Abstract
Introduction
A systematic literature review was undertaken to evaluate real-world use of ceftazidime-avibactam for infections due to aerobic Gram-negative organisms in adults with limited treatment options.
Methods
Literature searches retrieved peer-reviewed publications and abstracts from major international infectious disease congresses from January 2015 to February 2021. Results were screened using pre-defined criteria to limit the dataset to relevant publications (notable exclusions were paediatric data and outcomes data for bacteria intrinsically resistant to ceftazidime-avibactam). Data for included publications were subjected to qualitative synthesis.
Results
Seventy-three relevant publications (62 peer-reviewed articles; 10 abstracts) comprising 1926 patients treated with ceftazidime-avibactam (either alone or combined with other antimicrobials) and 1114 comparator/control patients were identified. All patients were hospitalised for serious illness and most had multiple comorbidities. The most common infections were pneumonia, bacteraemia, and skin/soft tissue, urinary tract, or abdominal infections; smaller numbers of patients with meningitis, febrile neutropenia, osteomyelitis, and cystic fibrosis were also included. Carbapenem-resistant or carbapenemase-producing Enterobacterales (CRE; n = 1718) and carbapenem-resistant, multidrug-resistant (MDR), and extensively drug-resistant Pseudomonas aeruginosa (n = 150) were the most common pathogens. Most publications reported positive outcomes for ceftazidime-avibactam treatment (clinical success rates ranged from 45 to 100% and reported 30-day mortality from 0 to 63%), which were statistically superior versus comparators in some studies. ceftazidime-avibactam resistance emergence occurred infrequently and mostly in Klebsiella pneumoniae carbapenemase (KPC)-producing K. pneumoniae strains.
Conclusion
This review provides qualitative evidence of successful use of ceftazidime-avibactam for the treatment of hospitalised patients with CRE and MDR P. aeruginosa infections with limited treatment options.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Antimicrobial resistance among Gram-negative bacteria, including Acinetobacter species, Enterobacterales, and Pseudomonas aeruginosa, represents a significant problem for healthcare systems worldwide. For regions with high local prevalence of extended-spectrum β-lactamase (ESBL)- and carbapenemase-producing organisms, treatment options for infections caused by such pathogens can be severely limited. |
A systematic literature review was undertaken to evaluate real-world use of ceftazidime-avibactam for infections due to aerobic Gram-negative organisms in adults with limited treatment options. |
What was learned from the study? |
Literature searches identified 73 publications reporting data for 1926 patients treated with ceftazidime-avibactam and 1114 comparator/control patients, including 26 case reports, 17 case series, 15 retrospective cohort/chart review studies, 12 retrospective comparative/case-control studies and 2 prospective observational studies. |
This review provides important insights into how ceftazidime-avibactam is being used in practice for the treatment of serious Gram-negative infections with limited treatment options, in particular, those caused by non-MBL-producing CRE and P. aeruginosa. |
Introduction
Antimicrobial resistance among Gram-negative bacteria, including Acinetobacter species, Enterobacterales, and Pseudomonas aeruginosa, represents a significant problem for healthcare systems worldwide, in particular for resistance epicentres with high local prevalence of extended-spectrum β-lactamase (ESBL)- and carbapenemase-producing organisms, where treatment options for infections caused by such pathogens can be severely limited [1]. The need for antimicrobial development to address third-generation cephalosporin-resistant and carbapenem-resistant Enterobacterales (CRE) and P. aeruginosa has been designated a critical priority by the World Health Organization [2].
Ceftazidime-avibactam, a combination of the anti-pseudomonal cephalosporin ceftazidime and the novel β-lactamase inhibitor avibactam, has in vitro activity against a broad range of Gram-negative bacteria, including highly resistant strains, such as ESBL-, AmpC-, and serine carbapenemase-producing Enterobacterales (CPE) and P. aeruginosa, but not against metallo-β-lactamase (MBL) producers [3, 4]. In an extensive phase 2 and 3 randomised controlled trial (RCT) programme, ceftazidime-avibactam has demonstrated efficacy generally comparable to carbapenem-based comparator regimens in the primary indications of complicated intra-abdominal infection (cIAI), complicated urinary tract infection (cUTI; including pyelonephritis), and hospital-acquired pneumonia (HAP/HABP; including ventilator-associated pneumonia [VAP/VABP]) [5,6,7,8,9,10,11], including against ceftazidime non-susceptible and multidrug-resistant (MDR) Enterobacterales and P. aeruginosa [6, 12]. Ceftazidime-avibactam (standard dose 2.5 g by 2-h intravenous infusions every 8 h [q8h], adjusted for patients with creatinine clearance [CrCL] ≥ 50 ml/min) is approved in the US for the treatment of adults with cIAI (co-administered with metronidazole), cUTI (including pyelonephritis) and HABP (including VABP), and cIAI or cUTI (including pyelonephritis) in children aged ≥ 3 months [13]. In Europe it is approved for cUTI (including pyelonephritis), cIAI, and HAP/VAP in adults, including for cases of bacteraemia associated with these infections, and for the treatment of infections due to aerobic Gram-negative organisms with limited treatment options [14]. It is also approved for children aged ≥ 3 months with cUTI (including pyelonephritis), cIAI, HAP/VAP, and infections due to aerobic Gram-negative organisms with limited treatment options [14]. Approval of ceftazidime-avibactam for the ‘limited treatment options’ indication in adults was based on microbiological data, demonstration of clinical efficacy in patients with cIAI and cUTI, and population pharmacokinetic (PK) data demonstrating adequate PK/pharmacodynamic (PD) target attainment with approved dosages [15]. By definition, there is a lack of RCT data describing outcomes of treatment for this diverse patient population. However, there is a growing body of published (predominantly retrospective and observational) data on the use of ceftazidime-avibactam in indications for which there are limited treatment options. This systematic literature review provides a qualitative synthesis of the clinical and microbiological outcomes of ceftazidime-avibactam treatment of infections caused by aerobic Gram-negative organisms in adult patients with limited treatment options. This article is based on published literature and does not contain any previously unreported studies with human participants or animals.
Methods
Definition of ‘Infections due to Aerobic Gram-Negative Organisms with Limited Treatment Options’
For the purposes of this review, a working definition of publications describing ‘aerobic Gram-negative infections in adult patients with limited treatment options’ was formulated. Publications reporting outcomes for patients with any of the approved ‘primary’ indications for ceftazidime-avibactam (cIAI, cUTI, HAP/HABP, or VAP/VABP) were only included if the infection(s) involved microbiological confirmation/suspicion of ESBL- and/or carbapenemase-producing Gram-negative organisms, excluding MBL producers. Other acute infections included in the working definition of ‘limited treatment options’ were those with microbiological confirmation/suspicion of involvement of ceftazidime-avibactam-susceptible Gram-negative bacteria (i.e., non-MBL-producing Enterobacterales or P. aeruginosa), such as primary or secondary bacteraemia/bloodstream infections including sepsis/toxic shock, bacterial meningitis, febrile neutropenia, device-related infections, transplant-related infections, bone and joint infections, cystic fibrosis-related bronchopulmonary infections, and skin and soft tissue infections (SSTI).
Literature Search
Methodology for conducting literature searches followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and guidance [16, 17]. A PubMed search using the search terms ‘ceftazidime AND avibactam’ (filters: none) was conducted in February 2021 to include publications from 2015 onwards. Additional searches were conducted to retrieve abstracts from major international infectious disease congresses [European Congress of Clinical Microbiology and Infectious Diseases, American Society for Microbiology (ASM) Microbe/International Conference on Antimicrobial Agents and Chemotherapy, and Infectious Diseases Society of America IDWeek] from 2015 to 2019.
All retrieved publication search results were screened by two researchers by titles and abstracts. The following publication types were excluded, with reasons for exclusion documented in each case: RCT data for any of the primary indications for ceftazidime-avibactam; in vitro/animal study data, including microbiological surveillance or population PK and PK/PD modelling; review articles, guidelines, commentaries/opinion pieces, and editorials not reporting original outcomes data for patients treated with ceftazidime-avibactam; publications primarily reporting outcomes of treatment for infections caused by pathogens not susceptible to ceftazidime-avibactam as defined in the European Summary of Product Characteristics [i.e., Staphylococcus aureus (methicillin-resistant and methicillin-sensitive), anaerobes, Enterococcus spp., Stenotrophomonas maltophilia, and Acinetobacter spp.] [14], or those describing outcomes of MBL-producing Gram-negative infections. Meta-analyses of published literature were also excluded from the analysis to avoid duplication of data; however, the source data/references within such articles were reviewed to ensure all relevant primary data were included.
Data Extraction
Included publications were tabulated in Microsoft Excel for data extraction. In addition to author/citation details, the following data (where reported) were extracted for each included publication: country/region; study design; number of sites (single or multiple); number of ceftazidime-avibactam and control/comparator participants and their characteristics [age, sex, type of infection, clinical isolate(s) and resistance mechanism(s), renal status, and intensive care unit (ICU) admittance]; primary/index infection and proportion of patients with bacteraemia; duration of ceftazidime-avibactam treatment and use of prior and concomitant antibiotics; timing relative to index infection and duration of ceftazidime-avibactam treatment; clinical and microbiological outcomes (for example, hospital, 30-day, or 90-day mortality), and rates of eradication, recurrence, and emergence of resistance.
Results
Literature Search
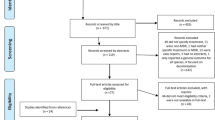
The literature search identified a total of 1262 publications, from which initial screening and further exclusion identified 73 relevant publications (Fig. 1). The included publications (63 journal articles and 10 congress abstracts) reported data for a total of 1926 patients treated with ceftazidime-avibactam and 1114 comparator/control patients and comprised 26 case reports, 17 case series, 16 retrospective cohort/chart review studies, 12 retrospective comparative/case-control studies, and 2 prospective observational studies (Table 1).
Most publications were from Europe (34; 47%) and North America (29; 40%), with five from China (7%) and two each from Latin America (3%) and the Middle East (3%). One publication (1%) included patients from Europe and Australia. Included publications involved patients with a variety of infections, including IAI, HAP/VAP, UTI, SSTI, primary and secondary bacteraemia, meningitis, osteomyelitis, and febrile neutropenia. Five publications (44 patients) included haematology/oncology patients; seven (35 patients) involved solid organ transplant (SOT) patients; six (14 patients) involved treatment of bronchopulmonary infections in patients with cystic fibrosis. Ten case studies (10 patients) included surgical and trauma patients. The most frequently identified pathogens were Enterobacterales (1718 patients), including CPE and CRE strains, and P. aeruginosa (150 patients), including carbapenem-resistant, MDR, and extensively drug-resistant (XDR) strains; 74 patients had other Gram-negative pathogens, including Burkholderia cepacia complex, Burkholderia multivorans, and Raoultella planticola.
Prospective Observational Studies
Data for 95 patients treated with ceftazidime-avibactam from two prospective studies were included [18, 19]. Both studies included patients with CRE infections; no decreased susceptibility/ceftazidime-avibactam resistance emergence was reported in either study.
Sousa et al. (2018) prospectively collected data on outcomes of ceftazidime-avibactam salvage therapy for 57 patients with OXA-48-producing K. pneumoniae infections during an outbreak caused by this pathogen at a Spanish hospital [18]. The most common primary infections were intra-abdominal [16 patients (28%)] or respiratory tract [15 patients (26%)]; 26 patients (57%) had bacteraemia and 51 (89%) had received prior antibiotics. All-cause mortality rates were 14% at 14 days and 22% at 30 days.
In a US prospective, multicentre observational study in patients with CRE bacteraemia (ceftazidime-avibactam, n = 38; colistin, n = 99), Van Duin et al. (2018) reported that inverse probability of treatment weighting (IPTW)-adjusted all-cause 30-day hospital mortality was significantly lower in the ceftazidime-avibactam group (9%) compared with the colistin group [32%; difference 23%, 95% confidence interval (CI) 9–35; P = 0.001]. Based on desirability of outcome ranking (DOOR) analysis, patients treated with ceftazidime-avibactam had an IPTW-adjusted probability of a better outcome of 64% (95% CI, 57–71) [19].
Retrospective Comparative/Case–Control Studies
Data for 481 patients treated with ceftazidime-avibactam from 12 retrospective comparative/case-control studies (Table 2) were retrieved from the literature search [20,21,22,23,24,25,26,27,28,29,30,31]. Each of these studies involved infections caused by CRE and most of the patients were bacteraemic; some included patients treated on a ‘compassionate use’ basis before the commercial availability of ceftazidime-avibactam (the drug was first approved in the US in 2015). Comparator treatments comprised best available therapy (BAT) and included polymyxin B, colistin, aminoglycosides and tigecycline alone or in combination with carbapenems and unspecified ‘salvage agents’ (Table 2). Clinical outcomes included clinical cure and 30- and 90-day mortality rates. Across these studies, efficacy outcomes for ceftazidime-avibactam (where reported) were in general numerically similar or superior to those reported for comparator treatments (Table 2). Emergence of resistance to ceftazidime-avibactam in three of eight patients with recurrent infections (38%) was reported in one congress abstract involving 35 solid organ transplant (SOT) patients with CRE infections (predominantly bacteraemia or pneumonia); however, the duration of ceftazidime-avibactam treatment, resistance mechanism(s), and timing of resistance emergence were not reported [29]. Of note, a non-comparative retrospective analysis by the same investigators (see below) reported an overall rate of ceftazidime-avibactam resistance emergence of 10% among 77 patients infected with CRE, of whom 4 were SOT recipients [32]. A separate study among 147 patients treated with KPC- or OXA-48-producing K. pneumoniae infections treated with ceftazidime-avibactam reported a lower rate of resistance emergence of 1% (2/147 patients); details of timings and mechanism(s) were not reported [25].
Retrospective Cohort/Chart Review Studies
Data for 1155 patients treated with ceftazidime-avibactam from 16 retrospective non-comparative cohort/chart review studies were identified (Table 3) [32,33,34,35,36,37,38,39,40,41,42,43,44,45,46], the majority of which involved CRE/CPE infections (981 patients); six publications included data for P. aeruginosa infections (124 patients). Emergence of resistance and/or reduced susceptibility to ceftazidime-avibactam was reported in seven of these publications, including one OXA-48-producing K. pneumoniae in a single patient [35]; in the remaining cases, the resistant strains were either K. pneumoniae carbapenemase (KPC)-producing K. pneumoniae or carbapenem-resistant K. pneumoniae (CRKP) with unreported resistance mechanisms [32,33,34,35, 46, 47]. Decreased sensitivity to ceftazidime-avibactam was reported in one patient with KPC-producing K. pneumoniae [39].
The largest retrospective cohort study, reported by Tumbarello et al. (2021), included 577 patients with KPC-producing K. pneumoniae infections, including 391 patients with bacteraemia. In this analysis, there was no difference in 30-day mortality for patients who received ceftazidime-avibactam combination or monotherapy regimens (26% vs. 25%, P = 0.79), and rates of resistance development and adverse events were low (both 3%) [47].
Another large retrospective cohort study, reported by Jorgensen et al. (2019), included 203 patients who received ceftazidime-avibactam for ≥ 72 h at six US hospitals (2015–2019) and included data for 117 patients with CRE and 63 with P. aeruginosa as well as infections with other Gram-negative and gram-positive pathogens [34]. In the overall cohort, clinical failure, 30-day mortality and 30-day recurrence occurred in 59 (29%), 35 (17%) and 12 (6%) patients, respectively, and outcomes were similar for patients infected with CRE or P. aeruginosa. Of note, ceftazidime-avibactam dosage adjustments for renal function were made for 92 patients but were considered inappropriate for 11 patients (effectively resulting in underdosing); among these patients, five (46%) experienced clinical failure and three (27%) died by day 30. Moreover, only 50 of 74 CRKP isolates underwent baseline testing for ceftazidime-avibactam susceptibility, of which 48 were susceptible [two isolates were ceftazidime-avibactam-resistant, one with New Delhi metallo-β-lactamase (NDM) and OXA-48 and a second with an unknown mechanism]. Similarly, only 27 of 63 P. aeruginosa isolates underwent baseline testing for ceftazidime-avibactam susceptibility, of which 26 were susceptible and one was resistant with NDM and OXA-type carbapenemases. Ceftazidime-avibactam resistance development on treatment was not detected in any of the 61 patients (30%) with available follow-up cultures. In total, 17 patients (8%) experienced potential drug-related adverse effects [34].
Shields et al. (2018) reported an analysis of 77 consecutive patients with CRE infections treated with ceftazidime-avibactam for ≥ 3 days in a US hospital [32]. Thirty- and 90-day survival rates were 81% and 69%, respectively. Clinical success was 55% overall but differed by infection site (pneumonia, 36%; bacteraemia, 75%; UTI, 88%). Pneumonia (P = 0.045) and receipt of renal replacement therapy [either intermittent haemodialysis or continuous renal replacement therapy (CRRT); P = 0.046] were associated with clinical failure in multivariate analysis. Microbiological failure occurred in 32% of patients and was more common among patients with KPC-3-producing CRE versus KPC-2-producing CRE infections (P = 0.002). Pneumonia was an independent predictor of microbiological failure (P = 0.007). Ceftazidime-avibactam resistance (all in K. pneumoniae harbouring variant KPC-3 enzymes) emerged in eight (10%) patients. Renal replacement therapy was an independent predictor of resistance development (P = 0.009). Importantly, the authors noted that ‘optimal dosing of ceftazidime-avibactam in patients with renal insufficiency, particularly those receiving CRRT, is an ongoing challenge for clinicians’. Of note, appropriate ceftazidime-avibactam dosage regimens for patients receiving CRRT have not been established, and there are currently no approved dose recommendations for such patients. Similar rates of resistance emergence were reported in a Spanish study of 47 patients with CRKP infections (Castón et al. 2020) and in an Italian study (Iannacone et al. 2020) of 23 patients with CRE infections [46] (Table 3).
In a further US retrospective, multicentre study in 60 severely ill patients with CRE infections treated with ceftazidime-avibactam, King el al. (2017) reported that in-hospital mortality, microbiological cure, and clinical success occurred in 32%, 53%, and 65% of patients, respectively; there were no documented cases of drug-related adverse events or on-therapy resistance emergence [37].
Vena et al. (2020) reviewed outcomes for 41 patients treated with ceftazidime-avibactam for infections [predominantly nosocomial pneumonia (NP) and primary bacteraemia] caused by non-CRE MDR Gram-negative bacteria (38 isolates of P. aeruginosa and 7 ESBL-positive Enterobacterales, with > 50% of isolates classified as XDR), including 4 patients with polymicrobial infections, in 13 Italian hospitals [42]. Most patients received ceftazidime-avibactam as part of a combination regimen following resistance emergence or failure of initial antibiotic treatments; despite relatively severe baseline disease status (median Charlson Comorbidity Index score of 4; 24% of patients admitted to ICU and 59% with sepsis/septic shock), clinical success occurred in 37 patients (90%). Three of the four patients who were clinical failures were undergoing CRRT; all received ceftazidime-avibactam 1.25 g q8h (as noted above, there are no approved ceftazidime-avibactam dosing recommendations for patients on CRRT). No adverse events related to ceftazidime-avibactam treatment occurred, and resistance development was not documented in any patient [42].
Temkin et al. (2017) analysed outcomes for 38 patients in Europe and Australia with infections caused by carbapenem-resistant organisms (36 with CRE and two with P. aeruginosa), 95% of whom received ceftazidime-avibactam as salvage therapy [41]. Clinical and/or microbiological cure occurred in 28 patients (74%). Five patients (21%) with documented microbiological cure died versus ten patients (71%) with no documented microbiological cure (P = 0.01).
Rahmati et al. (2017) and Krapp et al. (2017) reported outcomes for 11 and 6 patients, respectively, with KPC-producing K. pneumoniae infections treated with ceftazidime-avibactam in the US [38, 39]. In the cohort of 11 patients, the clinical success rate was 73%, 30-day survival was 82%, 90-day survival was 73%, and in-hospital mortality was 27% [39]. Mortality was 75% among four patients receiving CRRT or haemodialysis (P = 0.02); however, as noted above, approved ceftazidime-avibactam dosing recommendations are not currently available for patients on CRRT (moreover, ceftazidime-avibactam dosages were not reported in this congress abstract). Recurrence occurred in two patients (18%). Decreased sensitivity to ceftazidime-avibactam was noted in one patient (9%), and three patients (27%) had CRE isolated after ≥ 7 days of treatment [39]. In the cohort of six patients, five achieved clinical cure; however, two relapsed with the same KPC-3-producing K. pneumoniae strain within 3 weeks of completing ceftazidime-avibactam treatment [38]. One patient with pneumonia experienced clinical failure, despite having a documented ceftazidime-avibactam-susceptible CRKP strain [minimum inhibitory concentration (MIC) = 2 mg/l]. Thus, the overall clinical success rate in this small cohort was 50% [38].
In two retrospective cohort studies involving MDR or XDR P. aeruginosa infections (in 8 and 10 patients in Spain and the US, respectively) in various locations (including respiratory, blood, bone/joint, skin/wound, and meningitis), clinical success rates with ceftazidime-avibactam treatment were 50–80% [36, 40]. In the Spanish study, 30- and 90-day mortality rates were 13% and 38%, respectively [40]. In-hospital mortality in the US study was 20% [36]. Kuang et al. (2021) and Nwankwo et al. (2020) reported outcomes for 21 and 28 patients with a variety of MDR Gram-negative infections treated with ceftazidime-avibactam in the UK and China, respectively; Papadimitriou-Olivgeris et al. (2021) included a single patient treated with ceftazidime-avibactam in a wider analysis of 115 critically ill patients with K. pneumoniae bloodstream infections (Table 3).
Finally, Katchanov et al. (2018) described a prevalence study of patients infected or colonised with MDR Gram-negative bacteria in a German tertiary care hospital, in which ceftazidime-avibactam was administered to five patients with severe HAP (and bacteraemia in one case) during an outbreak of OXA-48- and CTX-M-14-producing K. pneumoniae [35]. In-hospital mortality occurred in all five patients (100%), and ceftazidime-avibactam resistance emergence on therapy was documented in one patient (three patients were not assessed for resistance emergence), prompting the authors to note that ‘novel β-lactam/β-lactamase inhibitor combinations are of limited usefulness in our setting because of the high prevalence of Ambler class B carbapenemases (in Pseudomonas spp. and Escherichia coli but not in Klebsiella spp.) and the emergence of non-susceptibility under therapy’. Of note, the five patients received 2, 57, 86, 8, and 61 days, respectively, of ceftazidime-avibactam therapy, implying a 30-day mortality rate of 2/5 (i.e., 40%) [35].
Case Series and Case Reports
Data for 169 patients treated with ceftazidime-avibactam from 17 case series (Table 4) were identified from the literature search [48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64]. One case series of three patients (Shields et al. 2017 [65]) was excluded from the literature search results because clinical outcomes for these patients are included in another larger case series published by the same group (Shields et al. 2016 [56]); data for the latter publication are summarised in Table 4. Most of the included case series involved CRE/CPE infections (141 patients); 10 publications included data for P. aeruginosa infections (24 patients). Three case series reported emergence of ceftazidime-avibactam resistance on therapy in two of ten patients (20%; one P. aeruginosa; one Citrobacter freundii with KPC detected in initial isolate and undetermined mechanism of ceftazidime-avibactam resistance) [55]; 3 of 37 patients (8%; all initial isolates were KPC-3-producing K. pneumoniae) [56] and one of two patients (50%) were treated for XDR P. aeruginosa infections [61].
The literature search identified 26 case reports with data for 26 patients (Table 5) treated with ceftazidime-avibactam [66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91]. As with the other included publications, most of the case reports involved CRE/CPE infections (16 patients, of which 1 was also infected with P. aeruginosa). Emergence of ceftazidime-avibactam resistance on treatment was reported in one patient each in two case reports involving K. pneumoniae bloodstream infections (one KPC-2; one KPC-3) [85, 89], and an increase in ceftazidime-avibactam MIC was reported for a paediatric patient with cystic fibrosis and acute pulmonary exacerbation caused by B. cepacia complex [81].
Discussion
This systematic review provides important insights into the real-world use of ceftazidime-avibactam treatment for adults with serious Gram-negative infections with limited treatment options. Although limited by potential publication bias (the tendency for positive results to be more likely to be published than negative results) and the qualitative approach necessitated by the inclusion of heterogenous data representing multiple patient populations, infection types, and bacterial pathogens, the included data are consistent with the broad definition of ‘limited treatment options’ in the European Summary of Product Characteristics for ceftazidime-avibactam [14] and generally reflect the expected usage of ceftazidime-avibactam in this setting.
As expected, most of the included publications involved severely ill patients with significant comorbidities; the main pathogens were CRE/CPE and P. aeruginosa. A large proportion of reported cases treated with ceftazidime-avibactam included either primary or secondary bacteraemia, and many involved combination treatment with other antibiotics. The relatively wide inclusion criteria for literature in the current analysis [notable exclusions were paediatric data and studies involving pathogens listed in the European Summary of Product Characteristics as non-susceptible to ceftazidime-avibactam (e.g., Acinetobacter spp. or MBL-producing isolates)] and the resulting large number of publications encompassing multiple patient populations, infection types, and various endpoints and definitions limit the possibility for quantitative analysis and warrant caution in generalising the findings. However, an advantage of this broad scope is the inevitable inclusion of several publications that have been subject to more formal analysis. Onorato et al. (2019) reported a meta-analysis of 11 studies including 396 patients with CRE or carbapenem-resistant P. aeruginosa infections treated with ceftazidime-avibactam [92]; these 11 studies were all included in the current review. The meta-analysis by Onorato et al. was designed to assess the impact of ceftazidime-avibactam monotherapy versus combination therapy with other antibiotics. Rates of mortality [38% for combination therapy and 31% for monotherapy; risk ratio (RR) 1.18, 95% CI 0.88–1.58; P = 0.259] and microbiological cure (65% vs. 63%, respectively; RR 1.04, 95% CI 0.85–1.28, P = 0.705) were comparable for ceftazidime-avibactam as monotherapy or as part of a combination regimen [92]. A similar network meta-analysis by Fiore et al. (2020) [93] included six observational studies, all of which are also included in the current review [18, 20, 23, 28, 30, 37]. Similarly, Dietl et al. (2020) reviewed 11 observational and comparative studies of ceftazidime-avibactam in patients with CPE infections (including 8 in patients with KPC- and 3 in patients with OXA-48-producing Enterobacterales) [94], all of which were also included in the current review [18, 19, 23, 28, 30, 31, 34, 38, 41, 49, 56]. Finally, a systematic review of severe infectious complications among patients with haematological malignancies identified three studies of ceftazidime-avibactam [95], of which two were included in the current review (the third was excluded as it involved treatment of an infection caused by an MBL-producing organism) [23, 53].
The current qualitative analysis is an attempt to complement, rather than replicate, the meta-analyses and RCTs that were excluded from our dataset [92,93,94,95,96,97,98,99,100]. Although lacking the statistical robustness of RCTs or meta-analyses, advantages of our qualitative approach include the broad inclusion criteria and focus on observational data, in contrast to the relatively selective inclusion criteria inherent in meta-analyses and RCTs. Given these broad inclusion criteria, our dataset inevitably included a varied and heterogeneous patient population and a mixture of different assessments and outcome measures; as such, we are unable to provide overall or aggregated efficacy data for ceftazidime-avibactam across the different publications that we included. Nevertheless, this review provides important insights into how ceftazidime-avibactam is being used in practice for the treatment of serious Gram-negative infections with limited treatment options, in particular, those caused by non-MBL-producing CRE and P. aeruginosa. In some cases, positive clinical outcomes were reported with documented reductions in renal toxicity compared with other possible treatment options (e.g., aminoglycosides and/or colistin/polymixins). The accumulating data from RCTs and real-world clinical experience with ceftazidime-avibactam have prompted its inclusion in various national and regional guidance documents and management protocols [101,102,103,104,105,106,107]. For example, guidance by the Infectious Diseases Society of America for the management antimicrobial-resistant Gram-negative infections recommends ceftazidime-avibactam as a preferred treatment option for CRE and P. aeruginosa infections both within and outside of the urinary tract [107].
The review also highlights areas in which further research may help elucidate optimal use of ceftazidime-avibactam (for example, in patients undergoing CRRT). In addition, and not unexpectedly given the nature of the limited treatment options setting (including complicated infections and extensive and complex comorbidities), the analysis included several publications reporting resistance emergence and/or reduced susceptibility during therapy with ceftazidime-avibactam [29, 32,33,34,35, 39, 47, 55, 56, 61, 85, 89]. A further study specifically evaluated outcomes for five patients with documented resistance ceftazidime-avibactam emergence [60]. Of note, information on the timing of resistance emergence relative to initiation of ceftazidime-avibactam therapy was reported inconsistently. As with all antimicrobial agents, use of ceftazidime-avibactam should be guided, wherever possible, by pathogen culture/phenotypic susceptibility results (ideally supported by genotypic detection and characterisation of any carbapenemases identified) and local resistance patterns. In cases where patients show signs of deterioration or failure to improve, it is important that purposeful microbiological sampling is undertaken to enable early detection of potential resistance development on therapy.
An important point to highlight regarding the inclusion criteria and data extraction is that although we endeavoured to exclude publications reporting off-label use of ceftazidime-avibactam, in some instances, we did include publications reporting data on the use of ceftazidime-avibactam outside of the approved European Summary of Product Characteristics. For example, some reported studies included patients undergoing CRRT [32, 42, 48, 50, 55], although there are no approved ceftazidime-avibactam dosing recommendations for such patients; similarly, some studies reported the use of doses of ceftazidime-avibactam (including adjustments for renal function) outside of the dosing recommendations in the European Summary of Product Characteristics. One case series reported on the use of ceftazidime-avibactam in surgical prophylaxis in two patients with cystic fibrosis undergoing lung transplantation [52] (this publication was nonetheless included because both patients also subsequently received ceftazidime-avibactam postoperatively for infections consistent with the ‘limited treatment options’ definition). Moreover, a few studies reported on the use of ceftazidime-avibactam in combination with other agents to treat infections caused by MBL-producing bacteria or species listed in the European Summary of Product Characteristics as inherently resistant to ceftazidime-avibactam (for example Acinetobacter species); we excluded such publications, except in a few instances where the data were reported alongside/within a larger patient group in which the reported use of ceftazidime-avibactam was otherwise consistent with approved labelling. A case series of three patients with XDR P. aeruginosa infections reported use of an off-label dose of ceftazidime-avibactam alongside aztreonam [61]; we nevertheless included these data since a second patient in the series received standard-dose ceftazidime-avibactam (plus colistin then aztreonam) and was reported to have on-therapy resistance emergence after 60 days of treatment.
Although it has been anecdotally reported that the standard ceftazidime-avibactam dosage regimen for patients with CrCL ≥ 50 ml/min (i.e., 2.5 g by 2-h intravenous infusions q8h) may be suitable for patients with pneumonia undergoing continuous venovenous haemodiafiltration [108, 109], it is important to note that appropriate ceftazidime-avibactam dosage regimens for patients receiving CRRT have not been established. In the retrospective study of 77 patients with CRE infections by Shields et al. (2018), 3 patients required CRRT, including 2 of the 5 patients with pneumonia [32]. Ceftazidime-avibactam doses for these three patients ranged from 0.94 g every 12 h (q12h) to 2.5 g q8h. The link between ceftazidime-avibactam underdosing and clinical failure in renal impairment was identified in the phase 3 RECLAIM trial [5] and has been further reviewed by Li et al. (2020) [110]. Considering the small sample size and variable ceftazidime-avibactam dosing in the CRRT subset in the analysis by Shields et al. [32], the reported statistical association between pneumonia and treatment failure should be interpreted with caution.
In conclusion, the data reviewed here demonstrate qualitative evidence of successful use of ceftazidime-avibactam for treatment of hospitalised patients with Gram-negative infections with limited treatment options, based on clinical and microbiological cure outcomes and mortality, including evidence of effectiveness against CRE and MDR P. aeruginosa. The review also highlights areas where further data are needed, for example, on the use of ceftazidime-avibactam in patients undergoing CRRT.
References
Theuretzbacher U. Global antimicrobial resistance in gram-negative pathogens and clinical need. Curr Opin Microbiol. 2017;39:106–12.
Tacconelli E, Carrara E, Savoldi A, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18(3):318–27.
Lagacé-Wiens P, Walkty A, Karlowsky JA. Ceftazidime-avibactam: an evidence-based review of its pharmacology and potential use in the treatment of gram-negative bacterial infections. Core Evid. 2014;9:13–25.
Shirley M. Ceftazidime-avibactam: a review in the treatment of serious gram-negative bacterial infections. Drugs. 2018;78(6):675–92.
Mazuski JE, Gasink LB, Armstrong J, et al. Efficacy and safety of ceftazidime-avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infection: results from a randomized, controlled, double-blind, phase 3 program. Clin Infect Dis. 2016;62(11):1380–9.
Carmeli Y, Armstrong J, Laud PJ, et al. ceftazidime-avibactam or best available therapy in patients with ceftazidime-resistant Enterobacteriaceae and Pseudomonas aeruginosa complicated urinary tract infections or complicated intra-abdominal infections (REPRISE): a randomised, pathogen-directed, phase 3 study. Lancet Infect Dis. 2016;16(6):661–73.
Wagenlehner FM, Sobel JD, Newell P, et al. Ceftazidime-avibactam versus doripenem for the treatment of complicated urinary tract infections, including acute pyelonephritis: RECAPTURE, a phase 3 randomized trial program. Clin Infect Dis. 2016;63(6):754–62.
Qin X, Tran BG, Kim MJ, et al. A randomised, double-blind, phase 3 study comparing the efficacy and safety of ceftazidime/avibactam plus metronidazole versus meropenem for complicated intra-abdominal infections in hospitalised adults in Asia. Int J Antimicrob Agents. 2017;49(5):579–88.
Torres A, Zhong N, Pachl J, et al. Ceftazidime-avibactam versus meropenem in nosocomial pneumonia, including ventilator-associated pneumonia (REPROVE): a randomised, double-blind, phase 3 non-inferiority trial. Lancet Infect Dis. 2018;18(3):285–95.
Lucasti C, Popescu I, Ramesh MK, Lipka J, Sable C. Comparative study of the efficacy and safety of ceftazidime/avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infections in hospitalized adults: results of a randomized, double-blind. Phase II trial J Antimicrob Chemother. 2013;68(5):1183–92.
Vazquez JA, Gonzalez Patzan LD, Stricklin D, et al. Efficacy and safety of ceftazidime-avibactam versus imipenem-cilastatin in the treatment of complicated urinary tract infections, including acute pyelonephritis, in hospitalized adults: results of a prospective, investigator-blinded, randomized study. Curr Med Res Opin. 2012;28(12):1921–31.
Stone GG, Newell P, Gasink LB, et al. Clinical activity of ceftazidime/avibactam against MDR Enterobacteriaceae and Pseudomonas aeruginosa: pooled data from the ceftazidime/avibactam Phase III clinical trial programme. J Antimicrob Chemother. 2018;73(9):2519–23.
Allergan. AVYCAZ (ceftazidime and avibactam) for injection, for intravenous use. 2020. https://www.allergan.com/assets/pdf/avycaz_pi. Accessed 19 February 2021.
Pfizer. Summary of Product Characteristics: Zavicefta 2 g/0.5 g powder for concentrate for solution for infusion. 2021. https://www.ema.europa.eu/documents/product-information/zavicefta-epar-product-information_en.pdf. Accessed 19 February 2021.
European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP). Zavicefta assessment report. 2016. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/004027/WC500210236.pdf. Accessed 19 February 2021.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9, W64.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65-94.
Sousa A, Perez-Rodriguez MT, Soto A, et al. Effectiveness of ceftazidime/avibactam as salvage therapy for treatment of infections due to OXA-48 carbapenemase-producing Enterobacteriaceae. J Antimicrob Chemother. 2018;73(11):3170–5.
van Duin D, Lok JJ, Earley M, et al. Colistin versus ceftazidime-avibactam in the treatment of infections due to carbapenem-resistant Enterobacteriaceae. Clin Infect Dis. 2018;66(2):163–71.
Alraddadi BM, Saeedi M, Qutub M, Alshukairi A, Hassanien A, Wali G. Efficacy of ceftazidime-avibactam in the treatment of infections due to carbapenem-resistant Enterobacteriaceae. BMC Infect Dis. 2019;19(1):772.
Bandali A, Deering C, Bias T, Gancher E. 2410. Clinical outcomes associated with various treatment options for infections caused by carbapenem–resistant enterobacteriaceae. Open Forum Infectious Diseases. 2018;5(suppl_1):S720-S1.
Borjan J, Shelburne SA, Shelburne SA, Bhatti MM, Aitken SL, Aitken SL. 2246. Improved Outcomes for Cancer Patients Treated With ceftazidime-avibactam vs. Polymyxin-Containing Regimens for Carbapenem-Resistant Enterobacteriaceae Bacteremia. Open Forum Infectious Diseases. 2019;6(Supplement_2):S768-S.
Castón JJ, Lacort-Peralta I, Martin-Davila P, et al. Clinical efficacy of ceftazidime/avibactam versus other active agents for the treatment of bacteremia due to carbapenemase-producing Enterobacteriaceae in hematologic patients. Int J Infect Dis. 2017;59:118–23.
John J, Nelson B, Macesic N. 2262. ceftazidime-avibactam vs. Polymyxin B in the Treatment of Infections Due to Carbapenem-Resistant Enterobacteriaceae. Open Forum Infectious Diseases. 2019;6(Supplement_2):S774-S.
Karaiskos I, Daikos GL, Gkoufa A, et al. Ceftazidime/avibactam in the era of carbapenemase-producing Klebsiella pneumoniae: experience from a national registry study. J Antimicrob Chemother. 2021;76(3):775–83.
Pillinger KE, Patel S, Stilwell A, et al. Factors Associated with Mortality in Carbapenem-Resistant Enterobacteriaceae Bacteremia: Focus on Antibiotic Therapy. Open Forum Infectious Diseases. 2017;4(suppl_1):S285-S.
Shen L, Lian C, Zhu B, et al. Bloodstream infections due to carbapenem-resistant klebsiella pneumoniae: a single-center retrospective study on risk factors and therapy options. Microb Drug Resist. 2021;27(2):227–33.
Shields RK, Nguyen MH, Chen L, et al. Ceftazidime-avibactam is superior to other treatment regimens against carbapenem-resistant Klebsiella pneumoniae bacteremia. Antimicrob Agents Chemother. 2017;61(8):e00883-e917.
Sun J, Clancy CJ, Shields RK, Nguyen M-H. 2667. Does ceftazidime-avibactam (CAZ-AVI) Improve Short- and Long-Term Outcomes Among Solid-Organ Transplant (SOT) Recipients with Carbapenem-Resistant Enterobacteriaceae (CRE) Infections? Open Forum Infectious Diseases. 2019;6(Supplement_2):S934-S5.
Tumbarello M, Trecarichi EM, Corona A, et al. Efficacy of ceftazidime-avibactam salvage therapy in patients with infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Clin Infect Dis. 2019;68(3):355–64.
Tsolaki V, Mantzarlis K, Mpakalis A, et al. ceftazidime-avibactam to treat life-threatening infections by carbapenem-resistant pathogens in critically ill mechanically ventilated patients. Antimicrob Agents Chemother. 2020;64(3):e02320-e2419.
Shields RK, Nguyen MH, Chen L, Press EG, Kreiswirth BN, Clancy CJ. Pneumonia and renal replacement therapy are risk factors for ceftazidime-avibactam treatment failures and resistance among patients with carbapenem-resistant Enterobacteriaceae infections. Antimicrob Agents Chemother. 2018;62(5):e02497-e2517.
Caston JJ, Gallo M, Garcia M, et al. Ceftazidime-avibactam in the treatment of infections caused by KPC-producing Klebsiella pneumoniae: factors associated with clinical efficacy in a single-center cohort. Int J Antimicrob Agents. 2020;56(3):106075.
Jorgensen SCJ, Trinh TD, Zasowski EJ, et al. Real-World Experience With Ceftazidime-Avibactam for Multidrug-Resistant Gram-Negative Bacterial Infections. Open Forum Infect Dis. 2019;6(12):ofz522.
Katchanov J, Asar L, Klupp EM, et al. Carbapenem-resistant Gram-negative pathogens in a German university medical center: prevalence, clinical implications and the role of novel beta-lactam/beta-lactamase inhibitor combinations. PLoS One. 2018;13(4):e0195757.
King M, Huang V, Gallagher J, Heil E. Outcomes with Ceftazidime/Avibactam in patients with carbapenem-resistant pseudomonas infections: a multi-center study. Open Forum Infectious Diseases. 2016;3(suppl_1).
King M, Heil E, Kuriakose S, et al. Multicenter study of outcomes with ceftazidime-avibactam in patients with carbapenem-resistant Enterobacteriaceae infections. Antimicrob Agents Chemother. 2017;61(7):e00449-e517.
Krapp F, Grant JL, Sutton SH, Ozer EA, Barr VO. Treating complicated carbapenem-resistant Enterobacteriaceae infections with ceftazidime/avibactam: a retrospective study with molecular strain characterisation. Int J Antimicrob Agents. 2017;49(6):770–3.
Rahmati E, Blodget E, She RC, Abbott JC, Bonomo RA, Spellberg B. Treatment of Carbapenem-Resistant Enterobacteriaceae Infections with Ceftazidime-Avibactam. Open Forum Infectious Diseases. 2017;4(suppl_1):S286-S.
Rodriguez-Nunez O, Ripa M, Morata L, et al. Evaluation of ceftazidime/avibactam for serious infections due to multidrug-resistant and extensively drug-resistant Pseudomonas aeruginosa. J Glob Antimicrob Resist. 2018;15:136–9.
Temkin E, Torre-Cisneros J, Beovic B, et al. Ceftazidime-avibactam as salvage therapy for infections caused by carbapenem-resistant organisms. Antimicrob Agents Chemother. 2017;61(2):e01964-e2016.
Vena A, Giacobbe DR, Castaldo N, et al. Clinical experience with ceftazidime-avibactam for the treatment of infections due to multidrug-resistant Gram-negative bacteria other than carbapenem-resistant Enterobacterales. Antibiotics (Basel, Switzerland). 2020;9(2):71.
Papadimitriou-Olivgeris M, Bartzavali C, Georgakopoulou A, et al. Mortality of pandrug-resistant klebsiella pneumoniae bloodstream infections in critically Ill patients: a retrospective cohort of 115 episodes. Antibiotics (Basel, Switzerland). 2021;10(1).
Kuang H, Zhong C, Wang Y, et al. Clinical characteristics and outcomes of patients with multidrug-resistant Gram-negative bacterial infections treated with ceftazidime/avibactam. J Glob Antimicrob Resist. 2020;23:404–7.
Nwankwo L, Butt Z, Schelenz S. Experience of Ceftazidime/avibactam in a UK tertiary cardiopulmonary specialist center. Expert Rev Anti Infect Ther. 2021;19(1):101–8.
Iannaccone M, Boattini M, Bianco G, Corcione S, Cavallo R, Costa C. Ceftazidime-avibactam susceptible to resistant KPC-producing Enterobacterales bloodstream infections: an observational study. J Chemother. 2020;32(3):160–2.
Tumbarello M, Raffaelli F, Giannella M, et al. Ceftazidime-avibactam use for KPC-Kp infections: a retrospective observational multicenter study. Clin Infect Dis. 2021.
Algwizani A, Alzunitan M, Alharbi A, et al. Experience with ceftazidime-avibactam treatment in a tertiary care center in Saudi Arabia. J Infect Public Health. 2018;11(6):793–5.
De la Calle C, Rodriguez O, Morata L, et al. Clinical characteristics and prognosis of infections caused by OXA-48 carbapenemase-producing Enterobacteriaceae in patients treated with ceftazidime-avibactam. Int J Antimicrob Agents. 2019;53(4):520–4.
Gallagher JC, McKinnell J. Compassionate use ceftazidime/avibactam (Cazavi) for carbapenem-resistant (Cr) Gram-negative rod (Gnr) infections. ASM Microbe; 2016 20 June; Boston, Massachusetts, USA; 2016.
Guimaraes T, Nouer SA, Martins RCR, et al. ceftazidime-avibactam as salvage therapy for infections caused by Enterobacteriales coresistant to carbapenems and polymyxins. Antimicrob Agents Chemother. 2019;63(10).
Los-Arcos I, Len O, Martin-Gomez MT, et al. Lung transplantation in two cystic fibrosis patients infected with previously pandrug-resistant Burkholderia cepacia complex treated with ceftazidime-avibactam. Infection. 2019;47(2):289–92.
Metafuni E, Criscuolo M, Spanu T, Sica S. ceftazidime-avibactam for Gram-negative multidrug-resistant bacteria in hematological patients: a single-center experience. Ann Hematol. 2019;98(6):1495–7.
Micozzi A, Ansuinelli M, Minotti C, et al. Ceftazidime-avibactam as empiric therapy in febrile neutropenic high-risk haematological patients, HMpts, colonized with carbapenem-resistant Klebsiella pneumoniae (CRKP). 28th European Congress of Clinical Microbioogy and Infectious Diseses (ECCMID); 2018 21–24 April; Madrid, Spain; 2018.
Santevecchi BA, Smith TT, MacVane SH. Clinical experience with ceftazidime/avibactam for treatment of antibiotic-resistant organisms other than Klebsiella pneumoniae. Int J Antimicrob Agents. 2018;51(4):629–35.
Shields RK, Potoski BA, Haidar G, et al. Clinical outcomes, drug toxicity, and emergence of ceftazidime-avibactam resistance among patients treated for carbapenem-resistant Enterobacteriaceae infections. Clin Infect Dis. 2016;63(12):1615–8.
Spoletini G, Etherington C, Shaw N, et al. Use of ceftazidime/avibactam for the treatment of MDR Pseudomonas aeruginosa and Burkholderia cepacia complex infections in cystic fibrosis: a case series. J Antimicrob Chemother. 2019;74(5):1425–9.
Wu G, Abraham T, Lee S. ceftazidime-avibactam for treatment of carbapenem-resistant Enterobacteriaceae bacteremia. Clin Infect Dis. 2016;63(8):1147–8.
Xipell M, Bodro M, Marco F, Losno RA, Cardozo C, Soriano A. Clinical experience with ceftazidime/avibactam in patients with severe infections, including meningitis and lung abscesses, caused by extensively drug-resistant Pseudomonas aeruginosa. Int J Antimicrob Agents. 2017;49(2):266–8.
van Asten SAV, Boattini M, Kraakman MEM, et al. Ceftazidime-avibactam resistance and restoration of carbapenem susceptibility in KPC-producing Klebsiella pneumoniae infections: A case series. J Infect Chemother. 2021.
Meschiari M, Franconi I, Bacca E, et al. Ceftazidime/avibactam and ceftolozane/tazobactam for the treatment of extensively drug-resistant Pseudomonas aeruginosa post-neurosurgical infections: three cases and a review of the literature. Infection. 2020.
Wang Z, Qian Y, Bai H, Yang J, Li X. Allograft hemorrhage as a manifestation of carbapenem-resistant Klebsiella pneumonia infection in kidney transplant recipients: Case series. Medicine (Baltimore). 2020;99(13):e18982.
Amore D, Pecoraro Y, Carillo C, et al. Use of ceftazidime-avibactam and ceftolozane-tazobactam after lung transplantation. Transpl Proc. 2020;52(5):1605–7.
Chen W, Sun L, Guo L, et al. Clinical outcomes of ceftazidime-avibactam in lung transplant recipients with infections caused by extensively drug-resistant gram-negative bacilli. Ann Transl Med. 2020;8(3):39.
Shields RK, Chen L, Cheng S, et al. Emergence of ceftazidime-avibactam resistance due to plasmid-borne blaKPC-3 mutations during treatment of carbapenem-resistant Klebsiella pneumoniae infections. Antimicrob Agents Chemother. 2017;61(3):e02097-e2116.
Barlow G, Morice A. Successful treatment of resistant Burkholderia multivorans infection in a patient with cystic fibrosis using ceftazidime/avibactam plus aztreonam. J Antimicrob Chemother. 2018;73(8):2270–1.
Bulbin A, Bono C, Philp T, Mariano N, Urban C. Successful treatment of Klebsiella pneumoniae harboring a Klebsiella pneumoniae carbapenemase isolated from lumbar wound infection and blood in a patient with hardware retention. Case Rep Infect Dis. 2017;2017:9028543.
Camargo JF, Simkins J, Beduschi T, et al. Successful treatment of carbapenemase-producing pandrug-resistant Klebsiella pneumoniae bacteremia. Antimicrob Agents Chemother. 2015;59(10):5903–8.
Canton-Bulnes ML, Hurtado Martinez A, Lopez-Cerero L, Arenzana Seisdedos A, Merino-Bohorquez V, Garnacho-Montero J. A case of pan-resistant Burkholderia cepacia complex bacteremic pneumonia, after lung transplantation treated with a targeted combination therapy. Transpl Infect Dis. 2019;21(2):e13034.
Carannante N, Pallotto C, Bernardo M, Di Caprio G, Tascini C. Treatment of a Klebsiella pneumoniae KPC cellulitis and gut decolonization with ceftazidime/avibactam in a migrant from Libya. J Chemother. 2018;30(3):183–4.
Caravaca-Fontan F, Jimenez-Alvaro S, Marcen-Letosa R, Fernandez-Rodriguez A, Rodriguez-Navarro CQ. ceftazidime-avibactam in urinary tract infections due to carbapenemase-producing Klebsiella in kidney transplantation. Nefrologia. 2015;35(4):412–3.
Dacco V, Claut L, Piconi S, et al. Successful ceftazidime-avibactam treatment of post-surgery Burkholderia multivorans genomovar II bacteremia and brain abscesses in a young lung transplanted woman with cystic fibrosis. Transpl Infect Dis. 2019;21(3):e13082.
De Leon-Borras R, Alvarez-Cardona J, Vidal JA, Guiot HM. Ceftazidime/avibactam for refractory bacteremia, vertebral diskitis/osteomyelitis with pre-vertebral abscess and bilateral psoas pyomyositis secondary to Klebsiella Pneumoniae carbapenemase-producing bacteria (KPC). P R Health Sci J. 2018;37(2):128–31.
Gofman N, To K, Whitman M, Garcia-Morales E. Successful treatment of ventriculitis caused by Pseudomonas aeruginosa and carbapenem-resistant Klebsiella pneumoniae with i.v. ceftazidime-avibactam and intrathecal amikacin. Am J Health Syst Pharm. 2018;75(13):953–7.
Gugliandolo A, Caio C, Mezzatesta ML, et al. Successful ceftazidime-avibactam treatment of MDR-KPC-positive Klebsiella pneumoniae infection in a patient with traumatic brain injury: A case report. Medicine (Baltimore). 2017;96(31):e7664.
Guedes M, Duro R, Fonseca T, Abreu I, Rocha-Pereira N. Carbapenemase-producing Klebsiella pneumoniae intra-abdominal infection successfully treated with ceftazidime/avibactam plus tigecycline. IDCases. 2020;20:e00745.
Gonzales Zamora JA, Corzo-Pedroza M, Romero Alvarez M, Martinez OV. Carbapenemase-producing Raoultella planticola: A rare cause of pneumonia and bacteremia. Diseases. 2018;6(4):94.
Holyk A, Belden V, Lee JJ, et al. Ceftazidime/avibactam use for carbapenem-resistant Klebsiella pneumoniae meningitis: a case report. J Antimicrob Chemother. 2018;73(1):254–6.
Iacovelli A, Spaziante M, Al Moghazi S, Giordano A, Ceccarelli G, Venditti M. A challenging case of carbapenemase-producing Klebsiella pneumoniae septic thrombophlebitis and right mural endocarditis successfully treated with ceftazidime/avibactam. Infection. 2018;46(5):721–4.
Jacobs DM, DiTursi S, Ruh C, et al. Combination treatment with extended-infusion ceftazidime/avibactam for a KPC-3-producing Klebsiella pneumoniae bacteraemia in a kidney and pancreas transplant patient. Int J Antimicrob Agents. 2016;48(2):225–7.
Nguyen TT, Condren M, Walter J. ceftazidime-avibactam for the treatment of multidrug resistant Burkholderia cepacia complex in a pediatric cystic fibrosis patient. Pediatr Pulmonol. 2020;55(2):283–4.
Park SC, Wailan AM, Barry KE, et al. Managing all the genotypic knowledge: approach to a septic patient colonized by different Enterobacteriales with unique carbapenemases. Antimicrob Agents Chemother. 2019;63(8):e00029-e119.
Parruti G, Frattari A, Polilli E, et al. Cure of recurring Klebsiella pneumoniae carbapenemase-producing Klebsiella pneumoniae septic shock episodes due to complicated soft tissue infection using a ceftazidime and avibactam-based regimen: a case report. J Med Case Rep. 2019;13(1):20.
Pingue V, Penati R, Nardone A, Franciotta D. Ceftazidime/avibactam neurotoxicity in an adult patient with normal renal function. Clin Microbiol Infect. 2020.
Raisanen K, Koivula I, Ilmavirta H, et al. Emergence of ceftazidime-avibactam-resistant Klebsiella pneumoniae during treatment, Finland, December 2018. Euro Surveill. 2019;24(19):1900256.
Rico-Nieto A, Moreno-Ramos F, Fernandez-Baillo N. Lumbar arthrodesis infection by multi-resistant Klebsiella pneumoniae, successfully treated with implant retention and ceftazidime/avibactam. Rev Esp Cir Ortop Traumatol. 2018;62(6):471–3.
Samuel S, Edwards NJ, Rojas LJ, et al. Ceftazidime-Avibactam for the treatment of post-neurosurgical meningitis caused by a Klebsiella pneumoniae carbapenemase (KPC)-Producing Klebsiella pneumoniae. Open Forum Infectious Diseases. 2016;3(suppl_1).
Schimmenti A, Brunetti E, Seminari E, Mariani B, Cambieri P, Orsolini P. Prosthetic joint infection from carbapenemase-resistant Klebsiella pneumoniae successfully treated with ceftazidime-avibactam. Case Rep Infect Dis. 2018;2018:1854805.
Shields RK, Nguyen MH, Press EG, Chen L, Kreiswirth BN, Clancy CJ. Emergence of ceftazidime-avibactam resistance and restoration of carbapenem susceptibility in Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: a case report and review of literature. Open Forum Infect Dis. 2017;4(3):ofx101.
Wang Z, Ma K, Chen Z, et al. Successful treatment of early post-transplant bloodstream and pulmonary infection caused by carbapenem-resistant klebsiella pneumoniae with a combination of ceftazidime-avibactam and carbapenem: a case report. Transplant Proc. 2020;52(9):2742–6.
Yasmin M, Hanrahan J, Marshall S, et al. Using therapeutic drug monitoring to treat KPC-producing klebsiella pneumoniae central nervous system infection with ceftazidime/avibactam. Open Forum Infect Dis. 2020;7(9):ofaa349.
Onorato L, Di Caprio G, Signoriello S, Coppola N. Efficacy of ceftazidime/avibactam in monotherapy or combination therapy against carbapenem-resistant Gram-negative bacteria: a meta-analysis. Int J Antimicrob Agents. 2019;54(6):735–40.
Fiore M, Alfieri A, Di Franco S, et al. Ceftazidime-avibactam combination therapy compared to ceftazidime-avibactam monotherapy for the treatment of severe infections due to carbapenem-resistant pathogens: a systematic review and network meta-analysis. Antibiotics (Basel, Switzerland). 2020;9(7).
Dietl B, Martinez LM, Calbo E, Garau J. Update on the role of ceftazidime-avibactam in the management of carbapenemase-producing Enterobacterales. Future Microbiol. 2020;15:473–84.
Criscuolo M, Trecarichi EM. Ceftazidime/avibactam and ceftolozane/tazobactam for multidrug-resistant gram negatives in patients with hematological malignancies: current experiences. Antibiotics (Basel, Switzerland). 2020;9(2).
Che H, Wang R, Wang J, Cai Y. Ceftazidime/avibactam versus carbapenems for the treatment of infections caused by Enterobacteriaceae: a meta-analysis of randomised controlled trials. Int J Antimicrob Agents. 2019;54(6):809–13.
Zhong H, Zhao XY, Zhang ZL, et al. Evaluation of the efficacy and safety of ceftazidime/avibactam in the treatment of Gram-negative bacterial infections: a systematic review and meta-analysis. Int J Antimicrob Agents. 2018;52(4):443–50.
Nguyen CP, Dan Do TN, Bruggemann R, et al. Clinical cure rate and cost-effectiveness of carbapenem-sparing beta-lactams vs. meropenem for Gram-negative infections: a systematic review, meta-analysis, and cost-effectiveness analysis. Int J Antimicrob Agents. 2019;54(6):790–7.
Sternbach N, Leibovici Weissman Y, Avni T, Yahav D. Efficacy and safety of ceftazidime/avibactam: a systematic review and meta-analysis. J Antimicrob Chemother. 2018;73(8):2021–9.
Isler B, Ezure Y, Romero JLG, Harris P, Stewart AG, Paterson DL. Is ceftazidime/avibactam an option for serious infections due to extended-spectrum-beta-lactamase- and AmpC-producing enterobacterales?: a Systematic Review and Meta-analysis. Antimicrob Agents Chemother. 2020;65(1).
Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the infectious diseases society of america and the american thoracic society. Clin Infect Dis. 2016;63(5):e61–111.
Torres A, Niederman MS, Chastre J, et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: Guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociacion Latinoamericana del Torax (ALAT). Eur Respir J. 2017;50(3):1700582.
National Institute for Health and Care Excellence. Pneumonia (hospital-acquired): antimicrobial prescribing. 2019. https://www.nice.org.uk/guidance/ng139. Accessed 15 July 2020.
Sartelli M, Chichom-Mefire A, Labricciosa FM, et al. The management of intra-abdominal infections from a global perspective: 2017 WSES guidelines for management of intra-abdominal infections. World J Emerg Surg. 2017;12:29.
European Association of Urology. Guidelines on Urological Infections. 2018. https://uroweb.org/wp-content/uploads/EAU-Guidelines-on-Urological-Infections-2018-large-text.pdf. Accessed 19 February 2021.
Hawkey PM, Warren RE, Livermore DM, et al. Treatment of infections caused by multidrug-resistant Gram-negative bacteria: report of the British Society for Antimicrobial Chemotherapy/Healthcare Infection Society/British Infection Association Joint Working Party. J Antimicrob Chemother. 2018;73(suppl_3):iii2-iii78.
Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. Infectious Diseases Society of America Antimicrobial Resistant Treatment Guidance: Gram-Negative Bacterial Infections. 2020. https://www.idsociety.org/practice-guideline/amr-guidance/. Accessed 17 November 2020.
Soukup P, Faust AC, Edpuganti V, Putnam WC, McKinnell JA. Steady-state ceftazidime-avibactam serum concentrations and dosing recommendations in a critically ill patient being treated for Pseudomonas aeruginosa pneumonia and undergoing continuous venovenous hemodiafiltration. Pharmacotherapy. 2019;39(12):1216–22.
Kline EG, Nguyen MHT, McCreary EK, et al. 1298. Population pharmacokinetics of ceftazidime-avibactam among critically-ill patients with and without receipt of continuous renal replacement therapy. Open Forum Infectious Diseases. 2020;7(Supplement_1):S663-S4.
Li J, Lovern M, Riccobene T, et al. Considerations in the selection of renal dosage adjustments for patients with serious infections and lessons learned from the development of ceftazidime-avibactam. Antimicrob Agents Chemother. 2020;64(4):e02105-e2119.
Acknowledgements
Funding
The journal’s Rapid Service Fee was funded by Pfizer. No payments or honoraria were made to the authors in respect of manuscript preparation. All authors provided input at each stage of manuscript development and approved the final version for submission.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Medical Writing Assistance
Medical writing support was provided by Mark Waterlow, BSc, CMPP, of Prime Medica Ltd, Knutsford, Cheshire, UK, and was funded by Pfizer.
Disclosures
Alex Soriano has received honoraria for lectures and advisory boards from Pfizer, Merck, Angelini, Menarini, and Shionogi and research grant funding from Pfizer. Yehuda Carmeli received institutional research funding from AstraZeneca for conducting clinical studies in the development of ceftazidime-avibactam. AstraZeneca’s rights to ceftazidime-avibactam were acquired by Pfizer in December 2016. Ali S. Omrani has consulted for Pfizer (2014–2020) and Gilead (2014–2020). Luke S. P. Moore has consulted for bioMerieux (2013–2020), DNAelectronics (2015), Dairy Crest (2017–2018), and Umovis Lab (2020), has received speaker fees from Profile Pharma (2018–2019) and Pfizer (2018–2020), has received research grants from the National Institute for Health Research (2013–2019), CW + Charity (2018–2019), and Leo Pharma (2016), and has received educational support from Eumedica (2016–2018). Margaret Tawadrous and Paurus Irani are employees of and shareholders in Pfizer.
Compliance with Ethics Guideline
This article is based on published literature and does not contain any previously unreported studies with human participants or animals.
Data Availability
This article is based on published literature.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Soriano, A., Carmeli, Y., Omrani, A.S. et al. Ceftazidime-Avibactam for the Treatment of Serious Gram-Negative Infections with Limited Treatment Options: A Systematic Literature Review. Infect Dis Ther 10, 1989–2034 (2021). https://doi.org/10.1007/s40121-021-00507-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-021-00507-6