37,38,39,40]. TETRAXIM™ (Sanofi) is a combined diphtheria, tetanus, acellular pertussis, and IPV (DTaP-IPV) vaccine. It has been licensed in South Korea since August 31, 2009, for active immunization against diphtheria, tetanus, pertussis, and poliomyelitis in infants and children. It has been evaluated for immunogenicity and safety in multiple randomized clinical trials including in South Korea [41]. Per the regulations from the Ministry of Food and Drug Safety (MFDS) in South Korea, data were collected in the present study in South Korea to confirm its safety profile in real-world clinical practice as a post-authorization commitment and when used per licensed indications (i.e., primary series and school-entry booster).
This post-marketing surveillance (PMS) study aimed to monitor and evaluate the safety of the DTaP-IPV vaccine over a period of 6 years in children aged between 2 months and 6 years who were given the DTaP-IPV vaccine in real-world clinical practice.
Methods
Study Design
This was a multicenter, observational PMS study, conducted in real-world practice for a duration of 6 years from the product approval date in accordance with the MFDS regulations (NCT01437423). The planned sample size was 600 participants in accordance with the MFDS notification. Participants were enrolled across eight centers from August 31, 2009, to August 30, 2015. The protocol for this study was approved by the MFDS and ethics committee before study initiation. The study was conducted in accordance with the guidelines established by the Declaration of Helsinki and MFDS Notification No. 2009-46 (basic standard for re-examination of new drug). Informed consent was obtained from all participants or their parents/legal representative before enrollment.
Study Participants
Participants aged between 2 months and 6 years were enrolled in the study. Participants were eligible if they had received ≥ 1 dose of primary vaccination as part of the primary vaccination schedule or the booster dose at 4–6 years of age with the study vaccine. Participants were not included in study analysis if they had off-label use of the vaccine.
Study Vaccine
The DTaP-IPV vaccine (TETRAXIM™) is available as a sterile suspension in a single-dose prefilled syringe. Each 0.5 mL dose of the vaccine contains antigens against four target pathogens, ≥ 30 international units (IU) of diphtheria toxoids, ≥ 40 IU of tetanus toxoids, two purified antigens of B. pertussis (25 μg of pertussis toxoid [PT] and 25 μg of filamentous hemagglutinin [FHA], each adsorbed onto aluminum salt), and three distinct poliovirus antigens (40 D-antigen units of type 1 poliovirus, 8 D-antigen units of type 2 poliovirus, and 32 D-antigen units of type 3 poliovirus; each produced on Vero cells). It is a licensed vaccine in South Korea for immunization against diphtheria, tetanus, pertussis, and poliomyelitis in infants and children. It was administered intramuscularly per local product labeling. It was administered as primary vaccination (at least one of the three successive doses at 2-month interval starting from 2 months of age) or as school-entry booster vaccination (children aged 4–6 years).
Data Collection
Demographic and other baseline data were collected on a case report form (CRF). Immediate events, within 30 min of the observation period after the vaccination, were also collected on a CRF on the day of vaccination. Participants and/or parents were given a diary card to record safety information on all pre-listed solicited injection site and systemic reactions and all adverse events (AEs), including unsolicited AEs/adverse drug reactions (ADRs) and serious AEs (SAEs) occurring up to 30 days after vaccination.
Study Outcomes
The study outcomes included occurrence of solicited ADRs (pre-listed injection site reactions and systemic reactions), unsolicited AEs/ADRs, unexpected AEs/ADRs, and SAEs/serious ADRs. Adverse drug reactions were defined as the AEs reported to be related to the study vaccine. By definition, all the solicited AEs were considered to have a causal relationship with the DTaP-IPV vaccine. Unsolicited AEs were defined as observed AEs that did not fulfil the conditions of solicited reactions, i.e., not pre-listed in the CRF in terms of diagnosis and onset window post-vaccination. Unsolicited ADRs were defined as unsolicited AEs that were considered related to the study vaccine. Unexpected ADRs were defined as the ADRs that were not reflected in the AE section of the local product label. SAEs were defined as any event that is fatal or life-threatening or causes hospitalization or the prolongation of hospitalization, or persistent or significant disabilities/incapacity, congenital anomalies, or birth defect, or other medically significant events that jeopardize the participant or require medical or surgical intervention to prevent one of the outcomes.
Per the summary of product characteristics, the safety profile of the study vaccine does not differ significantly between the different age groups. However, some AEs such as myalgia, malaise, and headache are specific to children ≥ 2 years of age [42]. The solicited (pre-listed) injection site AEs for participants for all age groups were tenderness/pain, erythema, and swelling. The solicited (pre-listed) systemic AEs were fever, vomiting, abnormal crying, drowsiness, appetite loss, and irritability for participants aged ≤ 23 months, and myalgia, malaise, and headache for participants aged 4–6 years old.
The intensity of the solicited and unsolicited AEs was measured by the participant’s parent/legal representative based on an intensity scale provided on the CRF. Solicited injection site reactions and systemic reactions were classified as grade 1 (mild), grade 2 (moderate), or grade 3 (severe). Unsolicited AEs were classified as grade 1 (mild—did not interfere with daily activities), grade 2 (moderate—interfered with daily activities), or grade 3 (severe—prevented daily activities).
The causality of each AE was assessed for all events occurring up to 30 days post-vaccination. It was assessed by the investigator as certain (valid time relationship to vaccine administration and cannot be explained by other concomitant drugs or other disorders), probable/likely (pertinent time relationship to vaccine administration and was not likely to be caused by other concomitant drugs or disorders), possible (pertinent time relationship to vaccine administration, but could be caused by other concomitant drugs or disorders), unlikely (not likely to have causal relationship with vaccine administration; temporary cases), conditional/unclassified (requires more data for proper assessment), or unassessable/unclassifiable (insufficient information exists).
Statistical Analysis
Primary analyses were descriptive in nature. Safety evaluation population was defined as all participants who received the DTaP-IPV vaccine. AEs were summarized using the WHO Adverse Reaction Terminology. Summary statistics were presented for AEs (MedDRA [Medical Dictionary for Regulatory Activities] preferred term). As required by the MFDS in South Korea, in order to determine the factors that may affect the incidence of AEs, univariate analysis (chi-square test or Fisher’s exact test) was conducted on the demographic and medical factors of participants. Gender distribution, current medical history, past medical history, previous vaccination history, simultaneous vaccination, and concomitant medication were assessed using the chi-square test, and age, renal disease (current medical history), and allergic history were assessed using Fisher’s exact test.
Results
Demographic and Baseline Characteristics
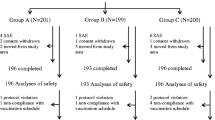
Among the 662 participants enrolled in the study, data from 647 outpatient participants were included in the safety analysis (Fig. 1). Overall, 602 participants had received ≥ 1 dose of the primary vaccination series, and 45 participants had received the school-entry booster dose. The exclusion criteria included failure to follow up (n = 12) and off-label vaccine usage (n = 3). The demographic and baseline characteristics are presented in Table 1. Overall, 50.5% of the participants were female. A total of 14.4% of participants had ongoing medical conditions at the time of vaccination. The most frequently reported current medical condition was bronchitis (3.9%), followed by upper respiratory tract infection (1.9%) and otitis media (1.4%). Overall, 34% of participants were on concomitant medications at the time of vaccination or during the safety follow-up (30-days period post-vaccination). The most frequently reported concomitant medications were for the treatment of cough and cold episodes (17.9%), followed by anti-asthmatics and other respiratory disease medications (16.1%), and nasal decongestant or other nasal preparations (15.9%). None of the study participants experienced diphtheria, tetanus, pertussis, or poliomyelitis during the study. Among 647 participants, 477 (73.7%) were vaccinated simultaneously with at least one other vaccine. The most frequent simultaneous vaccines were Haemophilus influenzae type b (37.9%), rotavirus (33.1%), hepatitis B (26.1%), and Streptococcus pneumoniae (16.2%) vaccines. Additionally, 58 (9%) participants were vaccinated with other vaccines within 4 weeks prior to the study vaccine. The most frequent vaccines administered within the past 4 weeks were hepatitis B (7%), H. influenzae type b (1.6%), and S. pneumoniae (1.2%) vaccines.
Safety Analysis
Overall, 268 AEs were reported by 181 (28%) participants (Table 2), of which 47 (17.5%) were solicited reactions, 220 (82.1%) were unsolicited AEs, and 1 (0.4%) was an unsolicited ADR. The most frequently reported AE was bronchitis (11.8%), followed by upper respiratory tract infection (4.6%), enteritis (3.1%), injection site tenderness (3.1%), and contact dermatitis (2.3%).
Immediate Adverse Events
No immediate (occurring within 30 min after vaccination) AEs were reported during this study.
Solicited Reactions
Overall, 47 solicited reactions were reported by 47 (7.3%) participants (Table 3), all of which were reported to have a causal relationship with the DTaP-IPV vaccine, by definition. All solicited reactions were mild in intensity, except fever. Two cases of fever were of moderate intensity (> 38.5 to ≤ 39.5 °C). Injection site tenderness was the most frequently observed solicited reaction, reported by 3.1% of participants (n = 20). All participants with solicited reactions recovered.
Unsolicited Adverse Events
Overall, 221 unsolicited AEs were reported (Table 4). The most frequently observed unsolicited AE was bronchitis (11.8% [n = 79]), followed by upper respiratory tract infection (4.6% [n = 32]), enteritis (3.1% [n = 20]), and contact dermatitis (2.3% [n = 15]). Only one case of irritability of mild intensity in one (0.2%) participant was reported to have a possible causal relationship with the DTaP-IPV vaccine. All other unsolicited AEs were reported to have no causal relationship with the study vaccine.
Most (91.4%) of the AEs were of mild intensity, and none of the unsolicited AEs were severe in intensity. Among the most frequently reported unsolicited AEs, 66/79 cases of bronchitis, 30/32 cases of upper respiratory tract infection, 19/20 cases of enteritis, and all 15 cases of contact dermatitis were of mild intensity. Thirteen cases of bronchitis were of moderate intensity, followed by four cases of otitis media, two of upper respiratory tract infection, two of fever, and one case each for bronchiolitis, enteritis, and diarrhea (Table 5). Participants with moderate-intensity AEs recovered within a few days. Overall, all participants with unsolicited AEs recovered.
Unexpected Adverse Events
Among 268 reported AEs, a total of 212 unexpected AEs were reported by 152 (23.5%) participants. The most frequently reported unexpected AE was bronchitis (11.8%), followed by upper respiratory tract infection (4.6%), enteritis (3.1%), and contact dermatitis (2.3%). None of the unexpected AEs were reported to have a causal relationship with the DTaP-IPV vaccine.
Serious Adverse Events
No SAEs were reported during the study.
Occurrence of Adverse Events by Demographic or Medical Characteristics
Demographic and medical factors, including gender, age, current medical history, past medical history, allergic history, previous vaccination history, simultaneous vaccination, and concomitant medications, were assessed for their effect on the incidence of AEs. Among the participants who reported AEs, 34% (n = 220) were on concomitant medications. Although results indicated that the incidence of AEs was higher among participants on concomitant medications (odds ratio [OR], 59.98; 95% confidence interval, 34.64–103.87; p < 0.0001), data from participants receiving the medication in response to an AE post-vaccination with DTaP-IPV were also included in the analysis. Other medical characteristics did not have a significant effect on the incidence of AEs (p > 0.05).
Discussion
This PMS study evaluated the safety of the DTaP-IPV vaccine in South Korea, after its approval, in participants aged between 2 and 6 months for the primary series and between 4 and 6 years for the school-entry booster dose. Results demonstrated an acceptable safety profile, although the sample size, which was driven by local regulatory requirements, would not necessarily allow the detection of rare AEs. All the solicited and unsolicited AEs were mild or moderate in intensity, and all participants recovered. No unexpected AEs were reported to have a causal relationship with the DTaP-IPV vaccine. There were no deaths or SAEs reported during the study, and no participant discontinued due to AEs.
The findings from this study are consistent with the information present on the local product label. They are also in line with the good safety profile of other DTaP-IPV vaccines reported from real-word use [19, 43]. Injection site AEs/ADRs are commonly reported for vaccines administered via the intramuscular route [44]. By definition, all the solicited injection site reactions, including injection site tenderness, erythema, and swelling, were considered related to the DTaP-IPV vaccine in this study. The occurrence of solicited reactions in this study was consistent with the information on the product label, where redness, pain, and swelling at the injection site were considered to be very common (≥ 1/10) AEs [42]. Fever was reported as the most common AE (11.9%) in another PMS study that assessed the safety profile of another DTaP-IPV in South Korea [19]. In contrast to this other PMS study and the product label of the study vaccine, which considers a fever ≥ 38 °C as a very common AE, the rate of fever was substantially lower in the current study (1.4%). The overall incidence of solicited reactions in this study was lower than that observed in a randomized clinical trial assessing the immunogenicity and safety of DTaP-IPV (primary vaccination series) in infants from South Korea [41], possibly because this PMS study evaluated the safety of the DTaP-IPV vaccine in real-world clinical practice.
Some unsolicited AEs were reported in this study. All were considered not related to the study vaccine, except one case of irritability. The majority of the other unsolicited AEs were diseases or conditions occurring commonly in infants and children.
In this study, all the unexpected AEs were determined to be unrelated to vaccination. Most of them were mild in severity, and all participants recovered.
Participants who had concomitant medications (any medication being taken at the time of study vaccination and/or within the 30-day post-vaccination period) reported a higher incidence of AEs in this study. However, this data should be interpreted with extreme caution as the data from these participants receiving the medication in response to an AE following vaccination were not excluded from the analysis, and therefore, introduced a bias in the analysis.
Clinical trials have demonstrated the safety of the DTaP-IPV combination vaccine in South Korea [41] and other countries [45,46,47,48,49,50,51,52,53], and their results were based on data obtained through selective inclusion and exclusion criteria and mainly included healthy participants. On the other hand, real-world post-licensure safety studies can complement knowledge regarding vaccine safety in the general population. Hence, such studies have important implications for the formation and implementation of national immunization policies. The data from this study support the overall assessment of the safety of the DTaP-IPV vaccine in real-world practice in South Korea in addition to the existing clinical trial data. The findings from this PMS study are consistent with the known safety profile of DTaP-IPV.
Combination vaccines simplify vaccine administration, permit inclusion of new vaccines to the childhood schedules, help in achieving higher vaccination coverage, and reduce vaccination costs. A clinical trial previously demonstrated no clinically significant difference between the reactogenicity of the DTaP-IPV vaccine versus the DTaP and IPV vaccines administered separately. The incidence of solicited injection site reactions (tenderness, erythema, and swelling) was 27.0–36.9% in participants who received DTaP–IPV, 11.6–23.9% for DTaP, and 6.0–20.2% for IPV. The incidence of severe injection site reactions was low and was similar between the combined and separate vaccine groups (0.2–0.6% in the DTaP-IPV group vs. 0.2–0.6% in the DTaP + IPV group). Overall, the proportion of solicited systemic reactions was similar between the two groups. Fever (≥ 37.4 °C, axillary) was observed in 9.5% and 7.1% of participants in the DTaP-IPV and DTaP + IPV groups, respectively. Other solicited systemic AEs were observed in 20.6–27.0% of participants in the DTaP-IPV group and in 19.1–24.5% in the DTaP + IPV group. Unsolicited AEs were reported in 72.8% and 73.9% of participants who received the DTaP-IPV and separate vaccines, respectively [41], indicating a similar safety profile between the two groups.
Vaccine safety in real-life practice is key for vaccine acceptance and adherence to vaccination programs. This will contribute to the achievement and maintenance of optimal vaccination coverage in the entire targeted populations to sustain high immunity against diphtheria, tetanus, pertussis, and polio. Vaccinating school-entry children who are a source of infection for younger infants, too young to be vaccinated or incompletely vaccinated, also confers herd protection. Monitoring of the safety of the DTaP-IPV vaccine will be continued through voluntary reporting of post-vaccination AEs and collection of safety information.
This study had some limitations. As the sample size was based on the regulatory requirement in South Korea, it was limited (approximately 600 participants), which would not necessarily allow the detection of rare AEs. Furthermore, the AEs were self-reported by parents, and this might have led to reporting bias. However, the vaccine safety management system in South Korea includes a rapid response system to ensure the documentation of rare or serious AEs post-vaccination [54].
Conclusion
No safety concerns related to the use of the DTaP-IPV vaccine in the real world were raised in participants 2–6 months old for the primary series and 4–6 years old for the school-entry booster dose in the Korean population. The review of safety results in this local PMS study reaffirms that the DTaP-IPV vaccine was well tolerated and can be given as part of routine immunization programs for active immunization against diphtheria, tetanus, pertussis, and poliomyelitis in infants and children.
References
Centers for Disease Control and Prevention (CDC). Diphtheria, tetanus, and pertussis vaccines. Available from https://www.cdc.gov/vaccinesafety/vaccines/dtap-tdap-vaccine.html.
Edwards KM, Decker MD. Pertussis vaccines. In: Plotkin SA, Orenstein WA, Offit PA, editors. Plotkin’s vaccines. 7th ed. Philadelphia: W.B. Saunders Company; 2017. p. 711–61.
Spector TB, Maziarz EK. Pertussis. Med Clin N Am. 2013;97(4):537–52.
Skoff TH, Kenyon C, Cocoros N, Liko J, Miller L, Kudish K, et al. Sources of infant pertussis infection in the United States. Pediatrics. 2015;136(4):635–41.
Jardine A, Conaty SJ, Lowbridge C, Thomas J, Staff M, Vally H. Who gives pertussis to infants? Source of infection for laboratory confirmed cases less than 12 months of age during an epidemic, Sydney, 2009. Commun Dis Intell Q Rep. 2010;34(2):116–21.
Forsyth K, Plotkin S, Tan T, Wirsing von König CH. Strategies to decrease pertussis transmission to infants. Pediatrics. 2015;135(6):e1475–82.
Nguyen VTN, Simon L. Pertussis: the whooping cough. Prim Care. 2018;45(3):423–31.
Nieves DJ, Heininger U. Bordetella pertussis. Microbiol Spectr. 2016;4(3).
Diphtheria vaccine: WHO position paper—August 2017. Releve Epidemiol Hebdomadaire. 2017;92(31):417–35.
Tetanus vaccines: WHO position paper—February 2017. Releve Epidemiol Hebdomadaire. 2017;92(6):53–76.
Pertussis vaccines: WHO position paper—September 2015. Releve Epidemiol Hebdomadaire. 2015;90(35):433–58.
Polio vaccines: WHO position paper—March, 2016. Releve Epidemiol Hebdomadaire. 2016;91(12):145–68.
Jackson DW, Rohani P. Perplexities of pertussis: recent global epidemiological trends and their potential causes. Epidemiol Infect. 2014;142(4):672–84.
Domenech de Cellès M, Magpantay FM, King AA, Rohani P. The pertussis enigma: reconciling epidemiology, immunology and evolution. Proc Biol Sci. 2016;283(1822):20152309.
Kim C, Yi S, Cho SI. Recent increase in pertussis incidence in Korea: an age-period-cohort analysis. Epidemiol Health. 2021;43: e2021053.
Mungall BA, Kim H, Oh KB. A systematic review of the burden of pertussis in South Korea. Hum Vaccin Immunother. 2021;17(6):1747–56.
Choe YJ, Park YJ, Jung C, Bae GR, Lee DH. National pertussis surveillance in South Korea 1955–2011: epidemiological and clinical trends. Int J Infect Dis IJID. 2012;16(12):e850–4.
Pertussis vaccines: WHO position paper, August 2015—recommendations. Vaccine. 2016;34(12):1423–5.
Lee SM, Kim SJ, Chen J, Song R, Kim JH, Devadiga R, et al. Post-marketing surveillance to assess the safety and tolerability of a combined diphtheria, tetanus, acellular pertussis and inactivated poliovirus vaccine (DTaP-IPV) in Korean children. Hum Vaccin Immunother. 2019;15(5):1145–53.
Jo DS, Kim J-H, Choi EH, Park SE, Kim Y-J, Kim YK, et al. Recommended immunization schedule for children and adolescents: the Korean Pediatric Society, 2013. Korean J Pediatr. 2013;56(6):231–4.
WHO vaccine-preventable diseases: monitoring system. 2020 global summary. Available from: https://apps.who.int/immunization_monitoring/globalsummary/coverages?c=KOR.
Lee SY, Han SB, Kang JH, Kim JS. Pertussis prevalence in Korean adolescents and adults with persistent cough. J Korean Med Sci. 2015;30(7):988–90.
Park S, Lee MG, Lee KH, Park YB, Yoo KH, Park JW, et al. A multicenter study of pertussis infection in adults with coughing in Korea: PCR-based study. Tubercul Respir Dis. 2012;73(5):266–72.
Yu JH, Jeong HJ, Kim SJ, Lee JY, Choe YJ, Choi EH, et al. Sustained vaccination coverage during the coronavirus disease 2019 epidemic in the Republic of Korea. Vaccines. 2020;9(1):2.
Choe YJ, Yang JJ, Park SK, Choi EH, Lee HJ. Comparative estimation of coverage between national immunization program vaccines and non-NIP vaccines in Korea. J Korean Med Sci. 2013;28(9):1283–8.
Abu-Raya B, Maertens K. Protection of the newborn through vaccination in pregnancy. Neoreviews. 2021;22(1):e25–39.
Kim C, Pae J, Kim WJ, Jang Y, Wie JH, Park IY, et al. Current status of pertussis vaccination during pregnancy and influencing factors in Korea. Taiwan J Obstet Gynecol. 2021;60(2):273–80.
Gao H, Lau EHY, Cowling BJ. Waning immunity after receipt of pertussis, diphtheria, tetanus and polio-related vaccines: a systematic review and meta-analysis. J Infect Dis. 2021;225:557–66.
Schwartz KL, Kwong JC, Deeks SL, Campitelli MA, Jamieson FB, Marchand-Austin A, et al. Effectiveness of pertussis vaccination and duration of immunity. CMAJ Can Med Assoc J. 2016;188(16):e399–406.
Pertussis vaccines: WHO position paper. Releve Epidemiol Hebdomadaire. 2010;85(40):385–400.
Kurova N, Timofeeva EV, Guiso N, Macina D. A cross-sectional study of Bordetella pertussis seroprevalence and estimated duration of vaccine protection against pertussis in St. Petersburg, Russia. Vaccine. 2018;36(52):7936–42.
Lugauer S, Heininger U, Cherry JD, Stehr K. Long-term clinical effectiveness of an acellular pertussis component vaccine and a whole cell pertussis component vaccine. Eur J Pediatr. 2002;161(3):142–6.
Salmaso S, Mastrantonio P, Tozzi AE, Stefanelli P, Anemona A, Ciofidegli Atti ML, et al. Sustained efficacy during the first 6 years of life of 3-component acellular pertussis vaccines administered in infancy: the Italian experience. Pediatrics. 2001;108(5):E81.
Carlsson RM, Trollfors B. Control of pertussis–lessons learnt from a 10-year surveillance programme in Sweden. Vaccine. 2009;27(42):5709–18.
Quinn HE, Snelling TL, Macartney KK, McIntyre PB. Duration of protection after first dose of acellular pertussis vaccine in infants. Pediatrics. 2014;133(3):e513–9.
Paradowska-Stankiewicz I, Rumik A, Bogusz J, Zbrzeźniak J, Rastawicki W, Śmietańska K, et al. Duration of protection against Bordetella pertussis infection elicited by whole-cell and acellular vaccine priming in Polish children and adolescents. Vaccine. 2021;39(41):6067–73.
Obando-Pacheco P, Rivero-Calle I, Gómez-Rial J, Rodríguez-Tenreiro Sánchez C, Martinón-Torres F. New perspectives for hexavalent vaccines. Vaccine. 2018;36(36):5485–94.
Tafreshi SH. Efficacy, safety, and formulation issues of the combined vaccines. Expert Rev Vaccines. 2020;19(10):949–58.
Edwards KM, Decker MD. Combination vaccines. Infect Dis Clin North Am. 2001;15(1):209–30.
Maman K, Zöllner Y, Greco D, Duru G, Sendyona S, Remy V. The value of childhood combination vaccines: from beliefs to evidence. Hum Vaccin Immunother. 2015;11(9):2132–41.
Lee SY, Hwang HS, Kim JH, Kim HH, Lee HS, Chung EH, et al. Immunogenicity and safety of a combined diphtheria, tetanus, acellular pertussis, and inactivated poliovirus vaccine (DTaP-IPV) compared to separate administration of standalone DTaP and IPV vaccines: a randomized, controlled study in infants in the Republic of Korea. Vaccine. 2011;29(8):1551–7.
Summary of Product Characteristics-Tetravac, Suspension for Injection [Version 12.2]. Available from: https://docetp.mpa.se/LMF/Tetravac%20suspension%20for%20injection%20ENG%20SmPC_09001be680001604.pdf.
Daley MF, Yih WK, Glanz JM, Hambidge SJ, Narwaney KJ, Yin R, et al. Safety of diphtheria, tetanus, acellular pertussis and inactivated poliovirus (DTaP-IPV) vaccine. Vaccine. 2014;32(25):3019–24.
Zanoni G, Migliorini M, Gallo T, Guidolin L, Schena D. Recurrent injection site reactions to vaccines: two clinical patterns of presentation. Vaccine. 2020;38(45):6985–9.
Vidor E, Plotkin SA. Immunogenicity of a two-component (PT&FHA) acellular pertussis vaccine in various combinations. Hum Vaccin. 2008;4(5):328–40.
Mallet E, Matisse N, Mathieu N, Langue J, Boisnard F, Soubeyrand B. Antibody persistence against diphtheria, tetanus, pertussis, poliomyelitis and Haemophilus influenzae type b (Hib) in 5-6-year-old children after primary vaccination and first booster with a pentavalent combined acellular pertussis vaccine: immunogenicity and tolerance of a tetravalent combined acellular pertussis vaccine given as a second booster. Vaccine. 2004;22(11–12):1415–22.
Pancharoen C, Chotpitayasunondh T, Chuenkitmongkol S, Ortiz E. Long-term immunogenicity assessment of a DTaP-IPV//PRP-T vaccine given at 2, 4, 6 and 18–19 months of age, and immunogenicity and safety of a DTaP-IPV vaccine given as a booster dose at 4 to 6 years of age in Thai children. Southeast Asian J Trop Med Public Health. 2012;43(3):687–98.
Langue J, David T, Roussel F, Pines E, Hoffenbach. Safety and immunogenicity of a DTaP-IPV and Act-Hib vaccines administered either combined or separately to infants at 2, 3, and 4 months of age. In Abstracts of the 15th European Societies for the Pediatric Infectious Diseases; Paris, France. 1997, Abstract 79.
Lagos R, Kotloff K, Hoffenbach A, San Martin O, Abrego P, Ureta AM, et al. Clinical acceptability and immunogenicity of a pentavalent parenteral combination vaccine containing diphtheria, tetanus, acellular pertussis, inactivated poliomyelitis and Haemophilus influenzae type b conjugate antigens in two-, four- and six-month-old Chilean infants. Pediatr Infect Dis J. 1998;17(4):294–304.
Kang JH, Lee HJ, Kim KH, Oh SH, Cha SH, Lee J, et al. The immunogenicity and safety of a combined DTaP-IPV//Hib vaccine compared with individual DTaP-IPV and Hib (PRP~T) vaccines: a randomized clinical trial in South Korean infants. J Korean Med Sci. 2016;31(9):1383–91.
Collins CL, Salt P, McCarthy N, Chantler T, Lane L, Hemme F, et al. Immunogenicity and safety of a low-dose diphtheria, tetanus and acellular pertussis combination vaccine with either inactivated or oral polio vaccine as a pre-school booster in UK children. Vaccine. 2004;22(31–32):4262–9.
Langue J, Matisse N, Pacoret P, Undreiner F, Boisnard F, Soubeyrand B. Persistence of antibodies at 5–6 years of age for children who had received a primary series vaccination with a pentavalent whole-cell pertussis vaccine and a first booster with a pentavalent acellular pertussis vaccine: immunogenicity and tolerance of second booster with a tetravalent acellular vaccine at 5–6 years of age. Vaccine. 2004;22(11–12):1406–14.
Ferrera G, Cuccia M, Mereu G, Icardi G, Bona G, Esposito S, et al. Booster vaccination of pre-school children with reduced-antigen-content diphtheria-tetanus-acellular pertussis-inactivated poliovirus vaccine co-administered with measles-mumps-rubella-varicella vaccine: a randomized, controlled trial in children primed according to a 2 + 1 schedule in infancy. Hum Vaccin Immunother. 2012;8(3):355–62.
Choe YJ, Bae G-R. Management of vaccine safety in Korea. Clin Exp Vaccine Res. 2013;2(1):40–5.
Acknowledgements
The authors would like to thank all participants who volunteered to take part in the study, the primary investigators, and their site staff. We thank Roopsha Brahma, PhD, for editorial assistance and manuscript coordination on behalf of Sanofi. The authors would also like to thank Hyun Jung Kim (Sanofi) and Catherine Huoi (Sanofi) for their contributions as the medical lead for South Korea and the global medical expert, respectively.
Funding
This study and the journal’s Rapid Service Fee were both funded by Sanofi.
Medical Writing Assistance and Other Assistance
Medical writing support for this manuscript was provided by Saili Dharadhar (Sanofi).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work, and have given their approval for this version to be published.
Author Contributions
All authors contributed to the material preparation and data analysis and/or interpretation. All authors read and approved the final manuscript and are accountable for accuracy and integrity of the data presented therein.
Disclosures
Emmanuel Vidor and Christine Manson are employees of Sanofi and may hold stocks in the company. The current affiliation of Young June Choe is Korea University Anam Hospital, 73 Goryeodae-ro Seongbuk-gu, Seoul 02841, South Korea. Young June Choe was an employee of Sanofi at the time of the study.
Compliance with Ethics Guidelines
The protocol for this study was approved by the MFDS and ethics committee before study initiation. The study was conducted in accordance with the guidelines established by the Declaration of Helsinki and MFDS Notification No. 2009-46 (basic standard for re-examination of new drug). Informed consent was obtained from all participants or their parents/legal representative before enrollment.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at https://www.vivli.org/.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Choe, Y.J., Vidor, E. & Manson, C. Post-Marketing Surveillance of Tetravalent Diphtheria-Tetanus-Acellular Pertussis and Inactivated Poliovirus (DTaP-IPV) Vaccine in South Korea, 2009 to 2015. Infect Dis Ther 11, 1479–1492 (2022). https://doi.org/10.1007/s40121-022-00650-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-022-00650-8