Abstract
Introduction
Hepatitis B virus (HBV) infection is associated with the onset of several major liver diseases. Inactive hepatitis B surface antigen (HBsAg) carriers (IHCs) may be successfully treated with PEGylated interferon-α2b (PEG-IFNα2b)-based antiviral therapy; however, studies on this treatment have been insufficient. In this study, we evaluated the efficacy and safety of PEG-IFNα2b treatment in IHCs.
Methods
Nineteen IHCs were treated with subcutaneous PEG-IFNα2b (180 μg/week) for 48 weeks (treatment group). Patients were followed up for 24 weeks after treatment discontinuation. Twenty untreated control patients were observed for 72 weeks (control group). HBsAg clearance (HBsAg < 0.05 IU/mL), HBsAg seroconversion, and alanine aminotransferase levels were monitored.
Results
Of the 19 patients treated with PEG-IFNα2b, 16 showed HBsAg loss (84.2%), and 13 showed HBsAg seroconversion (68.4%) at 72 weeks. All patients in the treatment group exhibited virological response (serum HBV DNA level < 10 IU/mL) at the time of drug withdrawal. In the control group, no patients experienced HBsAg loss during the observational period. There were no serious adverse events during treatment, and the therapy was well tolerated.
Conclusions
Short PEG-IFNα2b therapy in IHCs produced a high functional cure rate and good safety profile, suggesting that PEG-IFNα2b treatment may be the best choice for clinical cure of some IHCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Hepatitis B virus (HBV) infection is associated with the onset of several major liver diseases. | |
Inactive hepatitis B surface antigen (HBsAg) carriers (IHCs) may be successfully treated with PEGylated interferon-α2b (PEG-IFNα2b)-based antiviral therapy; however, studies on this treatment strategy have been insufficient. | |
In this study, we aimed to assess the feasibility and safety of PEG-IFNα2b treatment for HBsAg clearance in IHCs with different HBsAg levels. | |
Our results demonstrated that treatment with PEG-IFNα2b produced a high rate of HBsAg loss/seroconversion in inactive carriers with low HBsAg levels, suggesting that PEG-IFNα2b treatment may be the best choice for some IHCs to pursue a clinical cure. |
Introduction
Hepatitis B virus (HBV) infection is prevalent worldwide, and approximately 2 billion people have been infected with HBV, including 257 million with chronic infection, mainly in the Western Pacific and Africa (according to the World Health Organization’s Global Hepatitis Report, 2017). Approximately 887,000 people die annually from HBV-related end-stage liver diseases, including liver cirrhosis, liver failure, and hepatocellular carcinoma (HCC) [1]. The negative conversion of hepatitis B surface antigen (HBsAg) is related to improvements in liver function, histology, and long-term prognosis. Therefore, HBsAg has been identified as an ideal therapeutic target for the prevention and treatment of chronic hepatitis B (i.e., to achieve functional or clinical cure) [2, 3].
Patients who are considered inactive HBsAg carriers (IHCs) typically show positive HBsAg, negative hepatitis B e-antigen (HBeAg), low replication or undetectable HBV DNA, continuous normal alanine aminotransferase (ALT) or aspartate aminotransferase (AST) levels, and mild or no pathological changes in liver tissues. IHCs are generally stable and have a good prognosis; thus, antiviral treatment is not recommended. However, this inactive, stable carrier status is not always sustained. Indeed, 14–24% of IHCs may show reactivation due to immune disorders or gene mutations, resulting in abnormal liver function and/or even HBeAg recovery, and these patients may then develop HBeAg-negative or HBeAg-positive chronic hepatitis B [4,5,6,7]. A prospective follow-up study showed that the cumulative probability of hepatitis reactivation in IHCs increases annually, reaching approximately 20.2% over 25 years [8]. Additionally, IHCs exhibit a higher risk of HCC and liver disease-related death compared with patients without HBV infection [9]. IHCs are generally not clinically cured, and a meta-analysis showed that natural clearance of HBsAg occurs in IHCs and that the annual negative conversion rate is only 1.1% [3]. Persistent positive HBsAg may indicate an unstable inactive state in IHCs and could result in a reversal of the inactive state or promote progression to end-stage liver disease. Moreover, HBV can be easily reactivated by immunosuppression, leading to varying degrees of liver damage from mild inflammation to liver failure [10,11,12].
Subsequently, researchers have begun to explore the feasibility of antiviral treatment for IHCs and have suggested that some IHCs may benefit from treatment with these drugs. Thus, IHCs have been identified as a target population for clinical cure, and further studies are needed to improve HBsAg clearance rates in these patients. Importantly, some studies have shown that clinical cure may be possible in IHCs by treatment with PEGylated interferon-α2b (PEG-IFNα2b) [13, 14]; however, additional studies are needed to confirm these findings.
Accordingly, in this study, we assessed the feasibility and safety of PEG-IFNα2b treatment for HBsAg clearance and seroconversion in IHCs with different HBsAg levels.
Methods
Patient Selection
From March 2018 to April 2020, a retrospective cohort study was conducted in the Infectious Disease Outpatient Department of Xiangya Hospital of Central South University. We defined IHCs as patients who were HBsAg positive for more than 6 months, were HBeAg-negative/anti-HBe-positive, had low HBV-DNA levels (< 2000 IU/mL), and had normal ALT levels, according to the Prevention and Treatment Guidelines for Chronic Hepatitis B (2015 edition) [15]. IHCs who had not received treatment were included in the study. The exclusion criteria were as follows: (1) peripheral blood neutrophil (Neu) count < 1.5 × 109/L and platelet (PLT) count < 100 × 109/L; (2) combination with other viral hepatitis (hepatitis A, C, D, or E) or liver diseases (such as autoimmune liver disease, Wilson’s disease, alcoholic liver disease, or drug-induced hepatitis); (3) human immunodeficiency virus infection with important organ lesions; (4) combination with hyperthyroidism or hypothyroidism or other autoimmune diseases; (5) alcoholism or drug addiction; and (6) receiving chemotherapy or immunosuppressive therapy.
Patients in the treatment group included all those who received PEG-IFNα2b treatment with the aim of achieving HBsAg clearance and those who had completed 48 weeks of treatment with PEG-IFNα2b and 24 weeks of follow-up after completing the treatment. In total, 39 patients were included, including 20 controls and 19 IHCs matched for age, sex, and HBsAg levels and showing undetectable HBV DNA with persistently normal ALT levels.
Measurement of HBV DNA and HBsAg Levels
HBV DNA was quantified by fluorescence quantitative polymerase chain reaction with a detection limit of 10 IU/mL. HBsAg and anti-HBs antibody were quantified by HBsAg quantitative Elecsys (Roche Diagnostics GmbH, Germany) with a detection limit of 0.05 IU/mL for HBsAg. An anti-HBs antibody level greater than 10 IU/L was defined as positivity.
Treatment and Observation
The treated cohort included 19 patients who had received subcutaneous PEG-IFNα2b at a dose of 180 μg/week for 48 weeks and who were followed for 24 weeks after completing the treatment. This regimen was response-guided; that is, if HBsAg loss was achieved before week 24, patients continued to receive 24 weeks of PEG-IFN treatment (we defined these 24 weeks as “consolidation”), whereas if HBsAg loss was achieved after week 24, PEG-IFN treatment was continued until 48 weeks (this period between HBsAg loss and week 48 was also defined as “consolidation”). The control cohort comprised 20 matched patients who had finished 72 weeks of observation. None of the participants received immunosuppressive or oral antiviral drugs during the study period.
The primary observational endpoint was HBsAg clearance, and the secondary observational endpoint was HBsAg seroconversion. In the control group, there were no interventions for the duration of the 72-week observational period.
Assessment of Safety and Efficacy
In the treated patients and controls, serum HBsAg levels, anti-HBs levels, HBV DNA levels, Neu and PLT counts, and liver and kidney function were assessed once every 1–3 months. HBV DNA was detected via fluorescence quantitative (high-sensitivity) polymerase chain reaction, with a detection limit of 10 IU/mL (Shengxiang Company, Changsha City, China). Quantitative detection of hepatitis B was performed via Abbott chemiluminescence automatic immunoassays (Architect i2000 HBsAg quantitative assays; Abbott Laboratories, Abbott Park, IL, USA). The detection limit for HBsAg was 0.05 IU/mL, and samples with HBsAb levels greater than 10 IU/mL were considered positive. HBsAg loss was defined as an HBsAg concentration less than 0.05 IU/mL.
Statistical Analysis
Data were tested for normality, and those with normal distributions were described as means ± standard deviations, whereas those with skewed distributions were described as medians and interquartile ranges (Q1–Q3). Categorical variables were expressed as frequencies (%). According to the results of normality tests, t tests or nonparametric tests were used to compare continuous variables between groups. Categorical variables were compared using chi-squared tests or Fisher’s exact tests. Empower (http://www.empowerstats.com [X & Y Solutions, Inc., Boston, MA, USA]; R language pack [http://www.R-project.org]) statistical software was used for statistical analysis of the data. Results with P values less than 0.05 were considered significant.
Study Approval
The study protocol was approved by the Ethics Committee of Xiangya Hospital (approval no. 201906014) and was conducted in accordance with the guidelines of the Declaration of Helsinki and the principles of Good Clinical Practice. All patient data were anonymized, and written consent was obtained from all patients.
Results
Baseline Characteristics
Thirty-nine IHCs were included in this study (19 in the treatment group, 20 in the control group). Baseline characteristics of sex, age, infection mode, and ALT, HBV DNA, and HBsAg levels did not differ significantly between treatment and control groups (Table 1). The median baseline HBsAg level in the treatment group was slightly higher than that in the control group (8.86 [3.37–62.72] versus 7.54 [1.44–58.95] IU/mL). The proportion of patients with HBsAg levels less than 100 IU/mL in the treatment group was lower than that in the control group (84.2% versus 90.0%); however, the proportion of patients with HBsAg levels less than 10 IU/mL was higher in the treatment group than in the control group (52.6% versus 50.0%). Fourteen (73.6%) and 13 (65.0%) patients were HBV DNA-positive (> 10 IU/mL) at baseline in the treatment and control groups, respectively.
HBsAg Clearance and Seroconversion Rates
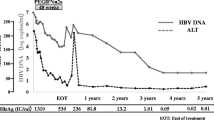
Among the 19 patients in the treatment group, nine and 10 patients were treated with PEG-IFNα2b for 36 and 48 weeks, respectively; thus, 52.6% of patients completed the 48-week treatment. Moreover, the nine patients who underwent a course of treatment less than 48 weeks all achieved HBsAg loss by week 12. Among these, seven patients were followed up after 24 weeks of consolidation treatment, whereas one halted treatment at week 24 because of hyperthyroidism. The HBsAg clearance and seroconversion rates in the treatment group were 84.2% (16/19) and 68.4% (13/19), respectively, at week 48 and at the end of follow-up (Fig. 1 and Table 1). After stratification according to baseline HBsAg levels, the HBsAg clearance rates were 93.3% (14/15) in patients with HBsAg levels less than 100 IU/mL and 100.0% (10/10) in patients with HBsAg levels less than 10 IU/mL. By contrast, during the 72-week observation period, no HBsAg clearance was detected in the control group. In 16 patients achieving HBsAg loss, the baseline HBsAg (IU/mL, median [Q1–Q3]) was 8.24 (3.37–62.72). In 13 patients achieving HBsAg seroconversion, the baseline HBsAg (IU/mL, median [Q1–Q3]) was 7.88 (3.29–40.82).
Undetectable HBV DNA Rates
Among the 19 patients in the treatment group, 14 were positive for HBV DNA. At week 12, 71.4% (10/14) of these patients showed a decrease in HBV DNA from greater than 10 IU/mL at baseline to undetectable levels after receiving PEG-IFNα2b treatment. At week 48, HBV DNA levels were less than 10 IU/mL for all patients in the treatment group. By contrast, none of the 13 patients in the control group, who showed HBV DNA levels greater than 10 IU/mL at baseline, exhibited significant changes during the follow-up period, indicating that HBV DNA clearance did not occur spontaneously (Table 1).
Safety
In the treatment group, eight patients reported fatigue and loss of appetite, four experienced alopecia, and two reported intermittent joint pain. Four patients (26.7%) showed decreased Neu counts (< 1.5 × 109/L), and six (40.0%) had thrombocytopenia (< 100 × 109/L). Two patients were found to have abnormal thyroid function upon re-examination. One patient showed hyperthyroidism at week 24 of treatment but showed recovery to normal at 24 weeks after stopping the treatment. Another patient was found to have hyperthyroidism at week 48 of treatment and is still being followed up. No neuropsychiatric adverse responses, such as depression, delirium, or irritability, were reported.
Thirteen patients (86.7%) showed elevated ALT or AST, and four patients (26.7%) had ALT levels > 2 × the upper limit of normal (ULN). Total bilirubin levels in four patients increased during treatment (all < 2 × ULN [< 34.2 μM]). Normalization of ALT levels coincided with HBsAg loss and/or the end of treatment and was maintained during the follow-up period.
Discussion
The HBsAg clearance rate in patients with chronic hepatitis B after long-term treatment with nucleoside analogues is not satisfactory. To achieve the strategic goal of global elimination of hepatitis B by 2030, clinicians continue to explore methods to improve clinical cure rates in patients with hepatitis B. In the 10 years since the start of the OSST study in 2009, evidence of a clinical cure for chronic hepatitis B has been accumulating in China [16]. Several clinical trials, such as New Switch [17], ICURE [18], Anchor [19], S-C [20], and the SWAP [21] studies, have been successively carried out, revealing sequential/combined therapy based on IFN as an important strategy for improving clinical cure rates in patients with chronic hepatitis B.
Accumulating evidence suggests that IHCs can further improve outcomes if HBsAg loss can be achieved by diminishing the risk of hepatitis relapse, cirrhosis, and HCC [4, 8, 22, 23]. Moreover, some IHCs would prefer to achieve HBsAg clearance through treatment as a result of social discrimination, spiritual pressure, or career selection. However, few studies have reported clinical cure rates in IHCs. In this context, the current study was designed to evaluate the efficacy and safety of PEG-IFNα2b in an IHC population. Most patients achieved clinical cure in the early stage of treatment (12 or 24 weeks). At 48 weeks, the cumulative HBsAg clearance and seroconversion rates in the treatment group were 84.2% and 68.4%, respectively, which were higher than those (44.7% and 38.3%, respectively) reported by Cao et al. [14]. This discrepancy may be related to the lower baseline HBsAg level in IHCs in the current study. By contrast, no HBsAg clearance was achieved in the control group during the observation period of 48 weeks, similar to the results of Li et al. [13]. Moreover, PEG-IFNα2b treatment promoted a virological response in IHCs. Indeed, after treatment with PEG-IFNα2b, HBV DNA was negative in most patients. These results suggested that IHCs could benefit from short-term PEG-IFNα2b treatment to achieve HBsAg clearance and seroconversion.
Further stratification according to baseline HBsAg levels showed that the HBsAg clearance rate was negatively correlated with HBsAg levels in the treatment group. Compared with a study conducted by Li et al. [13], the clinical cure rate of IHCs with baseline HBsAg < 100 IU/mL was higher (84.2% versus 65.0%), potentially because of the lower median baseline HBsAg level in the treatment group. At the Asia Pacific Annual Meeting on Liver Diseases 2020, an IFN-treatment study in IHCs with very low HBsAg levels (< 20 IU/mL) also confirmed the close relationship between low baseline HBsAg levels and high clinical cure rates. These studies showed that PEG-IFNα2b treatment was an excellent choice for IHCs with low HBsAg levels (< 100 IU/mL) and a goal of achieving clinical cure. Notably, lower baseline HBsAg levels were associated with greater therapeutic effects for PEG-IFNα2b. Additionally, the clinical course of PEG-IFNα2b treatment was significantly shorter in the current study than in those conducted by Cao et al. and Li et al. [13, 14], indicating that for IHCs with low HBsAg levels, a shorter course of PEG-IFNα treatment could result in clinical cure.
We observed good safety profiles in 19 IHCs after PEG-IFNα2b treatment, and no serious adverse reactions were reported. The main manifestations reported were fatigue, loss of appetite, alopecia, intermittent joint pain, neutropenia, and thrombocytopenia, with no cases of agranulocytosis or severe thrombocytopenia detected. Increased ALT or AST levels were frequently detected; however, few cases showed ALT levels > 2 × ULN, and no patients showed elevated ALT levels > 10 × ULN. Two cases of hyperthyroidism were detected after HBsAg clearance, with one patient recovering after 24 weeks of treatment, and the other displaying hyperthyroidism at 48 weeks of treatment. Overall, these results suggest that IHCs who achieved clinical cure after PEG-IFNα2b treatment may still experience adverse reactions, and safety profiles should be regularly reviewed.
Conclusions
This study had several limitations, including a small sample size, inability to further confirm the diagnosis of IHC with liver pathology, lack of HBV genotype detection, and limited follow-up time after treatment. However, despite these limitations, the results demonstrated that treatment with PEG-IFNα2b produced a high rate of HBsAg loss/seroconversion in IHCs with low HBsAg levels. Further studies and longer follow-up times are required to determine whether IHCs with HBsAg levels greater than 100 IU/mL can benefit from PEG-IFNα2b treatment.
References
Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Akinyemiju TF, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2018;4(11):1553–68.
Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10(1):1–98.
Terrault NA, Lok A, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67(4):1560–99.
Fattovich G, Olivari N, Pasino M, D’Onofrio M, Martone E, Donato F. Long-term outcome of chronic hepatitis B in Caucasian patients: mortality after 25 years. Gut. 2008;57(1):84–90.
Chu CM, Hung SJ, Lin J, Tai DI, Liaw YF. Natural history of hepatitis B e antigen to antibody seroconversion in patients with normal serum aminotransferase levels. Am J Med. 2004;116(12):829–34.
McMahon BJ, Holck P, Bulkow L, Snowball M. Serologic and clinical outcomes of 1536 Alaska Natives chronically infected with hepatitis B virus. Ann Intern Med. 2001;135(9):759–68.
Hsu YS, Chien RN, Yeh CT, et al. Long-term outcome after spontaneous HBeAg seroconversion in patients with chronic hepatitis B. Hepatology. 2002;35(6):1522–7.
Chu CM, Liaw YF. Spontaneous relapse of hepatitis in inactive HBsAg carriers. Hepatol Int. 2007;1(2):311–5.
Chen JD, Yang HI, Iloeje UH, et al. Carriers of inactive hepatitis B virus are still at risk for hepatocellular carcinoma and liver-related death. Gastroenterology. 2010;138(5):1747–54.
Chung SJ, Kim JK, Park MC, Park YB, Lee SK. Reactivation of hepatitis B viral infection in inactive HBsAg carriers following anti-tumor necrosis factor-alpha therapy. J Rheumatol. 2009;36(11):2416–20.
Hwang JP, Lok AS. Management of patients with hepatitis B who require immunosuppressive therapy. Nat Rev Gastroenterol Hepatol. 2014;11(4):209–19.
Huang SC, Yang HC, Kao JH. Hepatitis B reactivation: diagnosis and management. Expert Rev Gastroenterol Hepatol. 2020;14(7):565–78.
Li MH, Xie Y, Zhang L, et al. Hepatitis B surface antigen clearance in inactive hepatitis B surface antigen carriers treated with peginterferon alfa-2a. World J Hepatol. 2016;8(15):637–43.
Cao Z, Liu Y, Ma L, et al. A potent hepatitis B surface antigen response in subjects with inactive hepatitis B surface antigen carrier treated with pegylated-interferon alpha. Hepatology. 2017;66(4):1058–66.
Chinese Society of Hepatology, et al. The guideline of prevention and treatment for chronic hepatitis B: a 2015 update. Zhonghua Gan Zang Bing Za Zhi. 2015;23:888–905.
Han M, Jiang J, Hou J, et al. Sustained immune control in HBeAg-positive patients who switched from entecavir therapy to pegylated interferon-α2a: 1 year follow-up of the OSST study. Antivir Ther. 2016;21(4):337–44.
Hu P, Shang J, Zhang WH, et al. HBsAg loss with Pegylated-interferon alfa-2a in hepatitis B patients with partial response to nucleos(t)-ide analog: new switch study. Zhonghua Gan Zang Bing Za Zhi. 2018;26(10):756–64.
Mouzannar K, Liang TJ. Hepatitis B virus—recent therapeutic advances and challenges to cure. J Hepatol. 2020;73(3):694–5.
Wu D, Yan WM, Tan DM. Combination of NA, Peg-IFN α-2b and GMCSF enhanced HBsAb production in NA suppressed CHB patients (The Anchor A study): an interim analysis. Hepatology. 2018. Abstract(oral) 157.
Li GJ, Yu YQ, Chen SL, et al. Sequential combination therapy with pegylated interferon leads to loss of hepatitis B surface antigen and hepatitis B e antigen (HBeAg) seroconversion in HBeAg-positive chronic hepatitis B patients receiving long-term entecavir treatment. Antimicrob Agents Chemother. 2015;59(7):4121–8.
Switch or add-on PEGINTEREERON to chronic hepatitis B patients already on nucleos(t)ide analogue (SWAP study): final results. J Hepatol. 2019. AASLD2019, Abstracts-oral193.
Yang HI, Lu SN, Liaw YF, et al. Hepatitis B e antigen and the risk of hepatocellular carcinoma. N Engl J Med. 2002;347(3):168–74.
Huo TI, Wu JC, Lee PC, et al. Sero-clearance of hepatitis B surface antigen in chronic carriers does not necessarily imply a good prognosis. Hepatology. 1998;28(1):231–6.
Acknowledgements
We thank the study participants for volunteering for this study. We thank Editage for critical reading of the manuscript.
Funding
The collection, analysis, and interpretation of data; manuscript writing; and the Rapid Service Fee were supported by grants from the National Natural Sciences Foundation of China (grant no. 82070613), the National Natural Sciences Foundation of Hunan Province (grant no. 2019JJ30041), and the Innovation-Driven Project of Central South University (grant no. 2020CX044).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Yan Huang and Xuegong Fan designed the study. Ming Qi, Jinrui Xun, Chenjing Liao, Ju Zou, Haiyue Huang, and Liyuan Long collected and analyzed the data. Ruochan Chen wrote the manuscript. All authors read and approved the final manuscript.
Disclosures
Yan Huang, Xuegong Fan, Ming Qi, Jinrui Xun, Chenjing Liao, Ju Zou, Haiyue Huang, and Liyuan Long, Jun Chen and Ruochan Chen declare no competing nonfinancial/financial interests.
Compliance with Ethics Guidelines
The study protocol was approved by the Ethics Committee of Xiangya Hospital (approval no. 2017090156). The study was conducted in accordance with the guidelines of the Declaration of Helsinki and the principles of Good Clinical Practice. All patient data were anonymized, and written consent was obtained from all patients.
Data Availability
The data are available upon request. Interested scientific researchers can contact Dr. Ruochan Chen directly for further information.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Huang, Y., Qi, M., Liao, C. et al. Analysis of the Efficacy and Safety of PEGylated Interferon-α2b Treatment in Inactive Hepatitis B Surface Antigen Carriers. Infect Dis Ther 10, 2323–2331 (2021). https://doi.org/10.1007/s40121-021-00511-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-021-00511-w