Abstract
Introduction
Imipenem/cilastatin/relebactam (IMI/REL), a combination β-lactam antibiotic (imipenem) with a novel β-lactamase inhibitor (relebactam), is an efficacious and well-tolerated option for the treatment of hospitalized patients with gram-negative (GN) bacterial infections caused by carbapenem-non-susceptible (CNS) pathogens. This study examines cost-effectiveness of IMI/REL vs. colistin plus imipenem (CMS + IMI) for the treatment of infection(s) caused by confirmed CNS pathogens.
Methods
We developed an economic model comprised of a decision-tree depicting initial hospitalization, and a Markov model projecting long-term health and economic impacts following discharge. The decision tree, informed by clinical data from RESTORE-IMI 1 trial, modeled clinical outcomes (mortality, cure rate, and adverse events including nephrotoxicity) in the two comparison scenarios of IMI/REL versus CMS + IMI for patients with CNS GN infection. Subsequently, a Markov model translated these hospitalization stage outcomes (i.e., death or uncured infection) to long-term consequences such as quality-adjusted life years (QALYs). Sensitivity analyses were conducted to test the model robustness.
Results
IMI/REL compared to CMS + IMI demonstrated a higher cure rate (79.0% vs. 52.0%), lower mortality (15.2% vs. 39.0%), and reduced nephrotoxicity (14.6% vs. 56.4%). On average a patient treated with IMI/REL vs. CMS + IMI gained additional 3.7 QALYs over a lifetime. Higher drug acquisition costs for IMI/REL were offset by shorter hospital length of stay and lower AE-related costs, which result in net savings of $11,015 per patient. Sensitivity analyses suggested that IMI/REL has a high likelihood (greater than 95%) of being cost-effective at a US willingness-to-pay threshold of $100,000–150,000 per QALY.
Conclusions
For patients with confirmed CNS GN infection, IMI/REL could yield favorable clinical outcomes and may be cost-saving—as the higher IMI/REL drug acquisition cost is offset by reduced nephrotoxicity-related cost—for the US payer compared to CMS + IMI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Carbapenem-non-susceptible (CNS) gram-negative infections (GNIs) continues to grow globally and have very limited treatment options |
This study assessed cost and clinical effectiveness of imipenem/cilastatin/relebactam (IMI/REL) in treating confirmed CNS GNIs, compared to colistin plus imipenem (CMS + IMI) |
What was learned from the study? |
Higher drug acquisition cost for IMI/REL over CMS + IMI may be offset by savings from hospital resource use due to reduced nephrotoxicity risk of IMI/REL |
For treatment of confirmed CNS GNIs, IMI/REL could be cost-effective or even cost-saving for the US payers compared to CMS + IMI |
Introduction
Carbapenems are highly effective against many bacterial species and less vulnerable to most beta-lactam resistance infections and are therefore considered a reliable treatment for Gram-negative infections (GNIs). However, the emergence and dissemination of carbapenem non-susceptible (CNS) GNIs has been witnessed in the USA [1] and worldwide [2], which often has very limited treatment options [3, 4]. The 2019 Centers of Disease Control and Prevention (CDC) report continues to maintain an urgent threat status for infections caused by carbapenem-resistant Enterobacterales (CRE) [5]. According to the CDC, CRE alone caused 13,100 infections among hospitalized patients and about 1100 deaths in the USA in 2019 [5]. Mortality associated with CNS GNI in the USA range from 35% to 60%, with resistance shown to be an independent risk factor for death [6,7,8].
Among the limited existing treatment options for CNS GNIs are colistin and colistin-associated combination therapies owing to colistin’s broad antibacterial spectrum and historically low levels of resistance [3, 4]. However, it is well documented that colistin use is associated with nephrotoxicity [9, 10]. The Infectious Diseases Society of America has previously commented with concern that the pipeline for novel therapeutics to treat drug-resistant infections, including those caused by GN pathogens, was “lean” [11]. A recent review concluded that in the past decade only nine new GN antibacterial agents were brought to market, each with niche roles [12].
Imipenem/cilastatin/relebactam (IMI/REL), a combination of a β-lactam antibiotic (imipenem/cilastatin) and a novel β-lactamase inhibitor (relebactam), provides a treatment alternative with a favorable efficacy and tolerability profile for patients with CNS GNIs [13]. The novel β-lactamase inhibitor (relebactam) restores the activity of imipenem against imipenem-resistant isolates such as KPC-producing Enterobacterales and/or against Pseudomonas aeruginosa isolates showing CNS due to porin loss in combination with AmpC expression [13].
IMI/REL was evaluated in RESTORE-IMI 1, a randomized, controlled, double-blind, phase III study comparing IMI/REL with colistimethate sodium plus imipenem/cilastatin (CMS + IMI) [14]. At day 28, IMI/REL was associated with higher favorable clinical response (71.4% vs. 40.0%) and lower 28-day all-cause mortality (9.5% vs. 30.0%) compared with CMS + IMI. Moreover, in a pre-specified safety analysis, fewer patients treated with IMI/REL vs. CMS + IMI experienced drug-related adverse events (AE) (16.1% vs. 31.3%, p = 0.001), including significantly fewer events related to treatment-emergent nephrotoxicity (10.3% vs. 56.3%, p = 0.001) [14].
Although the efficacy and tolerability profile of IMI/REL has been investigated in RESTORE IMI 1, the economic implication associated with the use of IMI/REL for the treatment of CNS GNIs has not been assessed. This study aimed to determine the cost-effectiveness of IMI/REL vs. CMS + IMI for the treatment of infections caused by CNS pathogens, from a US third-party payer perspective.
Methods
Model Overview
As per the RESTORE IMI-1 [14] study population, our model considered hospital-admitted adult patients with both no improvement from prior treatment and confirmed CNS GN bacterial infection(s). The model consists of two components. The first is a short-term decision tree which depicts antibacterial interventions during the hospitalization; each intervention can yield one of three patient health states: cured, not cured, or death. The second component, a Markov model with annual cycles, projects long-term health outcomes (such as life years and quality-adjusted life years [QALYs]) following hospital discharge till death (capped at 100 years old).
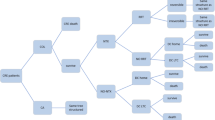
The decision tree (depicted in Fig. 1) begins with a treatment choice between IMI/REL and CMS + IMI for patients with a confirmed CNS infection. As the RESTORE IMI-1 trial population is those with confirmed CNS GNI with no improvement from prior treatment, to be consistent with the trial, this model did not explicitly model the efficacy of prior “failed” treatment. On the other hand, the model did consider salvage or extended therapy beyond the trial-defined treatment duration if a patient is not clinically cured. Salvage or extended therapy is not uncommon in real-world practice if an infection is not sufficiently managed or complications emerge. Therefore, the model included “switch to CMS + IMI” in the event that a patient is not cured by IMI/REL (i.e., salvage therapy), or “extended use of CMS + IMI” if a patient is not cured by CMS + IMI (i.e., extended therapy).
Patients who survived during hospitalization move to the Markov model, which was designed to capture lifetime costs and health-related quality of life. Cured patients will die at an age- and gender-specific rate, while uncured patients after receiving two lines of treatment are assumed to die within a year from index hospital admission (an assumption based on interviews of three infectious disease experts conducted by authors; this assumption was also tested in scenario analysis with alternative assumptions of 50% 1-year mortality rate or equal mortality with the general population [i.e., no additional mortality risk for the uncured]; see scenarios 21 and 22 in Appendix 5).
Model Settings
The cohort profile reflected the study population in RESTORE IMI-1 (average 56.7 years old and 76 kg body weight; 35.5% male). CNS GN infection types included the three types in RESTORE IMI-1 trial: hospital-acquired and ventilator-associated bacterial pneumonia (HABP/VABP) (35.5%), complicated intra-abdominal infection (cIAI) (12.9%), and complicated urinary tract infection (cUTI) (51.6%).
The model followed a 40-year lifetime horizon. Aligned with the reference case for the Institute for Clinical and Economic Review [15], our model adopted a US healthcare sector perspective, included direct costs incurred by third-party payers or integrated health systems, and captured direct health effects for patients. Results presented as costs and QALYs were discounted at an annual rate of 3.0% [15].
Clinical Efficacy
Key model input parameters are discussed here, with a complete list provided in Table 1. Clinical effectiveness and safety data for IMI/REL and CMS + IMI were sourced directly from the RESTORE IMI-1 trial [14]. Clinical response and all-cause mortality rates for IMI/REL and CMS + IMI were input into the model using the microbiological modified intent-to-treat (mMITT) analysis population [14]. The mMITT was the primary efficacy population in the trial, and defined as all patients from the ITT population, except those who did not receive any amount of a study drug, or who had a baseline bacterial pathogen that did not meet inclusion criteria (i.e., a pathogen that was non-susceptible to IMI, and susceptible to both IMI/REL and CMS + IMI). The mMITT population was aligned with the modeled target population. In addition to mMITT data, the trial also had a supplemental mMITT (SmMITT) population, which was defined as subjects whose baseline pathogens met susceptibility criteria based on the interpretive criteria used by the local laboratories. A scenario using the efficacy from the SmMITT population was explored in a sensitivity analysis (Appendix 5).
The primary endpoint in RESTORE IMI-1, favorable overall response (71.4% for IMI/REL and 70.0% for CMS + IMI), was a composite endpoint for which the definition varies across infection type. More specifically, favorable overall response was defined as 28-day survival post-randomization for patients with HABP/VABP, whereas for patients with cUTI or cIAI it was defined as 28-day clinical cure with (for cUTI) or without (for cIAI) sustained microbiological eradication. Because of inconsistent definitions across the infection types, the favorable overall response was not used in the base case analysis (but tested in scenario analysis, see Appendix 5). Instead, the favorable clinical response at 28 days, which was defined consistently as resolution of signs and symptoms of index infection (for more detailed description, see Appendix 1), was used in the model to measure the percentage of clinical cure. In addition, we used all-cause mortality through day 28 to measure the percentage of death. The above alternative endpoints not chosen in the base case were also explored in the sensitivity analysis (Appendix 5).
Adverse Events
Treatment emergent nephrotoxicity is well documented among patients treated with colistin [9, 16] and included in the base case. In addition, serious drug-related adverse events (AEs) observed in at least 5% of patients in one or more treatment groups during IV therapy and 14-day follow-up in RESTORE-IMI 1 were also included (Table 2).
Costs
The model considered GNI-treatment-associated direct costs borne by the US healthcare system, including drug treatment cost, hospital resource costs, and AE management cost. For the drug treatment cost, the wholesale acquisition costs for IMI/REL ($6688/25 vials 500 mg/250 mg units), CMS ($336/12 vials 150 mg), and IMI ($816/25 vials, 500 mg/500 mg) were sourced from the 2020 RED BOOK online database [13] and combined with trial-observed treatment durations to compute total acquisition costs [18]. Drug administration costs were not included in the model base case, as it is assumed these costs are implicitly captured by resource use (hospitalization) costs. For hospital resource costs, these were modeled using the average length of stay in the ICU and general ward reported in the literature [19], and stratified by clinical outcome (cured, uncured, or death). Daily ICU cost was sourced from Halpern and Halpern [20], and inpatient general ward costs was sourced from the Kaiser Family Foundation database 2018 [21]. The costs associated with AE management were based on the 2020 national inpatient average cost estimates for the corresponding International Classification of Diseases 10 (ICD-10) codes sourced from HCUPNet [22]. Lastly, all cost items were inflated to 2020 dollars using the Consumer Price Index sourced from the US Department of Labor Bureau of Labor Statistics [22, 23].
Utility
Health-related utility values during hospitalization were set to be dependent on a patient’s setting (ICU or general ward). More specifically, the base case utility for a patient was 0.68 in ICU [24] and 0.73 in a general ward [25]. The model did not account for any utility decrement associated with AEs during the initial hospitalization period. As patient utility during the initial hospitalization period was based on hospital setting (ICU and general ward), adding an additional per-AE-incidence utility loss would risk double-counting. The base case model assumed that patients’ quality of life returns to that of the general population following the initial discharge (i.e., the infection is cleared).
Analysis
The model considers the following clinical and economic outcomes: (1) percentage of patients cured and dead during hospitalization, (2) percentage of nephrotoxicity cases, (3) total healthcare cost (including drug acquisition, hospital resource use, long-term monitoring, and AEs), (4) life-time QALYs, and (5) incremental cost-effectiveness ratio (ICER) calculated as difference in total healthcare cost per death averted, or per nephrotoxicity case averted, or per QALY gained.
Sensitivity analyses were carried out to assess uncertainty and test key model assumptions. First, a one-way sensitivity analysis (OWSA) was conducted by varying individual model input parameters in turn to its upper and lower bounds (calculated from the 95% confidence interval of the assigned probability distribution) to identify key drivers of the ICER. Second, a two-way sensitivity analysis (TWSA) was conducted specifically to test the impact of trial efficacy due to limited sample size—in terms of 28-day mortality and favorable clinical cure rate—on the ICER. Third, in a probabilistic sensitivity analysis (PSA), all parameters subject to uncertainty were simultaneously randomly sampled from their assigned probability distributions for 10,000 iterations. Values of input parameters tested in the OWSA and PSA are listed in Appendix 3. Lastly, scenario analyses were conducted to explore the alternative to base case assumptions and model structural uncertainty (see Appendix 5).
This study was based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Results
Base Case
In terms of health outcomes, the model estimated favorable results for patients treated with IMI/REL compared with CMS + IMI, with an improved clinical cure rate (27.0%), reduced in-hospital mortality (− 23.8%), and reduced occurrence of nephrotoxicity (− 41.8%). The differences were smaller compared to the point estimates from the RESTORE IMI-1 mMITT population [14]; this was partially due to the model setting in which uncured patients in the IMI/REL arm would receive CMS + IMI as salvage therapy, while uncured patients in the CMS + IMI arm would receive extended treatment of CMS + IMI. Such a setting led to a higher cure rate in both arms and reduced mortality difference than observed in the trial. Differences in mortality and cure rates translate to a projected difference of 3.7 QALYs per treated patient over lifetime.
IMI/REL incurred higher acquisition costs of $9821 more per treated patient compared with CMS + IMI use. However, this higher drug cost was offset by savings ($9888) made from a shorter overall hospital LOS among patients treated with IMI/REL. Furthermore, when costs associated with AE management ($10,555 less for IMI/REL) and follow-up monitoring visits ($127 more for IMI/REL) are considered, IMI/REL would results in net savings of $11,090 compared with CMS + IMI per treated patient with confirmed CNS infection.
Combining the estimated health and economic outcomes, the model demonstrated that IMI/REL could potentially yield better health benefits while saving healthcare costs compared with CMS + IMI. For patients treated with IMI/REL, we observed negative ICERs of − $1988 per QALY gained, or − $46,579 per death averted, or − $26,521 per nephrotoxicity case averted. Negative ICERs indicate that IMI/REL for the treatment of confirmed CNS GNI, relative to CMS + IMI, could not only be cost-effective but also cost-saving.
Sensitivity Analysis
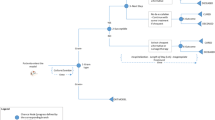
A Tornado diagram (Fig. 2) generated from the OWSA showed the impact of uncertainty around individual input parameters on the ICER per QALY gain. The parameters with the largest impact on the ICER were the in-hospital mortality and clinical response rates of CMS + IMI and IMI/REL. This was unsurprising considering that response and mortality rates determined the proportion of patients who enter the cured, uncured, and death states (thereby influencing treatment and resource use costs during hospitalization). In addition, response and mortality rates also established the proportion of patients who enter the long-term Markov model and, in turn, directly influenced QALY gains over a patient’s lifetime. Another finding from the tornado diagram was that, at a commonly referenced US ICER threshold of $100,000 per QALY gain [26], RECARBRIO remains a cost-effective treatment option at the lower and upper bounds of the response rates of RECARBRIO and CMS + IMI.
Deterministic sensitivity analysis. cUTI complicated urinary tract infection, HABP/VABP hospital-acquired bacterial pneumonia/ventilator-associated bacterial pneumonia, mMITT microbiological modified intent-to-treat, IMI/REL imipenem/cilastatin/relebactam, CMS + IMI colistimethate sodium plus imipenem/cilastatin
The two parameters of in-hospital mortality and clinical response rates—the most influential yet highly uncertain because of a limited trial size—were then jointly assessed to determine how combined uncertainty of the two parameters could impact the base case conclusion (in other words, when would IMI/REL no longer be cost-effective over CMS + IMI?). Figure 3 depicts, on a map of mortality and clinical response rate differences between IMI/REL and CMS + IMI, the areas in which IMI/REL (gray) or CMS + IMI (orange) is cost-effective using an ICER threshold of $100,000 per QALY gained [26]. It shows that IMI/REL maintains the status of being cost-effective within the entire area bounded by large 95% confidence intervals of mortality and clinical rates from RESTORE IMI-1 trial (depicted by a dashed line eclipse). Even at equal efficacy (triangle), IMI/REL is still cost-effective, which could be attributable to its lower nephrotoxicity. Furthermore, the robustness of the conclusion of IMI/REL being cost-effective is tested and confirmed in the probabilistic sensitivity analysis (Appendix 4) and scenario analysis (Appendix 5). In the scenario analysis, when the favorable overall response from RESTORE IMI-1 was used in place of the clinical response rate (scenario 16), IMI/REL was no longer cost-saving over CMS + IMI but had a positive ICER of $12,083 per QALY gain (lower than the threshold of $100,000 per QALY gain). In addition, the scenario analysis also suggested that the time horizon used in the Markov model (lifetime in the base case; tested in-hospital period only, 1, 5, 15, and 25 years) had significant impact of QALY values but did not alter the conclusion of IMI/REL dominating CMS + IMI.
Discussion
This study found that, compared to the currently available treatment option of CMS + IMI for confirmed CNS GNIs, IMI/REL has the potential to achieve better clinical outcomes without a net increase in treatment cost of CNS GNIs. This conclusion was robust against a wide range of possible scenarios tested. This finding was of importance, given high rates of mortality and economic burden caused by CNS GNIs in the USA and globally. It was estimated that CNS Enterobacterales infections alone cost the USA $2.2 billion annually for hospitals and payers, and $2.8 billion for society [8]. This burden underpins the maintained CDC threat status [5] and the need for new effective agents against CNS GNIs. It would be logical to assume that any benefits of reduced mortality, improved patient outcomes, and reduced cost/burden to the healthcare system would have a positive impact holistically.
All economic evaluations of new medical technologies are rooted in their comparative effectiveness against the current standard of care. Uncertainty in comparative effectiveness translates into uncertainty in economic value. In this study, uncertainty around the 28-day mortality and the clinical response rate among patients receiving IMI/REL versus CMS + IMI was moderately high, as a result of the limited trial sample size (mMITT n = 21 IMI/REL, n = 10 CMS + IMI), despite the observation of a powered statistically significant reduction in treatment-emergent nephrotoxicity outcomes. The small trial sample size was attributable to the restrictive inclusion criteria in which eligible patients had to both lack clinical improvement on prior therapy and have CNS pathogens [14]. A US study identified only 4.5% of GNIs in a national sample were CNS [27]; nonetheless, CNS pathogens especially CNS Enterobacterales were increasing in recent years in the USA [28] as well as in Europe [29]. Given the substantial excess mortality and economic burden associated with CNS pathogens, assessment of IMI/REL’s value is crucial to understand its potential impact in treating carbapenem-resistant pathogens. As high-quality/robust real-world evidence for IMI/REL begins to develop, further assessment of the economic value should be conducted.
As a result of differences in pathogen/resistance mechanism coverage, susceptibility profile, and patient and trial characteristics, we did not compare IMI/REL to other novel antibacterial therapies such as ceftazidime/avibactam and meropenem/vaborbactam. In the absence of direct comparative evidence between these antibacterial agents and the aforementioned heterogeneity, it was determined that data synthesis methods such as network meta-analysis would be unreliable [30,31,32]. Recognizing the limitations of established treatment options and the ethos of good antimicrobial stewardship, it is important to consider the collective “option value” that comes with having multiple efficacious and well-tolerated treatment options, rather than trying to determine superiority and the displacement of these newer agents. Rather, providers should consider patient risk factors, their local epidemiology and antibiograms of resistant microorganisms in when selecting from antibacterial therapy, including those newer agents when included on formulary. choosing these new agents. For instance, IMI/REL along with ceftazidime/avibactam and meropenem/vaborbactam are effective against the resistant mechanisms of ESBL, AmpC, and KPC [33, 34]; and ceftazidime/avibactam and IMI/REL both have good activity against multidrug resistant P. aeruginosa [34, 35].
Limitations
This analysis is not without limitations. First, as discussed previously, the RESTORE-IMI 1 trial had a relatively limited cohort size, including 21 patients in the IMI/REL treatment arm and 10 patients in the CMS + IMI arm. By infection type, 11 patients with HABP/VABP, four patients with cIAI, and 16 patients with cUTI were included. Although the associated high uncertainty was addressed by conducting extensive sensitivity analyses in this study, the small sample size may limit generalizability of the economic results with regard to the real-world population. Second, the assumption that uncured CNS GN patients would die within the first year is uncertain and based on clinical opinion, the authors recognized that the study cohort included cUTI and cIAI, which may represent less severe infections compared with HABP/VABP. This assumption may bias our results in favor of IMI/REL as more uncured patients were observed in CMS+IMI arm. Nonetheless, the scenario analysis suggested that using lower mortality values for uncured patients (scenarios 21 and 22 in Appendix 5) would not alter the study conclusion. Third, omitting AE-related utility decrements is a limitation of the model; nonetheless, because the short-term AE-related utility loss is small in comparison to long-term utility loss or death, it is anticipated that the inclusion of AE-related utility would have a negligible effect on the cost-effectiveness outcomes over a lifetime horizon. Lastly, this study did not account for any external benefits that the introduction/of a novel GN antibacterial agent may bring, such as a reduction in the development of antibacterial resistance due to overuse of a limited options, and/or reduced risk of resistance transmission [36, 37].
Conclusion
This study shows that, despite an increased treatment cost compared with the colistin based therapy, IMI/REL for the treatment of confirmed CNS GNI has advantages in terms of clinical outcomes as well as overall/net cost savings. Therefore, IMI/REL is likely cost-effective compared to CMS + IMI when considering a US Payer perspective, and as such represents a valuable option for the management of patients with CN GNI.
Change history
24 June 2022
A Correction to this paper has been published: https://doi.org/10.1007/s40121-022-00660-6
References
Endimiani A, Hujer A M, Perez F, et al. Characterization of bla KPC-containing Klebsiella pneumoniae isolates detected in different institutions in the Eastern USA. J Antimicrob Chemother. 2009;63(3):427–37.
Meletis G. Carbapenem resistance: overview of the problem and future perspectives.Therap Adv Infect Dis. 2016;3(1):15–21.
van Duin D, Kaye KS, Neuner EA, Bonomo RA. Carbapenem-resistant Enterobacteriaceae: a review of treatment and outcomes. Diagn Microbiol Infect Dis. 2013;75(2):115–20.
Peri AM, et al. Antimicrobial treatment challenges in the era of carbapenem resistance. Diagn Microbiol Infect Dis. 2019;94(4):413–25.
Center of Disease Control and Prevention, 2019 AR Threats Report. 2020
Paramythiotou E, Routsi C. Association between infections caused by multidrug-resistant gram-negative bacteria and mortality in critically ill patients. World J Crit Care Med. 2016;5(2):111.
Kollef KE, Schramm GE, Wills AR, et al. Predictors of 30-day mortality and hospital costs in patients with ventilator-associated pneumonia attributed to potentially antibiotic-resistant gram-negative bacteria. Chest. 2008;134(2):281–7.
Bartsch S, et al. Potential economic burden of carbapenem-resistant Enterobacteriaceae (CRE) in the United States. Clin Microbiol Infect. 2017;23(1):48.e9-48.e16.
Shokouhi S and Sahraei Z. A review on colistin nephrotoxicity. Eur J Clin Pharmacol. 2015;71(7):801–10.
Yahav D, Farbman L, Leibovici L, Paul M. Colistin: new lessons on an old antibiotic. Clin Microbiol Infect. 2012;18(1):18–29.
Boucher HW, Talbot GH, Bradley JS, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48(1):1–12.
Pontefract BA, Ho HT, Crain A, Kharel MK, Nybo SE. Drugs for gram-negative bugs from 2010–2019: a decade in review. Open forum infectious diseases. Oxford: Oxford University Press US; 2020.
Sahra S, Jahangir A, Hamadi R, Jahangir A, Glaser A. Clinical and microbiologic efficacy and safety of imipenem/cilastatin/relebactam in complicated infections: a meta-analysis. Infect Chemother. 2021;53(2):271.
Motsch J, Murta de Oliveira C, Stus V, et al. RESTORE-IMI 1: a multicenter, randomized, double-blind trial comparing efficacy and safety of imipenem/relebactam vs colistin plus imipenem in patients with imipenem-nonsusceptible bacterial infections. Clin Infect Dis. 2020;70(9):1799–808.
Institute for Clinical and Economic Review. ICER’s reference case for economic evaluations: principles and rationale. 2018. https://icer-review.org/wp-content/uploads/2018/07/ICER_Reference_Case_July-2018.pdf. Accessed 21 May 2019.
Eljaaly K, Bidell MR, Gandhi RG, et al. Colistin nephrotoxicity: meta-analysis of randomized controlled trials. Open forum infectious diseases. Oxford: Oxford University Press US; 2021.
IBM Microdex. The Red Book Online. 2020. https://www.micromedexsolutions.com/home/dispatch/ssl/true.
Johann Motsch et al. RESTORE-IMI 1: a multicenter, randomized, double-blind trial comparing efficacy and safety of imipenem/relebactam vs colistin plus imipenem in patients with imipenem-nonsusceptible bacterial infections. 2020. https://pubmed.ncbi.nlm.nih.gov/31400759/. Accessed 25 Mar 2021.
Alexander E L, Loutit J, Tumbarello M, et al. Carbapenem-resistant enterobacteriaceae infections: results from a retrospective series and implications for the design of prospective clinical trials. Open Forum Infect Dis. 2017;4(2):ofx063.
Halpern NA, Pastores SM. Critical care medicine beds, use, occupancy, and costs in the united states: a methodological review. Crit Care Med. 2015;43(11):2452–9.
Kaiser Family Foundation. Hospital adjusted expenses per inpatient day, 2018. 2020. https://www.kff.org/health-costs/state-indicator/expenses-per-inpatient-day/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D#. Accessed 16 Nov 2020.
Agency for Health and Research Quality. Healthcare Cost and Utilization Project (HCUP). 2020. https://hcupnet.ahrq.gov/#setup.
US Bureau of Labor Statistics. Consumer Price Index 2020. https://www.bls.gov/cpi/.
Whittington MD, Atherly AJ, Curtis DJ, et al. Recommendations for methicillin-resistant Staphylococcus aureus prevention in adult ICUs: a cost-effectiveness analysis. Crit Care Med. 2017;45(8):1304–10.
Lee BY, Wiringa AE, Bailey RR, et al. The economic effect of screening orthopaedic surgery patients preoperatively for methicillin-resistant Staphylococcus aureus. Infect Control Hosp Epidemiol. 2010;31(11):1130–8.
Institute for Clinical and Economic Review (ICER). ICER’s reference case for economic evaluations: principles and rationale. 2018. https://icer-review.org/wp-content/uploads/2018/07/ICER_Reference_Case_July-2018.pdf. Accessed 21 Aug 2019.
Cai B, Echols R, Magee G, et al. Prevalence of carbapenem-resistant Gram-negative infections in the United States predominated by Acinetobacter baumannii and Pseudomonas aeruginosa. In: Open forum infectious diseases. Oxford: Oxford University Press; 2017.
Jernigan JA, Hatfield KM, Wolford H, et al. Multidrug-resistant bacterial infections in US hospitalized patients, 2012–2017. N Engl J Med. 2020;382(14):1309–19.
Control. E C f D P a. Antimicrobial resistance in the EU/EEA (EARS-Net) Annual Epidemiological Report for 2019 2020. https://www.ecdc.europa.eu/sites/default/files/documents/surveillance-antimicrobial-resistance-Europe-2019.pdf. Accessed 20 Feb 2021.
O'Donnell JN, Rhodes NJ, Lopez J, Jett R, Scheetz MH, et al. Carbapenems vs alternative β-lactams for the treatment of nosocomial pneumonia: a systematic review and meta-analysis. Int J Antimicrob Agents. 2018;52(4):451–8.
Singh KP, Li G, Mitrani-Gold FS, et al. Systematic review and meta-analysis of antimicrobial treatment effect estimation in complicated urinary tract infection. Antimicrob Agents Chemother. 2013;57(11):5284–90.
Thom H, Thompson J, Scott D, et al. Comparative efficacy of antibiotics for the treatment of acute bacterial skin and skin structure infections (ABSSSI): a systematic review and network meta-analysis. Curr Med Res Opin. 2015;31(8):1539–51.
Hayden DA, White BP, Bennett KK. Review of ceftazidime-avibactam, meropenem-vaborbactam, and imipenem/cilastatin-relebactam to target klebsiella pneumoniae carbapenemase-producing enterobacterales. J Pharm Technol. 2020;36(5):202–10.
Yusuf E, Bax HI, Verkaik NJ, van Westreenen M. An update on eight “new” antibiotics against multidrug-resistant gram-negative bacteria. J Clin Med. 2021;10(5):1068.
Noval M, Banoub M, Claeys KC, Heil E. The battle is on: new beta-lactams for the treatment of multidrug-resistant Gram-negative organisms. Curr Infect Dis Rep. 2020;22(1):1–9.
Schaffer SK, West P, Towse A, et al. Assessing the value of new antibiotics: additional elements of value for health technology assessment decisions. London: Office of Health Economics; 2017.
Rothery C, Woods B, Schmitt L, et al., Framework for value assessment of new antimicrobials. Policy Research Unit in Economic Evaluations of Health & Care Interventions: New York, NY, USA, 2018.
Motsch J, Murta de Oliveira C, Stus V, et al. RESTORE-IMI 1: A Multicenter, Randomized, Double-blind Trial Comparing Efficacy and Safety of Imipenem/Relebactam vs Colistin Plus Imipenem in Patients With Imipenem-nonsusceptible Bacterial Infections. Clin Infect Dis. 2019
The Electronic Medicines Compendium (eMC). Summary of Product Characteristics. 2019. https://www.medicines.org.uk/emc.
Bonine N G, Berger A, Altincatal A, et al. Impact of delayed appropriate antibiotic therapy on patient outcomes by antibiotic resistance status from serious gram-negative bacterial infections. Am J Med Sci. 2019;357(2):103–10.
Lodise T, Yang J, Puzniak LA, Dillon R, Kollef M. Healthcare resource utilization of ceftolozane/tazobactam versus meropenem for ventilated nosocomial pneumonia from the randomized, controlled, double-blind ASPECT-NP trial. Infect Dis Ther. 2020;9(4):953–66.
Szende A, Janssen B, and Cabases J, Self-reported population health: an international perspective based on EQ-5D, ed. EQ-5D. 2014: SpringerOpen.
Acknowledgements
Funding
Funding for this research was provided by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey, USA (MSD). MSD provided funding for the journal’s Rapid Service Fee.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors are responsible for the work described in this paper. All authors were involved in at least one of the following: [conception, design of work or acquisition, analysis, interpretation of data] and [drafting the manuscript and/or revising/reviewing the manuscript for important intellectual content]. All authors provided final approval of the version to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosures
Jaesh Naik, Matthew Massello, and Lewis Ralph are employees of Bresmed, which received a collaborative contract from Merck & Co., Inc., Kenilworth, NJ, USA. Joe Yang and Ryan Dillon are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA (MSD) and may own stock and/or hold stock options in Merck & Co., Inc., Kenilworth, NJ, USA.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA’s data sharing policy, including restrictions, is available at http://engagezone.msd.com/ds_documentation.php. Requests for access to the clinical study data can be submitted through the EngageZone site or via email to dataaccess@merck.com.
Prior Presentation
This work was presented, in part, at the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) 2021 virtual meeting during May 18–20. All accepted abstracts were posted in the ISPOR journal Value in Health.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Yang, J., Naik, J., Massello, M. et al. Cost-Effectiveness of Imipenem/Cilastatin/Relebactam Compared with Colistin in Treatment of Gram-Negative Infections Caused by Carbapenem-Non-Susceptible Organisms. Infect Dis Ther 11, 1443–1457 (2022). https://doi.org/10.1007/s40121-022-00607-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-022-00607-x