Abstract
Introduction
The Philippines pediatric national immunization program (NIP) included the 13-valent pneumococcal conjugate vaccine manufactured by Pfizer (PCV13-PFE) since 2015. Uptake has been slow in particular regions, with coverage only reaching all regions in 2019. Given affordability challenges in the context of higher coverage, this study seeks to determine whether universal coverage across all regions of the Philippines with PCV13-PFE will provide good value for money compared with 10-valent PCV alternatives manufactured by GlaxoSmithKline (PCV10-GSK) or Serum Institute of India (PCV10-SII).
Methods
A decision analytic model is adapted for this cost-effectiveness analysis in the Philippines. Clinical and economic input parameters are taken from published sources. Future disease is predicted using age-stratified and population-level observed serotype dynamics. Total cases of pneumococcal disease, deaths, direct and indirect healthcare costs, and quality-adjusted life years (QALYs) gained are discounted 7% annually and modeled for each PCV. Given clinical uncertainty, PCV10-SII outcomes are reported as ranges. Incremental cost-effectiveness ratios (ICERs) are calculated for PCV13-PFE versus lower-valent PCVs (PCV10-GSK or PCV10-SII) from a societal perspective over 10 years.
Results
Nationwide PCV13-PFE use over 10 years is estimated to avert 375,831 more cases, save 53,189 additional lives, and gain 153,349 QALYs compared with PCV10-GSK. This equates to cost-savings of PHP 12.27 billion after vaccine costs are accounted for. Similarly, PCV13-PFE is more effective and cost-saving compared with PCV10-SII. Switching programs to PCV10-SII would result in more cases of disease (313,797 – 666,889), more deaths (22,759 – 72,435), and lost QALYs (108,061 – 266,108), equating to a net economic loss (PHP 359.82 million – 14.41 billion). PCV13-PFE remains cost-effective in the presence of parameter uncertainty.
Conclusion
PCV13-PFE would prevent exceedingly more cases and deaths compared with lower-valent PCVs. Additionally, the PCV13-PFE program is estimated to continue providing cost-savings, offering the best value for money to achieve universal PCV coverage in the Philippines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Economic evaluations of pneumococcal conjugate vaccines (PCVs) in the Philippines can help inform decision makers on which PCV will provide the best value for money to obtain universal PCV coverage for Filipino infants. |
This study assessed whether the 13-valent PCV (PCV13-PFE) would continue to provide good value for money with increased vaccine uptake as compared with switching the national immunization program to include either 10-valent PCV (PCV10) alternative. |
What was learned from the study? |
We estimate that PCV13-PFE would avert additional cases, save more lives, and provide further healthcare and societal cost-savings over 10 years when compared with either PCV10 alternative. |
PCV13-PFE is estimated to provide good value for money into the future and would be the most affordable option to achieve universal PCV coverage in the Philippines. |
Introduction
Streptococcus pneumoniae (S. pneumoniae) is the leading cause of lower respiratory infection morbidity and mortality causing over 1.1 million deaths globally, which is more deaths than all other infectious disease etiologies combined [1]. Pneumococcal disease manifestations can either be invasive, such as meningitis, bacteremia, or bacteremic pneumonia, or non-invasive, such as nonbacteremic pneumonia and acute otitis media (AOM). In the Philippines, pneumococcal diseases pose a substantial burden among children. It has been estimated that over 211,000 cases of pneumonia and invasive pneumococcal disease (IPD) due to S. pneumoniae occurred in children less than 5 years of age during 2015 [2].
Vaccines are available to prevent pneumococcal infections in children, including both a 13-valent pneumococcal conjugate vaccine (PCV13-PFE, formulated by Pfizer Inc.) covering 13 serotypes of S. pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F) and a 10-valent PCV manufactured by GlaxoSmithKline (PCV10-GSK), which contains 10 serotypes (i.e., the same serotypes as PCV13-PFE minus 3, 6A, and 19A).
Since the introduction of PCVs in national immunization programs (NIPs), numerous cost-effectiveness analyses have been conducted of PCVs versus no vaccination and comparing PCV13-PFE and PCV10-GSK. In member states of the Association of Southeast Asian Nations (ASEAN), findings from a recent systematic review by Wang et al. (2021) suggest that PCVs significantly reduce the mortality and morbidity of pneumococcal diseases and are typically cost-effective compared to no vaccination [3]. Within and between studies comparing PCV13-PFE and PCV10-GSK, model assumptions and input parameters, specifically local vaccine acquisition costs, the inclusion or exclusion of indirect effects (serotype replacement and herd protection), and serotype- and pneumococcal disease-specific effectiveness, are shown to be highly influential on cost-effectiveness results [3]. In the systematic review, two studies compared PCV13-PFE versus PCV10-GSK in the Philippines, namely Haasis et al. [4] and Zhang et al. [5].
The economic evaluation of PCVs conducted by Haasis et al. demonstrated that both PCV10-GSK and PCV13-PFE offered better value for money compared with no vaccination in the Philippines, although PCV13-PFE was shown to be even more cost-effective than PCV10-GSK [4]. Given the results of this assessment, the Philippine government chose to include PCV13-PFE in the pediatric national immunization program (NIP). Since 2015, PCV13-PFE has been available for Filipino infants in a 3 + 0 vaccination schedule, which includes a three-dose primary immunization series without a booster.
Despite this progressive advancement in public health, the overall level of PCV uptake and schedule completion remains concerningly low, ranging from 30% to 60% between 2015 and 2019 in Philippine regions with access [6]. PCV access expanded to all regions as of 2019, with National Capital Region and Region 4A and 4B as the last regions to receive PCV13. Rates improved marginally during 2019 for the regions that had access in previous years, ranging from 43% to 83%; however, regions that were newly included in 2019 have extremely low uptake, and all regions’ rates remain below the national target [7].
In efforts to improve national uptake rates and in light of multidose vial availability, a health technology reassessment was commissioned by the Philippine government in 2020 to reassess the cost-effectiveness of PCV13-PFE and PCV10-GSK [6]. The policy question of the 2020 reassessment was to determine which of the two available PCVs would provide the greatest value for money when expanding PCV access in the NIP. The reassessment report by the Health Technology Assessment Council (HTAC) contained a cost–utility analysis with a scenario of 90% vaccine uptake to best answer whether PCV13-PFE or PCV10-GSK is more cost-effective in the context of high vaccine uptake in the Philippines. Results suggested that PCV13-PFE was both less costly and more effective (dominant) over PCV10-GSK. The estimated clinical and economic outcomes demonstrated that PCV13-PFE resulted in larger overall cost-savings compared with PCV10-GSK for the Philippines, with over 45,000 additional averted cases and 4000 additional lives saved from IPD, pneumonia, and AOM. PCV13-PFE’s greater value for money was attributable to the vaccine’s broader serotype coverage, demonstrating that a PCV with coverage for three additional serotypes (3, 6A, and 19A) is important for the prevention of pneumococcal disease.
Economic model assumptions for PCV evaluations will become increasingly important given that a new PCV10 manufactured by Serum Institute of India (PCV10-SII) recently received prequalification from the World Health Organization (WHO) [8]. This vaccine includes a different serotype composition than the other PCV10, containing the same serotypes as PCV13-PFE minus 3, 4, and 18C. Recent publications have stated that PCV10-SII will be priced lower than existing PCVs, and governments are looking to minimize spending in all sectors, including the healthcare sector, especially within the context of the 2019 coronavirus (COVID-19) pandemic’s impact on economies [9]. Countries may prioritize perceived affordability by choosing to procure a lower cost PCV without comprehensively considering the benefits that may be forgone (disease cases averted, potential lives saved, and economic cost-offsets) as a result of switching from a higher-valent to a lower-valent vaccine [10, 11].
Clinical uncertainty exists with any new health intervention, such as the newly licensed PCV10-SII; therefore, economic evaluations need to incorporate in their analyses removing protection against certain serotypes that would no longer be contained within this formulation, including serotypes 3, 4, and 18C. In the Philippines, removing vaccine pressure against these serotypes could have unknown associated impacts, and real-world data suggest that switching from a higher-valent to a lower-valent PCV can result in an increase of pneumococcal disease from the re-emergence of newly unprotected serotypes. For example, Belgium switched from PCV13-PFE to PCV10-GSK in 2015 because of potential vaccine-related cost-savings and a low perceived risk of disease re-emergence given the local epidemiology (i.e., suppressed vaccine-serotype disease) [12]. Thereafter, the incidence of IPD due to newly unprotected serotypes, primarily serotype 19A in the case of PCV10-GSK, increased exponentially and the Belgium Superior Health Council made a proactive recommendation to return to PCV13-PFE [13]. One might need to consider a similar possibility for serotypes 3, 4, and 18C in the case of a switch to PCV10-SII.
Furthermore, PCV10-SII has been licensed on the basis of safety and immunogenicity, but there are no efficacy or effectiveness data for PCV10-SII demonstrating its impact on invasive or non-invasive pneumococcal disease (pneumonia and otitis media), nasopharyngeal carriage, or herd effects. Thus, the potential impacts of removing protection against PCV serotypes in the Philippines, coupled with limited clinical evidence for serotype-specific efficacy/effectiveness, generate additional uncertainties, which need to be reflected in economic evaluations that include PCV10-SII as a comparator.
Therefore, the overall objective of this study is to determine whether maintaining PCV13-PFE is a cost-effective option when increasing vaccine uptake to 90% for Filipino infants nationally, as compared with switching to lower-valent alternatives, PCV10-GSK and PCV10-SII. First, we assess the cost-effectiveness of PCV13-PFE versus PCV10-GSK using a different model framework and assumptions than the 2020 HTAC cost–utility analysis to determine if results and conclusions are consistent. Second, given that PCV10-SII might be available in the Philippines in the future, we assess the cost-effectiveness of PCV13-PFE versus PCV10-SII accounting for PCV10-SII’s clinical uncertainty.
Methods
Model Structure
The study uses a previously published Microsoft Excel-based decision analytic model that leverages historical real-world surveillance data to predict future clinical and economic outcomes depending on local contextual factors [10, 11, 14,15,16,17]. The model predicts prospective serotype behavior based on observed retrospective serotype dynamics with and without vaccine pressure among different population age groups. Age-specific disease incidence attributable to each serotype contained in PCVs is modeled independently and non-vaccine serotypes are modeled together. This methodology captures vaccine pressure on covered serotypes and replacement of non-vaccine serotypes as observed from surveillance data. The projected incidence in pneumococcal disease is used to calculate total cases, deaths, and economic cost outcomes associated with each PCV program.
We adapted this model for the Philippines to estimate the public health and economic impact of continued use of PCV13-PFE compared with a switch to either PCV10-GSK or PCV10-SII in the NIP using a three-dose schedule. Outcomes from the study include total cases of IPD (meningitis and bacteremia), pneumococcal hospitalized and non-hospitalized pneumonia, pneumococcal AOM, meningitis and AOM-related disease sequelae, pneumococcal deaths, direct and indirect healthcare costs, life-years gained, and quality-adjusted life years (QALYs) gained for each of the three PCVs, discounted at an annual rate of 7% as recommended by the Philippine Methods Guide for Health Technology Assessment [18]. An estimated 4,080,768 Philippine children under 2 years of age were eligible for the PCV in 2020 and 90% of infants are assumed to be vaccinated under a three-dose vaccination schedule each year [19]. The cost-effectiveness of maintaining a PCV13-PFE NIP compared to switching to lower-valent PCVs (PCV10-GSK or PCV10-SII) from a societal perspective is assessed by calculating incremental cost-effectiveness ratios (ICERs) over 10 years. This modeling analysis is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Epidemiologic Parameters
Invasive Pneumococcal Disease (IPD) Incidence
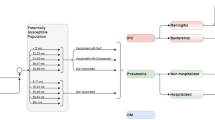
IPD is defined in the model as a combination of meningitis and bacteremia incidence reported by age group. Age group-specific meningitis and bacteremia incidence in the Philippines is sourced from the 2020 HTA reassessment, with the total incidence and the proportion of IPD caused by meningitis reported by age group (Table 1) [6]. The serotype-specific IPD incidence is estimated using the serotype distribution by age group from passive laboratory-based surveillance from the Research Institute for Tropical Medicine (RITM) that the HTAC summarizes in their report [6]. With this reported serotype distribution, the serotype coverage provided by PCV13-PFE, PCV10-GSK, and PCV10-SII was calculated for modeled age groups (Fig. 1).
Non-Invasive Pneumococcal Disease Incidence
Pneumococcal pneumonia incidence is calculated separately for inpatient and outpatient pneumonia. The reported age group-specific incidence of all-cause inpatient (i.e., hospitalized) pneumonia is sourced from the 2020 HTA reassessment [6]. As recommended in Haasis et al.’s analysis, all-cause outpatient (i.e., non-hospitalized) pneumonia cases are estimated based on a 60:40 ratio between hospitalized and non-hospitalized pneumonia (Table 1) [4]. In accordance with the HTAC’s report, 10.47% of all-cause hospitalized and non-hospitalized pneumonia is due to S. pneumoniae across all ages.
The incidence of all-cause AOM is also sourced from the 2020 HTA reassessment and estimated only for children below 18 years of age, given that it is predominantly diagnosed in children (Table 1) [6]. Because the HTAC does not report the proportion of all-cause AOM resulting from S. pneumoniae infection, this estimate is taken from a systematic review of bacteria detected among patients with AOM in Asia (26.4%) [20]. As calculated in other PCV cost-effectiveness studies, post-2020 incidence of pneumonia and AOM in the Philippines from either maintaining PCV13-PFE or switching to a 10-valent PCV is estimated to be proportional to the relative change in forecasted IPD cases each year based on the assumption that serotype distributions equally affect cases of invasive and non-invasive pneumococcal disease [10, 11, 14,15,16,17].
Mortality
General population all-cause mortality for each age group is calculated using population estimates and number of deaths obtained from the Philippine Department of Health (DOH) (Table 1) [21]. Based on case fatality rates reported by the HTAC, the risk of death from pneumococcal meningitis, pneumococcal bacteremia, and hospitalized pneumonia is estimated to be 12.9%, 40.0%, and 4.7%, respectively [6]. Diagnoses of AOM and non-hospitalized pneumonia are assumed to not increase mortality, consistent with other PCV cost-effectiveness analyses [10, 11].
Disease Sequalae
Disease sequalae can occur following episodes of pneumococcal disease. In alignment with the HTAC’s report, 3.64%, 2.18%, and 4.73% of meningitis cases are estimated to result in epilepsy, hearing loss, or neurodevelopmental impairment, respectively. Additionally, 20.0% of patients diagnosed with AOM are estimated to suffer from hearing loss [6]. Sequalae from other IPD manifestations such as bacteremia are not considered in the model to remain consistent with the sequalae modeled by the 2020 HTA reassessment.
Economic Parameters
Vaccine-associated and direct and indirect disease-related costs are included in the model to estimate the costs incurred from the societal perspective. All costs are reported in Philippine pesos (PHP) and discounted at a rate of 7% annually. Because vaccine acquisition costs are confidential, the PCV13-PFE and PCV10-GSK prices used in the model are the 2020 vaccine prices listed by the Pan American Health Organization (PAHO) Expanded Program on Immunization, namely $14.50 USD (PHP 704.43) and $12.85 USD (PHP 624.27) respectively [22]. The acquisition cost of PCV10-SII is still unknown because it is not yet available on the market. Therefore, PCV10-SII price is estimated by applying a 30% price discount to the PCV13-PFE PAHO price, as suggested by a recent publication by the Serum Institute of India [9]. The cost of administering the vaccine is estimated at PHP 2.50 per dose, as reported by the HTAC [6].
Direct and indirect costs incurred from each case of bacteremia, meningitis, hospitalized pneumonia, and AOM for all age groups are from hospital bill and insurance claims data published by PhilHealth [23]. Indirect costs associated with each disease are calculated as the difference between the actual hospital bill and claimed amount to PhilHealth among both the government and private payers. Because there are no non-hospitalized pneumonia costs reported by PhilHealth, these costs are obtained from the 2020 HTA reassessment that were derived from an expert panel [6]. Sequalae-related costs are also consistent with the costs reported by HTAC, which are based on PhilHealth data. Averaged across all age groups, meningitis-associated sequalae are estimated to cost PHP 35,241 for epilepsy and PHP 20,514 for neurological impairment, and meningitis and AOM-associated hearing loss is estimated to cost PHP 42,153.
Utility Parameters
Utility parameters for each age group used in past PCV cost-effectiveness analyses are applied to individuals without pneumococcal disease (Table 1) [10, 16, 17, 24]. For each disease occurrence, an annual utility decrement is subtracted from the baseline utility weight. Decrements for each case of bacteremia, meningitis, hospitalized pneumonia, and AOM correspond with the 2020 HTA reassessment, which are sourced from a Thai study and estimated to be 0.0148, 0.0362, 0.0090, and 0.0016, respectively [25]. As a result of a lack of non-hospitalized pneumonia data, the utility decrement is assumed to be the same as AOM. Lifetime utility decrements for meningitis or AOM-related sequalae are also sourced from Kulpeng et al. [25] for epilepsy (0.36), neurological impairment (0.31), and hearing loss (0.45). Like costs, utilities are discounted at a rate of 7% annually [25].
Base Case Analysis
Using the decision analytic model and input parameters, we estimated total disease cases, deaths, costs, and QALYs by assuming PCV13-PFE is either maintained in the NIP or is replaced with either lower-valent PCV10. For the first objective, we assess the cost-effectiveness of maintaining a PCV13-PFE NIP versus switching to PCV10-GSK. The change in IPD incidence for 2021 onwards is forecasted using the trend in serotype-specific IPD incidence as observed in the UK post PCV13-PFE implementation and in Finland post PCV10-GSK implementation. These country trendlines are chosen because of their established PCV programs, high PCV uptake, and active IPD surveillance systems. United States (US) and Colombia trendlines are also further explored and tested in sensitivity analyses for PCV13-PFE and PCV10-GSK, respectively.
For the second objective, we assess the cost-effectiveness of maintaining a PCV13-PFE NIP versus switching to PCV10-SII. Substantial uncertainty exists surrounding PCV10-SII clinical effects as a new entrant to the market. Specifically, neither clinical trial nor real-world data are available to support its protection against non-invasive pneumococcal disease (pneumonia or otitis media) or impact on nasopharyngeal carriage, and it is the first PCV to exclude serotypes 4 and 18C from the formulation. Data are therefore used from PCV13-PFE’s real-world impact in the UK for PCV10-SII serotype dynamics. To account for clinical uncertainty, we conduct the analysis for a range of plausible values to capture the lower and upper bound of results using either evidence-based or assumption-based PCV10-SII modeling scenarios. For the upper bound, vaccine effectiveness against IPD, pneumonia, and AOM in vaccinated children and herd protection among unvaccinated individuals are assumed to be equal to PCV13-PFE for serotypes covered by PCV10-SII. Additionally, serotypes 4 and 18C are assumed to remain stable with no disease replacement or re-emergence. Thus, this assumption-based scenario calculates the upper bound of PCV10-SII’s public health and economic impact and cost-effectiveness. For the lower bound of the range, effectiveness inputs reflect existing clinical data for PCV10-SII, with no protection against pneumonia or AOM and no reduction in disease among unvaccinated individuals. Additionally, serotype replacement is based on observed serotype trends from other countries that transition from a higher-valent to a lower-valent PCV. As such, newly unprotected serotypes 4 and 18C are modeled to re-emerge by a factor of 150%, evidenced by surveillance data of countries that have replaced PCV13-PFE with PCV10-GSK [12].
Sensitivity Analysis
Sensitivity analyses are conducted by adjusting input parameters based on plausible ranges to test the robustness of the results. In the first set of sensitivity analyses, direct and indirect healthcare costs by disease and age group are adjusted ± 20%. Disutilities associated with disease episodes and sequalae across all ages are similarly varied by ± 20%. In another, we alter the analysis perspective to the payer perspective, whereby we only include direct costs incurred to the healthcare system. To capture the public health and economic effects of each PCV program after a shorter and longer period, we calculate the ICER with a time horizon of 5 and 20 years. Given the uncertainty in using serotype-specific IPD incidence trends as observed in the UK and in Finland for the Philippines, we test these trendlines using alternative country data. For PCV10-GSK and PCV13-PFE, serotype trendlines data are used from Colombia and the USA, respectively, to provide alternative projections of future serotype dynamics. These alternative country trendlines are chosen because Colombia and the USA have long-term data on PCV10-GSK and PCV13-PFE use, respectively. We also test PCV10-SII trendlines with US data for the common serotypes shared with PCV13-PFE, with serotype trends for newly unprotected serotypes 4 and 18C adjusted based on the evidence-based and assumption-based scenarios (detailed above). As for PCV uptake rates in the Philippines, we conduct a scenario in which the uptake rate is decreased to 70% to mimic actual vaccine uptake in the current NIP. Finally, given that changing the vaccine price had the greatest influence in the ICER among all parameters in the 2020 HTA reassessment, we vary the prices of PCV10-GSK and PCV10-SII in price threshold analyses.
Results
Base Case Results
Table 2 captures the overall number of disease cases, deaths, life-years, QALYs, and costs of each PCV arm, as well as the incremental results of either maintaining PCV13-PFE or switching to a lower-valent PCV10-GSK or PCV10-SII. Compared to a PCV10-GSK NIP, maintaining PCV13-PFE is estimated to avert 375,831 more disease cases and save 53,189 more lives over the next 10 years. Similarly, continuing PCV13-PFE vaccination rather than switching to PCV10-SII would prevent between 313,797 and 666,889 disease cases and between 22,759 and 72,435 deaths over a 10-year period. Consequently, the decreased cases and deaths from pneumococcal disease resulting from maintaining a PCV13-PFE NIP would save 153,349 QALYs compared to PCV10-GSK vaccination and between 108,061 and 266,108 QALYs compared to PCV10-SII vaccination.
PCV13-PFE vaccine-related costs are approximately PHP 3.16 billion and PHP 8.33 billion more costly than implementing PCV10-GSK or PCV10-SII in the Philippines across 10 years, respectively. Vaccine costs are offset by the disease-related direct medical costs and indirect costs averted from the incremental serotype coverage of PCV13-PFE. As a result, PCV13-PFE vaccination is estimated to prevent PHP 15.43 billion in societal costs as compared to PCV10-GSK vaccination, saving PHP 12.27 billion when vaccine costs are accounted for. Likewise, PCV13-PFE vaccination would avert substantial societal costs to the Philippines compared to a PCV10-SII NIP (between PHP 8.69 billion and 22.74 billion), saving a total of between PHP 359.82 million and 14.41 billion from averted disease cases across 10 years. Overall, PCV13-PFE vaccination is estimated to not only prevent additional disease cases and deaths than PCV10-GSK or PCV10-SII but also be most cost-saving to the Philippines as compared to either 10-valent PCV.
Scenario Analysis Results
Table 3 summarizes the results of the scenario analysis. When either direct and indirect healthcare costs or disease and sequalae utility decrements are adjusted by ± 20%, PCV13-PFE remains consistently dominant compared with either 10-valent vaccine, except for the scenario where healthcare costs are decreased by 20%. In this scenario, PCV13-PFE is cost-effective versus PCV10-SII under the upper bound assumption-based scenario (ICER = 10,556) based on the threshold of PHP 150,000 per QALY gained specified by the Philippine Department of Health [6]. When we alter the analysis to the payer perspective by only including direct costs, PCV13-PFE remains dominant compared to PCV10-GSK and is cost-effective compared to PCV10-SII with an ICER between 1312 and 45,063. If the time horizon is shortened or extended to 5 and 20 years, respectively, PCV13-PFE remains consistently dominant compared to switching to PCV10-GSK and PCV10-SII, except for when the time horizon is extended to 20 years and the comparator is PCV10-SII under the upper bound assumption-based scenario (ICER = 39,938). PCV13-PFE remains dominant when alternative country trendlines are used to predict the change in disease incidence following widespread vaccination. Additionally, decreasing the PCV uptake rate from 90% to 70% does not alter the results of the base case analysis, with PCV13-PFE remaining dominant as compared to PCV10-GSK and PCV10-SII under either assumption-based or evidence-based scenarios.
Figure 2 captures the change in the ICER when the price of PCV10-GSK and PCV10-SII is adjusted in relation to the PCV13-PFE 2020 PAHO price. PCV13-PFE remains dominant as compared to PCV10-GSK priced up to 55% lower than PCV13-PFE. Meanwhile, PCV13-PFE remains dominant over PCV10-SII priced up to 31% and 81% lower than PCV13-PFE under an assumption-based scenario (upper bound) and evidence-based scenario (lower bound), respectively. The only instance the ICER of PCV13-PFE versus a lower-valent vaccine is above the cost-effectiveness threshold of PHP 150,000 per QALY gained is when PCV10-SII under the upper bound assumption-based scenario is procured at a price that is 90% lower than PCV13-PFE.
Discussion
Our study assessed whether increasing vaccine uptake rate and continuing PCV13-PFE in the Philippines pediatric NIP is cost-effective compared with switching to lower-valent alternatives. We estimate that PCV13-PFE would avert additional cases, save more lives, and provide further healthcare and societal cost-savings over 10 years compared with either PCV10 option. This economic evaluation demonstrates that PCV13-PFE would continue to provide good value for money and would be the most cost-saving strategy to achieve universal PCV coverage in the Philippines.
One of the study objectives is to compare whether results for PCV13-PFE versus PCV10-GSK in this analysis are similar to the cost–utility analysis results conducted in the Philippines 2020 HTA reassessment. We find that this study’s results are directionally consistent even when using different model frameworks and assumptions. Both the HTA reassessment and this analysis concluded that PCV10-GSK was dominated by PCV13-PFE, implying that PCV13-PFE should be maintained in the Philippines NIP. In our base case analysis, PCV13-PFE is estimated to dominate PCV10-GSK by incrementally averting over 375,000 pneumococcal disease cases, saving over 53,000 lives, gaining over 153,000 QALYs, and providing cost-savings of over PHP 12.2 billion. In the cost–utility analysis conducted by HTAC, PCV10-GSK was found to gain less QALYs (incremental QALY = −0.0131 for both single-dose and multidose vials) and cost more (incremental costs = PHP 470 for single-dose vial and PHP 221 for multidose vial) than PCV13-PFE assuming 90% vaccine uptake. Vaccine cost has a major impact on ICERs, as shown in both studies’ sensitivity analyses. Even though price impacts the ICER in our analysis, PCV13-PFE remains cost-effective at the willingness-to-pay threshold of PHP 150,000 per QALY when holding PCV13-PFE at $14.50 USD (PHP 704.43) per dose and reducing the price of PCV10-GSK to zero. Apart from the cost-effectiveness analysis, a separate budget impact analysis was conducted in the 2020 HTA reassessment which determined that PCV10-GSK would be more affordable, with the driving factor being lower cumulative total vaccine costs. However, both the HTAC and our study’s cost-effectiveness analyses included disease cost-offsets and vaccine costs and demonstrated that PCV13-PFE would save more overall net costs than PCV10-GSK, leading to our conclusion that PCV13-PFE would ultimately be more affordable.
Besides comparing the cost-effectiveness of PCV13-PFE versus PCV10-GSK, another study objective is to assess the cost-effectiveness of PCV13-PFE versus PCV10-SII in the presence of clinical uncertainty. PCV10-SII safety and immunogenicity continue to be studied, although vaccine efficacy or effectiveness does not seem to be part of the clinical development program. Consequently, many clinical effects remain unknown, which therefore creates difficulties in determining base case parameters when conducting economic analyses. Additionally, PCV10-SII was compared to PCV10-GSK in the phase 3 pivotal non-inferiority study, which is inconsistent with the WHO guidance that recommends comparators for PCV non-inferiority trials be driven by the number of common serotypes [26,27,28]. By not using PCV13-PFE as the comparator, there are no matched immunogenicity responses for 6A and 19A, both of which are now benchmarked against the least immunogenic serotype contained in PCV10-GSK’s formulation. A non-matched response with respect to 19A is problematic because this serotype requires a particularly strong immune response due to a high correlate of protection threshold, like that afforded by PCV13-PFE [27, 29]. Furthermore, PCV correlates of protection against pneumonia and OM have not been established and there are no data on PCV10-SII showing an impact on these outcomes. Finally, there are no clinical data on nasopharyngeal carriage or real-world evidence of herd effects. To our knowledge, this is the first economic evaluation in the Philippines to include PCV10-SII as a comparator and assess its clinical uncertainty. Our cost-effectiveness analysis estimates that maintaining PCV13-PFE in the NIP would remain cost-saving regardless of uncertainty in the base case clinical parameters (i.e., PCV13-PFE is found to save overall costs compared against both the upper and lower bound scenarios of PCV10-SII); thus a PCV13-PFE universal coverage NIP represents both better value for money and greater affordability compared with PCV10-SII infant vaccination in the Philippines.
Considering that the Philippines aims to increase uptake of PCVs and expand to universal vaccination coverage, it is important to continue vaccine pressure on currently covered serotypes to achieve the largest potential cost-savings and public health impact. Serotypes contained in PCV13-PFE have high disease potential and may re-emerge if a lower-valent PCV were to replace it. For example, when PCV10-GSK replaced PCV13-PFE in the Belgian NIP during 2015 and 2016, a rise in IPD isolates was observed in children less than 2 years old within a 2-year period, primarily driven by over a tenfold increase (1000%) in serotype 19A. This decision resulted in an unforeseen disease outbreak in previously covered serotypes with no observed cross-protection against 19A from PCV10-GSK, yielding a negative overall public health and economic impact [12]. This Belgian experience serves as a useful illustration of the limitations of modeling disease re-emergence in the absence of prior evidence for previously covered vaccine serotypes. In our study, serotype replacement trends were modeled to behave similarly to dynamics observed in Finland for PCV10-GSK. As for PCV10-SII, serotypes 4 and 18C were set to emerge at maximum 150% in the lower limit and were set to 0% (i.e., disease would remain stable) in the upper limit. Therefore, the analyses from this study are likely conservative and may underestimate the true impact of switching PCVs in light of serotype re-emergence data observed in Belgium. A Belgium case study that quantifies this underestimation was recently published, demonstrating that vaccine-type disease re-emergence may be more substantial than modeling exercises predict [13].
The risk of switching PCVs under the premise of affordability and potential vaccine-related cost-savings in the current context might therefore be misguided. In 2019, the Philippines reported outbreaks of vaccine preventable disease, such as dengue, diphtheria, measles, and polio, as a result of reduced protection from waning vaccination rates, with the Department of Health declaring a national dengue epidemic [30]. Decreasing protection against pneumococcal disease by transitioning to a lower-valent PCV may contribute to additional infectious outbreaks of vaccine-preventable diseases in the Philippines. Additionally, the Philippines was one of the most impacted ASEAN member states from the COVID-19 pandemic, with over 1,544,585 cases and 25,650 deaths as of July 2021 [31, 32]. Global disease containment measures (social distancing and stay-at-home procedures) resulted in the biggest fall in Philippine gross domestic product and the highest unemployment rate historically observed [33]. In these unprecedented times, choosing the health intervention with the greatest public health impact should be prioritized to prevent further setbacks and losing ground previously gained in the Philippine NIP.
Our study has some limitations. The introduction of PCVs in the Philippines has resulted in substantial declines in pneumococcal disease caused by serotypes included in PCV13-PFE, but the exact impact over the past years cannot be determined with precision because of limited disease surveillance. For our analysis, we overcame this by using data from other countries’ PCV programs with strong surveillance systems to inform future disease projections in the Philippines. Given that herd effects are often observed when high vaccine uptake in a pediatric PCV NIP is achieved, data from other countries with higher uptake might even be more appropriate for modeling outcomes. For example, historical observed data from Finland and the UK for PCV10-GSK and PCV13-PFE, respectively, may better predict future disease outcomes under a universal PCV program in the Philippines as compared to historical local data with current uptake levels ranging from 30% to 60%. Sensitivity analyses were conducted to test the uncertainty in future serotype dynamics with alternative country (USA and Colombia) trendlines, which consistently demonstrated that PCV13-PFE remains dominant over either PCV10 option. An additional limitation is that this study used serotype distribution data from the RITM, which is a passive surveillance system with reporting from select sentinel sites. Although this is the best data from the national surveillance system that exists, results need to be interpreted with caution given the lack of contemporary epidemiologic surveillance data for all ages. Moreover, most of our data were sourced from the cost–utility analysis conducted in the 2020 HTA PCV reassessment and are subject to the same inherent limitations. All costing and disease incidence data were taken from PhilHealth claims and may be underestimated (i.e., cases, actual treatment costs, and out-of-pocket costs not reported) or overestimated (i.e., upcoding or human errors). Finally, this study did not incorporate any differences between single and multidose vial wastage rates, schedule administration nuances, or schedule adherence and completion. Completion of vaccination series and adherence to vaccination schedules are important to prevent breakthrough infections, and the inclusion of a booster in a schedule can increase the duration of direct protection and produce stronger indirect effects.
Conclusion
PCV13-PFE is estimated to not only improve public health by averting additional pneumococcal disease cases and saving more lives, but also would save substantial future healthcare and societal costs compared with PCV10-GSK and PCV10-SII. Thus, switching from a higher-valent to a lower-valent PCV under the premise of affordability and the promise of short-term budget cost-savings in the current Philippine context would have a negative clinical and economic impact. This economic evaluation can help inform decision makers on which PCV will provide the best value for money for obtaining universal PCV coverage in Filipino infants.
References
Global Burden of Disease Study 2016 Lower Respiratory Infections Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18(11):1191–210.
Wahl B, O’Brien KL, Greenbaum A, et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000–15. Lancet Glob Health. 2018;6(7):e744–57.
Wang BC, Chaiyakunapruk N, Zhu S, et al. A systematic literature review of economic evaluations of pneumococcal conjugate vaccines in east and southeast Asia (2006–2019). Expert Rev Vaccines. 2021. https://doi.org/10.1080/14760584.2021.1894933.
Haasis MA, Ceria JA, Kulpeng W, Teerawattananon Y, Alejandria M. Do pneumococcal conjugate vaccines represent good value for money in a lower-middle income country? A cost-utility analysis in the Philippines. PLoS ONE. 2015;10(7): e0131156.
Zhang X-H, Nievera MC, Carlos J, et al. Cost-effectiveness analysis of pneumococcal vaccination with the pneumococcal polysaccharide NTHi protein D conjugate vaccine in the Philippines. Value Health Reg Issues. 2014;3:156–66.
Briones JR, Ceria-Pereña JA, Uy GD, Obmaña SM, Cabaluna IT. Reassessment of 10- versus 13-valent pneumococcal conjugate vaccines (PCV) in the Philippines. Manila: Republic of the Philippines Department of Health; 2020.
Epidemiology Bureau Republic of the Philippines Department of Health. Field Health Services Information System 2019 Annual Report. September 30, 2020.
World Health Organization. World Health Organization. Prequalification of medical products (IVDs, medicines, vaccines and immunization devices, vector control) - list of prequalified vaccines: Pneumosil 2020. https://extranet.who.int/pqweb/content/pneumosil%C2%AE. Accessed 1 June 2021.
Alderson MR, Sethna V, Newhouse LC, Lamola S, Dhere R. Development strategy and lessons learned for a 10-valent pneumococcal conjugate vaccine (PNEUMOSIL®). Hum Vaccine Immunother. 2021. https://doi.org/10.1080/21645515.2021.1874219.
Wasserman M, Palacios MG, Grajales AG, et al. Modeling the sustained use of the 13-valent pneumococcal conjugate vaccine compared to switching to the 10-valent vaccine in Mexico. Hum Vaccine Immunother. 2019;15(3):560–9.
Wilson M, Wasserman M, Jadavi T, et al. Clinical and economic impact of a potential switch from 13-valent to 10-valent pneumococcal conjugate infant vaccination in Canada. Infect Dis Ther. 2018;7(3):353–71.
Desmet S, Lagrou K, Wyndham-Thomas C, et al. Dynamic changes in paediatric invasive pneumococcal disease after sequential switches of conjugate vaccine in Belgium: a national retrospective observational study. Lancet Infect Dis. 2021;21(1):127–36.
Wilson MR, McDade CL, Perdrizet JE, et al. Validation of a novel forecasting method for estimating the impact of switching pneumococcal conjugate programs: evidence from Belgium. Infect Dis Ther. 2021;10(3):1765–78.
Kim H-Y, Park S-B, Kang E-S, et al. Cost-effectiveness of a national immunization program with the 13-valent pneumococcal conjugate vaccine compared with the 10-valent pneumococcal conjugate vaccine in South Korea. Hum Vaccine Immunother. 2021;17(3):909–18.
Perdrizet J, Santana CFS, Senna T, et al. Cost-effectiveness analysis of replacing the 10-valent pneumococcal conjugate vaccine (PCV10) with the 13-valent pneumococcal conjugate vaccine (PCV13) in Brazil infants. Hum Vaccine Immunother. 2021;17(4):1162–72.
Pugh S, Wasserman M, Moffatt M, et al. Estimating the impact of switching from a lower to higher valent pneumococcal conjugate vaccine in Colombia, Finland, and The Netherlands: a cost-effectiveness analysis. Infect Dis Ther. 2020;9(2):305–24.
Shafie AA, Ahmad N, Naidoo J, et al. Estimating the population health and economic impacts of introducing a pneumococcal conjugate vaccine in Malaysia- an economic evaluation. Hum Vaccine Immunother. 2020;16(7):1719–27.
Health Technology Assessment Unit, Republic of the Philippines Department of Health. Philippine HTA Methods Guide. 2020. https://hta.doh.gov.ph/philippine-hta-methods-guide/.
United Nations, Department of Economic and Social Affairs, Population Division. World population prospects 2019. 2019. Online Edition Rev. 1. https://population.un.org/wpp/Download/Standard/Interpolated/. Accessed 1 June 2021.
Ngo CC, Massa HM, Thornton RB, Cripps AW. Predominant bacteria detected from the middle ear fluid of children experiencing otitis media: a systematic review. PLoS ONE. 2016;11(3): e0150949.
Epidemiology Bureau, Republic of the Philippines Department of Health. The 2018 Philippine Health Statistics. 2020. https://doh.gov.ph/sites/default/files/publications/2018%20Philippine%20Health%20Statistics.pdf.
Pan American Health Organization, World Health Organization. Expanded program of immunization vaccine prices for year 2020. 2020. https://www.paho.org/hq/index.php?option=com_docman&view=download&category_slug=vaccines-9979&alias=51457-revolving-fund-vaccine-prices-2020&Itemid=270&lang=en. Accessed 1 June 2021.
Obmaña SM, Health Technology Assessment Unit DoH, Philippines. PhilHealth Costing Data Summary for PCV.pdf. Philippines: Pfizer; 2021 (personal communication).
Mittmann N, Trakas K, Risebrough N, Liu BA. Utility scores for chronic conditions in a community-dwelling population. Pharmacoeconomics. 1999;15(4):369–76.
Kulpeng W, Leelahavarong P, Rattanavipapong W, et al. Cost-utility analysis of 10- and 13-valent pneumococcal conjugate vaccines: protection at what price in the Thai context? Vaccine. 2013;31(26):2839–47.
Clarke E, Bashorun A, Adigweme I, et al. Immunogenicity and safety of a novel ten-valent pneumococcal conjugate vaccine in healthy infants in The Gambia: a phase 3, randomised, double-blind, non-inferiority trial. Lancet Infect Dis. 2021;21(6):834–46.
Madhi SA, Knoll MD. An affordable pneumococcal conjugate vaccine after 20 years. Lancet Infect Dis. 2021;21(6):751–3.
WHO Expert Committee on Biological Standardization. Recommendations to assure the quality, safety and efficacy of pneumococcal conjugate vaccines, Annex 3, TRS No 977. Replacement of WHO Technical Report Series, No. 927, Annex 2. 2013.
Andrews NJ, Waight PA, Burbidge P, et al. Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: a postlicensure indirect cohort study. Lancet Infect Dis. 2014;14(9):839–46.
The Lancet Infectious Diseases. Infectious disease crisis in the Philippines. Lancet Infect Dis. 2019;19(12):1265.
Rampal L, Liew BS, Choolani M, et al. Battling COVID-19 pandemic waves in six South-East Asian countries: a real-time consensus review. Med J Malays. 2020;75:613–25.
Republic of the Philippines Department of Health. COVID-19 Tracker 2021. 2021. https://doh.gov.ph/covid19tracker. Accessed 08 July 2021.
Lim JA. The Philippine Economy During the COVID Pandemic. Ateneo De Manila University Department of Economics-Ateneo Center for Economic Research and Development; 2020. http://ateneo.edu/ls/soss/economics/wp-2020-16-philippine-economy-during-covid-pandemic.
Acknowledgements
Funding
This study and the journal’s rapid service fee was funded by Pfizer Inc.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to the conception, design, and implementation of research for this study, the analysis of results, and the writing of the manuscript.
Disclosures
Johnna Perdrizet and Emily K Horn are employees of Pfizer Inc and may own stock or stock options. Winniefer Nua and Judith Perez-Peralta are employees of Pfizer Philippines and may own stock or stock options. Jennifer Nailes, Jamie Santos, and Anna Ong-Lim have nothing to disclose for this study.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
All data generated or analyzed during this study are included in this published article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Perdrizet, J., Horn, E.K., Nua, W. et al. Cost-Effectiveness of the 13-Valent Pneumococcal Conjugate Vaccine (PCV13) Versus Lower-Valent Alternatives in Filipino Infants. Infect Dis Ther 10, 2625–2642 (2021). https://doi.org/10.1007/s40121-021-00538-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-021-00538-z