Abstract
Introduction
The coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has rapidly spread throughout China and worldwide. Little is known about the dynamic changes in the patient immune responses to SARS-CoV-2 and how different responses are correlated with disease severity and outcomes.
Methods
Seventy-four patients with confirmed COVID-19 were enrolled in this prospective research. The demographic information, medical history, symptoms, signs and laboratory results were analyzed and compared between severe and non-severe patients. The leukocytes, lymphocyte subsets and inflammatory cytokines were longitudinally collected.
Results
Of the 74 patients included, 17 suffered from severe disease. The severe patients tended be older (65.29 ± 12.33 years vs. 45.37 ± 18.66 years) and had a greater degree of underlying disease (41.18% vs. 24.56%), lower baseline lymphocyte counts [0.64 (0.46–0.95) × 109 vs. 1.27 (0.95–1.70) × 109], higher neutrophil–lymphocyte ratios [NLRs; 3.76 (3.15–5.51) vs. 2.07 (1.48–2.93)] and lower baseline eosinophil counts [0 (0–0.01) × 109 vs. 0.03 (0.01–0.06) × 109] than those in non-severe patients. The baseline helper T (Th) cells (335.47 vs. 666.46/μl), suppressor T(Ts) cells (158 vs. 334/μl), B cells (95 vs. 210/μl) and natural killer (NK) cells (52 vs. 122/μl) were significantly decreased in severe cases compared to that in non-severe cases. In addition, the baseline neutrophils were positively correlated with the severity of COVID-19, and the baseline lymphocytes were negatively correlated with the severity of COVID-19. The dynamic change of T cells, Th cells and IFN-γ in the severe cases were parallel to the amelioration of the disease.
Conclusions
Collectively, our study provides novel information on the kinetics of the immune responses in a cohort of COVID-19 patients with different disease severities. Furthermore, our study indicates that both innate and adaptive immune responses correlate with better clinical outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
COVID-19, caused by SARS-CoV-2, has rapidly spread throughout the world. |
Little is known about the dynamic changes in patient immune responses to SARS-CoV-2. |
Seventeen patients (23%) were diagnosed with severe infections, and 57 patients (77%) were categorized as non-severe. |
Both innate and adaptive immune responses correlate with better clinical outcomes of COVID-19. |
Digital Features
This article is published with digital features, including a summary slide to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.14578500.
Introduction
First reported in Wuhan in December 2019, coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has rapidly spread throughout China and worldwide [1,2,3]. Viral genome sequencing revealed SARS-CoV-2 to be a member of the β-coronavirus family, which also includes the Middle East Syndrome Coronavirus (MERS-CoV) and Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) [4, 5]. As a result of its rapid global spread and high infectiousness, the World Health Organization (WHO) declared the COVID-19 outbreak a “Public Health Emergency of International Concern” (PHEIC) on 30 January 2020. According to the official report released by the WHO, as of 22 March 2021, a total of 122,992,844 laboratory-confirmed cases have been found worldwide, with 2,711,071 of these cases being fatal. During the pandemic times, government and public health organizations have enforced several restrictions and recommendations, including continue social distancing, isolation and home confinement [6]. Subsequently, significantly diminished volumes of habitual physical activity and social connections have been observed in large populations, negatively impacting eating behaviours, sleeping patterns, social participation, mental health and life satisfaction [6,7,8,9]. Thus, to promote social inclusion and mitigate the negative psychosocial effects during COVID-19, implementation of national strategies based on digital technology is strongly suggested [6, 7, 10, 11].
The major symptoms of COVID-19 are acute viral pneumonia as well as extrapulmonary manifestations [1, 3, 12]. Patients with SARS-CoV-2 infections present a wide range of disease severity, varying from asymptomatic to critical pneumonia with respiratory failure. The immune response is an important defense against viral infections and is often found to correlate with disease severity and prognosis [13, 14]. Currently, the pathogenesis of the pulmonary and extrapulmonary manifestations of COVID-19 remains poorly understood, and our understanding of the factors that affect disease severity is limited, although older age, underlying illness, lymphopenia and “cytokine storm” have been reported, in line with SARS and MERS [1, 2, 15, 16]. However, little is known about the dynamic changes in the immune response and inflammatory cytokines and their correlation with disease severity and outcomes during infection by SARS-CoV-2.
We sought to investigate the kinetics of the immune response and how this is correlated with disease severity and outcomes in patients with COVID-19. Our study provides several novel findings in the human immune response during disease progression, indicating that SARS-CoV-2 might mainly act on different types of immune cells, such as T cells, B cells and NK cells, and induce an inflammatory cytokine storm in the body. Additionally, both innate and adaptive immune responses are correlated with clinical outcomes. Such dynamic immune responses are implicated in the disease’s progression to a more severe and lethal process, suggesting loss of the immune regulation between protective and altered responses due to exacerbation of the inflammatory components. We hope that our study will provide a better understanding of host-pathogen interactions and dynamic host immune responses to SARS-CoV-2 and that it may help in uncovering the underlying mechanisms contributing to COVID-19 pathogenesis, clinical severity classification and disease progression to design an immune intervention or preventive vaccine for COVID-19 in the foreseeable future.
Methods
Study Design and Participants
All 74 patients included in this study had been confirmed as having COVID-19 and were admitted to the First Hospital of Changsha, Changsha, China, from January 29 to February 15, 2020. The First Hospital of Changsha was designated as “the specific hospital for the treatment of severe patients with COVID-19 in Changsha” by the government during the epidemic. This study was approved by the ethics committee of the First Hospital of Changsha City (no. 2020SK3013). Written informed consent was obtained from all patients.
Definition of Severe and Non-severe Infections
COVID-19 was confirmed by detecting the presence of SARS-CoV-2 RNA in the nasopharyngeal swab samples using a virus nucleic acid detection kit (Sheng Xiang Medical Biotechnology Co., Ltd., no. 20203400064), according to the manufacturer’s protocol. Curing was defined as absence of fever for at least 3 days, substantial improvement in both lungs on chest CT, clinical remission of respiratory symptoms and two throat-swab samples negative for SARS-CoV-2 RNA obtained at least 24 h apart. The disease severity in all the hospitalized COVID-19 patients was assessed according to the highest grade reached during the whole hospitalization, based on the Seventh Revised Trial Version of the Novel Coronavirus Pneumonia Diagnosis and Treatment Guidance. Four patients evolved from non-severe to severe in our study. Patients received treatments such as lopinavir/ritonavir, a-interferon, Arbidol or combination therapy. A severe case was defined according to the following criteria: (1) respiratory distress with a respiratory rate > 30 per min; (2) pulse oximeter oxygen saturation ≤ 93% in the resting state while breathing ambient air; (3) arterial blood oxygen partial pressure (PaO2)/oxygen concentration (FiO2) ≤ 300 mmHg (1 mmHg = 0.133 kPa). All other patients were categorized as non-severe, including mild cases and ordinary cases. Mild cases are defined as the clinical symptoms being mild and no pneumonia manifestation found on imaging. Ordinary cases are defined as patients have symptoms like fever and respiratory tract symptoms, etc., and pneumonia manifestation can be seen on imaging. Asymptomatic patients were not included in our study.
Data Collection
Primary data, including demographic information, medical history, symptoms, signs, laboratory results, radiological and therapeutic characteristics, were collected from electronic medical records. The day of admission was defined as Day 1. Laboratory tests included analysis of routine blood, lymphocyte subsets, infection-related biomarkers and inflammatory cytokines, which were analyzed at different time points (Day 1, Day 8, Day 15, Day 20 and Day 25). The total number of lymphocytes in peripheral blood was counted using a hemocytometer. Lymphocyte subset percentages were determined using FACSCalibur (Becton Dickinson Co., Ltd). The absolute numbers of different lymphocyte subsets were calculated by multiplying the percentages by the total lymphocyte count. The levels of inflammatory cytokines were also determined using FACSCalibur according to the manufacturer’s instructions (Becton Dickinson Co., no. P010002, Tian Jin Kuang Bo Co., no. 20180072).
Flow Cytometry to Measure Immune Cell Quantitation, Classification and Quantitation of Cytokines
Immune cell quantitation and classification were determined using a BD Multitest™ IMK kit (BD Co., Ltd.) according to the manufacturer’s instructions, which were used with BD FACSCalibur™ flow cytometers to determine the percentages and absolute counts of the following mature human lymphocyte subsets in peripheral whole blood for immunophenotyping: T lymphocytes (CD3+), B lymphocytes (CD19+), natural killer (NK) lymphocytes (CD3–CD16+ and/or CD56+), helper/inducer T lymphocytes (CD3+CD4+) and suppressor/cytotoxic T lymphocytes (CD3+CD8+). BDMultitest™ CD3/CD8/CD45/CD4 contains FITC-labeled CD3, clone SK7; PE-labeled CD8, clone SK1; PerCP-labeled CD45, clone 2D1 (HLe-1); and APC-labeled CD4, clone SK3. BDMultitest™ CD3/CD16+CD56/CD45/CD19 contains FITC-labeled CD3, clone SK7; PE-labeled CD16, clone B73.1, and PE-labeled CD56, clone NCAM 16.2; PerCP-labeled CD45, clone 2D1 (HLe-1); and APC-labeled CD19, clone SJ25C1. We used the BD™ CBA Human Th1/Th2/Th17 Cytokine Kit (BD Co., Ltd.) to measure interleukin-2 (IL-2), interleukin-4(IL-4), interleukin-6 (IL-6), interleukin-10 (IL-10), tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ) and interleukin-17A (IL-17A) protein levels in patients according to the manufacturer’s instructions. After acquiring samples on the flow cytometer, we used FCAP Array™ software to generate results in graphical and tabular format. All the samples were measured in duplicate, and the mean values were used for analysis.
Statistical Analysis
Continuous variables were expressed as means ± standard deviation (SD) or medians with ranges, and categorical variables were expressed as frequencies and percentages. Continuous variables were compared using a t test (for a normal distribution) or a Mann-Whitney U test (for a skewed distribution). The chi-squared test or Fisher’s exact test were used to compare categorical variables. A multivariate logistic regression analysis was performed by taking the severity of COVID-19 (yes or no) as dependent variable, and variables found significant during univariate analysis were selected as independent variables. The longitudinal data from repeated measures were compared by the generalized linear mixed model. P < 0.05 was considered statistically significant. SPSS statistical software (Macintosh version 26.0, IBM, Armonk, NY, USA), and GraphPad PRISM 5.0 software (GraphPad Software, San Diego, CA, USA) and EmpowerStats (X&Y Solutions, Inc, Boston, MA, USA) were used for statistical analysis.
Results
Demographic and Clinical Characteristics
As of February 15, 2020, 74 patients who were confirmed to have COVID-19 on admission to the First Hospital of Changsha were included in our study (Table 1). Among these, 17 patients (23%) were clinically diagnosed with severe infections, with the remaining 57 patients being categorized as non-severe. The average patient age was 49.95 ± 19.28 years, and 35 patients (47.30%) were men. A total of 67 patients (90.54%) had a history of exposure to potential transmission sources (having a history of travel or residence in Wuhan and its surrounding areas or contact with infected individuals). Of the 74 patients, 21 (28.38%) had underlying diseases, including hypertension, diabetes, liver cirrhosis and cardiovascular diseases. A higher percentage of comorbidities was found in the severe patients (41.18%, n = 7) than that in the non-severe patients (24.56%, n = 14). Compared with non-severe patients, the severe patients were significantly older (65.29 ± 12.33 years vs. 45.37 ± 18.66 years; P < 0.001). There was no significant difference in sex between the severe and non-severe patients (P = 0.595). The most common symptoms revealed by our study were fever (54.05%), dry cough (41.89%), fatigue (32.43%), pharyngalgia (13.51%) and shortness of breath (12.16%). Moreover, severe patients were significantly more likely to suffer from fever (76.47% vs. 47.37%) and shortness of breath (35.29% vs. 5.26%) compared to non-severe patients.
Laboratory Findings
The laboratory findings in patients with different degrees of disease severity are shown and compared in Table 2. Among the 74 patients who underwent laboratory examinations on admission, most tended to have lower lymphocyte counts, elevated enzyme markers (i.e., creatine kinase, lactate dehydrogenase and aspartate aminotransferase) and infection-related biomarkers (i.e., erythrocyte sedimentation rate and C-reactive protein) levels compared to laboratory reference ranges. There were also numerous differences in blood cell counts, infection-related biomarkers, enzymes and other biochemical markers between the severe and non-severe patients. Severe patients tended to have a higher percentage of neutrophils (78.04% vs. 61.19%; P < 0.001), much lower lymphocytes counts [0.64 (0.46–0.95) × 109 vs. 1.27 (0.95–1.70) × 109; P < 0.001], higher neutrophil-to-lymphocyte ratios (NLRs) [3.76 (3.15–5.51) vs. 2.07 (1.48–2.93); P < 0.001] and lower eosinophil counts [0 (0–0.01) vs. 0.03 (0.01–0.06); P < 0.001]. Compared to non-severe patients, severe patients presented higher C-reactive protein levels, erythrocyte sedimentation rates, lactate dehydrogenase levels, aspartate aminotransferase levels, D-dimer levels, creatine kinase levels and lower albumin levels (P < 0.05, all).
Lymphocyte Subset Analysis
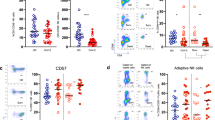
Subtypes of lymphocytes were detected in all of the 74 patients (Table 3). The total number of T cells, B cells and natural killer (NK) cells was significantly decreased in patients with COVID-19, and this was more evident in the severe group (675 vs. 1379/μl; P < 0.001) than in the non-severe group. In patients with COVID-19, NK cells were below normal levels, and T and B cells were both within the lower levels of the normal range. The levels of the three main subsets of lymphocytes were shown to be more suppressed in severe cases, as their counts were nearly half of those in non-severe patients (T cells: 500 vs. 1014/μl, P < 0.001; B cells: 95 vs. 210/μl, P < 0.001; NK cells: 52 vs. 122/μl, P < 0.001).
Different subsets of T cells were further analyzed, including helper T cells (CD3+CD4+), suppressor T cells (CD3+CD8+) and regulatory T cells (CD3+CD4+CD25+CD127low+). The levels of both helper T cells (CD3+CD4+) and suppressor T cells (CD3+CD8+) were decreased in patients with COVID-19, and this was more pronounced in severe patients compared to that in non-severe patients (335.47 vs. 666.46/μl, P < 0.001; 158 vs. 334 μl, P < 0.001). However, there was no significant difference in the percentage of regulatory T cells between severe and non-severe cases (P = 0.617). The helper T cell/suppressor T cell ratio (Th/Ts) remained in the normal range, and there was no difference between the two subgroups.
Immune Cell Factors Related to the Severity of COVID-19
The result of univariable analysis demonstrated that immune cells including lymphocytes, neutrophils, eosinophils, T cells, NK cells, Th cells and Ts cells were related to the severity of COVID-19. In multivariate logistic regression analysis, we observed that neutrophils [OR (95% CI) 3.79 (1.07, 13.46), P = 0.039] were independent risk factors for assessing the severity of COVID-19, and lymphocytes [OR (95% CI) 0.01 (0.00, 0.57), P = 0.027] were protective factors related to the severity of COVID-19 (Table 4). However, the correlation of B cells [OR (95% CI): 1.01 (1.00, 1.02), P = 0.049] and Th cells [OR (95% CI): 0.99 (0.99, 1.00), P = 0.019] with disease severity is not clear because of the small differences and relatively small sample size in our study.
Kinetics of Immune Response and Correlation with Disease Severity and Outcome
We analyzed the kinetics of white blood cells associated with disease severity and outcomes in patients with COVID-19. Significant increases in the neutrophil counts of the severe group were observed at day 8 and 15 compared to the non-severe group (Fig. 1A). With improved patient conditions, the number of neutrophils in severe patients decreased significantly after day 15. We took a generalized linear mixed model to find that the severity of disease (F = 0.719, P = 0.459) and curing time (F = 3.132, P = 0.136) were not related to neutrophil counts. The lymphocytes in the severe group were significantly lower than in the non-severe group at day 1, 8, 15, 20 and 25 (Fig. 1B). With improved patient conditions, the number of lymphocytes in severe patients gradually increased. We found the severity of disease (F = 11.244, P = 0.044) was related to lymphocyte counts, but curing time (F = 3.228, P = 0.115) was not related to lymphocyte counts in a generalized linear mixed model. At day 1, eosinophils of the severe group were significantly decreased compared to the non-severe group (Fig. 1C). At other time points, we found no significant differences in eosinophil counts between the two groups.
We further analyzed the kinetics of lymphocyte subsets associated with disease severity and outcomes in patients with COVID-19. A similar trend was observed in T cells (Fig. 2A), Ts cells (Fig. 2B) and Th cells (Fig. 2C). In severe patients, the numbers of T cells, Th cells and Ts cells increased from day 1 to day 20 and decreased after day 20. Before day 15, the numbers of T cells, Th cells and Ts cells in the severe group were significantly lower than in the non-severe group. We found that the severity of disease (F = 6.208, P = 0.047) and curing time (F = 4.730, P = 0.017) were related to T cells, and the severity of disease (F = 16.747, P = 0.009) and curing time (F = 10.727, P = 0.002) were also related to Th cells. No significant differences in NK cells were observed between the two groups during the whole observation period (Fig. 2D).
We analyzed the kinetic changes of inflammatory cytokine levels, including IL-2 (Fig. 3A), IL-4 (Fig. 3B), IL-6 (Fig. 3C), IL-10 (Fig. 3D), IL-17A (Fig. 3E), IFN-γ (Fig. 3F) and TNF–α (Fig. 3G). There were no significant differences in the levels of IL-2, IL-4, IL-17A, IFN-γ and TNF–α between the non-severe group and severe group at day 1, 8, 15, 20 and 25. All of them showed a gradual downward trend. However, in the generalized linear mixed model, we found the severity of disease (F = 10.535, P = 0.048) and curing time (F = 10.439, P = 0.023) were related to IFN-γ levels, and curing time (F = 39.345, P < 0.001) was related to TNF–α levels. IL-6 levels showed sustained increases in the severe group compared to the non-severe group until day 20. The IL-6 levels of the non-severe group remained basically unchanged. At day 1, 8, 15 and 20, the IL-6 levels in severe patients were significantly higher than non-severe patients. The IL-10 levels showed sustained increases in the severe group from day 1 to day 8. Reductions in serum IL-10 levels in the severe group started at day 8. At day 1, 8 and 15, IL-10 levels in the severe group were significantly increased compared to the non-severe group.
Discussion
The rapid and wide spread of SARS-CoV-2 infection in China and in the world has resulted in a tremendous loss of safety in peoples’ lives [17]. In this study, we systematically analyzed the clinical characteristics and dynamic changes in the immune response including changes in proinflammatory cytokines in 74 patients with different degrees of disease severity. Although the number of patients included in our study is limited, our study provides several novel findings, including the observations that SARS-CoV-2 might mainly act on lymphocytes, induces an inflammatory cytokine storm in the body and generates a series of immune responses. In addition, the dynamic changes in multiple immune cells and cytokines that we have observed, as well as their association with disease severity and outcomes during hospitalization, might help us develop effective treatment strategies and a preventive vaccine to treat and control COVID-19 in the near future. Our research helps us more clearly delineate the progression of COVID-19 in humans and also provides a scientific basis for a better understanding of its pathogenesis.
In total, old age and shortness of breath were more common in severe patients. Lymphopenia, including T cells, B cells and NK cells, and an increase in NLR were common among patients with COVID-19 and were more pronounced in the severe patients. These results are in accordance with other studies and the findings of limited autopsies and biopsies [18,19,20], which reported markedly shrunken spleens and a significant reduction in lymphocytes (Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment, 7th edition). Immune cells are important effectors of a host’s immune system and play crucial roles in anti-viral infections [21]. The protective response is T cell dependent, with CD4 helping B cells, geared toward the production of specific neutralizing antibodies, and cytotoxic CD8 cells capable of eliminating infected cells [22]. It is worth noting that 80% of the infiltrating cells in COVID-19 are CD8 [23]. CD4+ T helper cells coordinate immunity by releasing various cytokines, while CD8+ suppressor T cells directly kill the target cells during viral infections [16]. B cells perform their humoral immune function by releasing neutralizing antibodies, presenting antigens and regulating immune function. NK cells can kill non-specific target cells infected by virus and play an important role in the early anti-viral response [24]. The reduction in lymphocyte counts may allow SARS-CoV-2 to spread and progress in the early stages of an infection. Based on these data, we suggest that COVID-19 might damage immune cells, including T cells, B cells and NK cells, and that the immune system is impaired to various degrees, and this correlates with disease severity.
We also noted that the most severe patients presented higher neutrophil counts and lower lymphocytes counts, i.e., there was an increase in NLR compared to that in the non-severe patients. Transient or persistent leukocytosis, primarily due to increased levels of neutrophils, is a well-known phenomenon in systemic inflammation and infections [25, 26]. Our results are consistent with data from several studies [2, 18], suggesting that there is a serious disturbance in the internal environment, secondary bacterial infections and potential critical condition in these severe patients. These significant changes in white blood cells prompted us to quantify inflammatory cytokines. Consistently, a wide range of inflammation-related biomarkers and cytokines was elevated, and this was more evident in severe patients, suggesting that an inflammatory cytokine storm may have a role in disease progression.
Eosinophils are generally considered as multifunctional cells that function as part of the innate immune system and are associated with allergic and parasitic inflammation responses. However, several studies have shown that there is an intricate correlation between eosinophils and severe infectious diseases, including bacterial and viral infections [27, 28]. The translocation of eosinophils from the lungs of mice infected with influenza virus has been shown to reduce morbidity and viral burden, improve lung function and increase the levels of CD8(+) T cell in the airways [29]. Our study found that eosinopenia was common among patients with COVID-19, and this was more significant in the severe patients. Moreover, the number of eosinophils steadily increased as the patient’s condition improved. These data imply that eosinopenia might be considered to be a potential marker of disease severity in COVID-19 patients and that eosinophils might have a protective role in SARS-CoV-2 infections.
To investigate dynamic changes in the immune response, we further analyzed the kinetics of the immune response that were associated with clinical resolution of COVID-19. All of the recovered patients exhibited a gradual and persistent increase in lymphocyte counts, including helper T cells, suppressor T cells and NK cells, strongly suggesting that both the innate and adaptive immune systems play a protective role in fighting the SARS-CoV-2 infection. Plasma levels of cytokines and chemokines are also increased in COVID-19, but are higher in severe infections and include IL-2, IL-2R, IL-6, IL-7, IL-8 IL-10, IP10, MIP1A and TNF-α [2, 22]. High levels of plasmatic IL-6 have been consistently reported in COVID-19 and even appear to be associated with poor prognosis and risk of death [29]. Thus, its measurement has been proposed as a good biomarker to monitor these patients. Additionally, as the patients improved, the levels of most inflammatory cytokines examined, including IFN-γ, IL-10, IL-17A, IL-2, IL-4, IL-6 and TNF–α, generally decreased, indicating their potential as biomarkers and indicators of disease severity and prognosis.
There are several limitations in our study. First, it is a single-center study with a relatively small sample size. We propose that a larger cohort of patients with COVID-19 should be used to assess the dynamic changes in the immune response to avoid any potential bias. Second, all the patients studied here had recovered from COVID-19; thus, we do not have any information on the processes that occur in patients who do not recover from COVID-19. Third, only part of the immune response could be analyzed in our hospital. The immune response to SARS-CoV-2 in humans should be characterized in much more detail in the future. Fourth, asymptomatic and dead cases were not included in this study, which was unable to reflect the immune kinetics changes in all patients. In addition, Kwon et al. showed high viral load in the respiratory tract and excessive production of cytokines and chemokines were significantly associated with the severity of COVID-19 [30]. We would further study the relationship between immune response kinetics and the progression of COVID-19 (such as SARS-CoV-2 virus load, SARS-CoV-2 virus RNA shedding, imaging improvement, etc.)
Conclusions
Collectively, our study provides novel information toward understanding the kinetics of the immune response in a cohort of COVID-19 patients with different degrees of disease severity. Furthermore, our study indicates that both innate and adaptive immune responses are correlated with clinical outcomes. We hope that this study has provided evidence that sets the stage for identifying the predictors of outcomes and also potential intervention strategies for COVID-19.
References
Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;395(10223):497–506.
Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71(15):762–8.
Henry BM, Vikse J. Clinical characteristics of Covid-19 in China. N Engl J Med. 2020;382(19):1860–1.
Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–74.
Malik YS, Sircar S, Bhat S, et al. Emerging novel coronavirus (2019-nCoV)-current scenario, evolutionary perspective based on genome analysis and recent developments. Vet Q. 2020;40(1):68–76.
Ammar A, Chtourou H, Boukhris O, et al. COVID-19 home confinement negatively impacts social participation and life satisfaction: a worldwide multicenter study. Int J Environ Res Public Health. 2020;17(17):6237.
Bentlage E, Ammar A, How D, et al. Practical recommendations for maintaining active lifestyle during the COVID-19 pandemic: a systematic literature review. Int J Environ Res Public Health. 2020;17(17):6265.
Ammar A, Trabelsi K, Brach M, et al. Effects of home confinement on mental health and lifestyle behaviours during the COVID-19 outbreak: insights from the ECLB-COVID19 multicentre study. Biol Sport. 2021;38(1):9–21.
Ammar A, Brach M, Trabelsi K, et al. Effects of COVID-19 home confinement on eating behaviour and physical activity: results of the ECLB-COVID19 international online survey. Nutrients. 2020;12(6):1583.
Chtourou H, Trabelsi K, Hmida C, et al. Staying physically active during the quarantine and self-isolation period for controlling and mitigating the COVID-19 pandemic: a systematic overview of the literature. Front Psychol. 2020;11:1708.
Souissi MA, Ammar A, Trabelsi O, et al. Distance motor learning during the COVID-19 induced confinement: video feedback with a pedagogical activity improves the snatch technique in young athletes. Int J Environ Res Public Health. 2021;18(6):3069.
To KK, Tsang OT, Leung WS, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20(5):565–74.
Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39(5):529–39.
Min CK, Cheon S, Ha NY, et al. Comparative and kinetic analysis of viral shedding and immunological responses in MERS patients representing a broad spectrum of disease severity. Sci Rep. 2016;6:25359.
Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–13.
Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38(1):1–9.
Ding Q, Lu P, Fan Y, Xia Y, Liu M. The clinical characteristics of pneumonia patients coinfected with 2019 novel coronavirus and influenza virus in Wuhan. China J Med Virol. 2020;92(9):1549–55.
Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–9.
Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–33.
Zhu Y, Liu YL, Li ZP, et al. Clinical and CT imaging features of 2019 novel coronavirus disease (COVID-19). J Infect. 2020;18:147–78.
Li CK, Wu H, Yan H, et al. T cell responses to whole SARS coronavirus in humans. J Immunol. 2008;181(8):5490–500.
García LF. Immune response, inflammation, and the clinical spectrum of COVID-19. Front Immunol. 2020;11:1441.
Li G, Fan Y, Lai Y, et al. Coronavirus infections and immune responses. J Med Virol. 2020;92(4):424–32.
Thornton J. Don’t forget chronic lung and immune conditions during covid-19, says WHO. BMJ. 2020;368:m1192.
Berhane M, Melku M, Amsalu A, Enawgaw B, Getaneh Z, Asrie F. The role of neutrophil to lymphocyte count ratio in the differential diagnosis of pulmonary tuberculosis and bacterial community-acquired pneumonia: a cross-sectional study at Ayder and Mekelle Hospitals, Ethiopia. Clin Lab. 2019;65(4).
Liu X, Shen Y, Wang H, Ge Q, Fei A, Pan S. Prognostic significance of neutrophil-to-lymphocyte ratio in patients with sepsis: a prospective observational study. Mediators Inflamm. 2016;2016:8191254.
Percopo CM, Dyer KD, Ochkur SI, et al. Activated mouse eosinophils protect against lethal respiratory virus infection. Blood. 2014;123(5):743–52.
Samarasinghe AE, Melo RC, Duan S, et al. Eosinophils promote antiviral immunity in mice infected with influenza a virus. J Immunol. 2017;198(8):3214–26.
Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–43.
Bae S, Cha HH, Jung J, Kim MJ, Lee MJ, Choi SH, Chung JW, Shin EC, Kim SH. Factors of severity in patients with COVID-19: cytokine/chemokine concentrations, viral load, and antibody responses. Am J Trop Med Hyg. 2020;103(6):2412–8.
Acknowledgements
We would like to thank the participants of the study. We would like to thank all the hospital directors and laboratory staff of the study hospitals for their selfless dedication. We thank Brain for his critical reading of our manuscript
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
The collection, analysis and interpretation of data and writing the manuscript were supported by grants from Innovative Major Emergency Project Funding against the New Coronavirus Pneumonia in Changsha City and Hunan Province (nos. 2020SK3013, 2020SK3014, 2020SK3045, kq2001006 and kq2001053), National Natural Sciences Foundation of Hunan province (no. 2019JJ30041), National Natural Sciences Foundation of China (NO. 82070613) and Innovation-Driven Project of Central South University (NO.2020CX044). The journal’s Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this study, take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Authorship Contributions
Fang Zheng, Yuanlin Xie, Ning Li, and Jiyang Liu designed the study. Fang Zheng, Xin Tan and Yaxiong Huang collected the data. Ruochan Chen and Run Yao analyzed the data and wrote the manuscript. All authors read and approved the final manuscript.
Disclosures
Fang Zheng, Ruochan Chen, Run Yao, Yaxiong Huang, Xin Tan, Jiyang Liu, Ning Li and Yuanlin Xie declare that they have no competing interests.
Compliance with Ethics Guidelines
The study was approved by the Ethics Committee of First Hospital of Changsha (KL-2020006). The study was conducted in accordance with the guidelines of the Declaration of Helsinki and the principles of Good Clinical Practice. All patients provided written informed consent to include their clinical and biological data in the manuscript for scientific purposes. Data of the patients submitted were anonymized.
Data Availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Zheng, F., Chen, R., Yao, R. et al. Dynamic Changes in the Immune Response Correlate with Disease Severity and Outcomes During Infection with SARS-CoV-2. Infect Dis Ther 10, 1391–1405 (2021). https://doi.org/10.1007/s40121-021-00458-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-021-00458-y