Abstract
Up to 10 years ago the most common approach to the treatment of pediatric MS (ped-MS) was to start with IFNB or GA (so-called first-line therapies or moderate-efficacy disease-modifying therapies [ME-DMTs]) and to switch to more aggressive treatments (or high-efficacy disease-modifying therapies [HE-DMTs]) in non-responder patients. The use of HE-DMTs as first choice was recommended in selected cases with an active, aggressive form of MS. Indications for the treatment of ped-MS were essentially derived from data of observational studies. Recently, results of three randomized clinical trials have been published as well as data from many observational studies evaluating the effect of new and more active DMTs, with clear evidence that HE-DMTs are more effective than ME-DMTs. Therefore, the paradigm of treatment for patients with MS onset before 18 years of age should be changed, offering treatment with HE-DMTs as first option, because of their superior effectiveness to prevent relapses and disease progression. HE-DMTs present an overall reassuring safety profile and obtain better adherence to treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
•Differently from the adult form, no recent and specific guidelines are available for pediatric multiple sclerosis (ped-MS). Recommendations for its treatment were published many years ago when only a few disease-modifying treatments (DMTs) were available, suggesting initiating the treatment of ped-MS patients with interferon-beta (IFNB) or glatiramer acetate (GA) and using more aggressive treatments in selected patients with an active and aggressive form of MS or not responding to IFNB or GA |
•New and more efficacious DMTs are now available in many countries, and many observational studies have been published on their use in children and adolescents with MS, some of them including a large number of patients and cohorts with a long-term follow-up; three randomized clinical trials on ped-MS have been recently published |
•There is increasing evidence from observational studies in ped-MS evaluating the clinical effect of moderate-efficacy DMTs (ME-DMTs, essentially IFNB, GA, dimethyl fumarate, teriflunomide) and high-efficacy DMTs (natalizumab, fingolimod, rituximab, alemtuzumab, ocrelizumab and, according to some authors, dimethyl fumarate) showing that the latter have a stronger effect on reducing relapse rate, disability and MRI activity (in studies that have included this finding). Comparative studies have been recently published showing the overall superiority of HE-DMTs vs ME-DMTs in clinical and MRI measures of disease activity |
•Therefore, there is an urgent need to define new protocols for the treatment of ped-MS. The “old” approach, which suggested initiating the treatment of ped-MS patients with ME-DMTs, carefully assessing their effectiveness, safety and tolerability during the follow-up, and switching to more aggressive treatments if there is evidence of disease activity or concerns about safety/tolerability, should be modified in favor of using new and more active DMTs because of their superiority in measures of clinical and MRI outcome, better tolerability, with a lower rate of discontinuation, and, at present, reassuring safety profile. The issue of safety needs to be better assessed in large long-term studies |
Introduction
Many medications are currently available for the treatment of relapsing-remitting (RR) multiple sclerosis (MS), some of them also for the primary and secondary progressive form, if evidence of disease activity is demonstrated. The indications, results, limitations, safety issues and monitoring procedures of disease-modifying therapies (DMTs) have been accurately described regarding their use in adults with MS in international [1, 2] and country-specific guidelines [3,4,5,6]. Pediatric multiple sclerosis (ped-MS), which accounts for 4.4–7.8% of all MS cases in national registries [7, 8], is not included in these guidelines or, if included, is only shortly mentioned [5, 6].
No recent and specific guidelines are available for ped-MS with onset before 18 years of age. Recommendations for its treatment were published some years ago [9,10,11,12,13], when only a few DMTs were available. Since ped-MS is characterized by a higher relapse rate and lower age to reach mild and severe disability compared to the adult form, the recommendation of the International Pediatric MS Study group was to treat all pediatric patients with interferon-beta (IFNB) or glatiramer acetate (GA) soon after diagnosis is made [13]. More recently, practical indications for the treatment of ped-MS have been reported in some countries, limited to the specific utilization in that context [14, 15], and therefore they cannot be generalized elsewhere.
Only a few randomized clinical trials have been performed in ped-MS (see below); however, many observational studies have been performed evaluating the effects of DMTs in ped-MS, gathering information on their effectiveness, safety and tolerability (for review, see [9,10,11,12, 16,17,18,19,20]).
Furthermore, new DMTs are now available in many countries, and many studies have been published on their use in children and adolescents with MS. For these reasons, there is an urgent need to define protocols for the optimal treatment of ped-MS.
Up to 10 years ago, the most common approach in the treatment of ped-MS was to start prescribing first-line DMTs, essentially IFNB or GA, proposing the use of more aggressive treatments (essentially natalizumab [Nat]) in non-responder or active MS patients [11]. Since new and more efficacious DMTs are now available and studies have been published on their use (particularly Nat, fingolimod, rituximab, alemtuzumab, ocrelizumab) in ped-MS, the objective of this article is to discuss the issues that could suggest considering early treatment with high-efficacy (HF) DMTs.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Overview of Results of DMTs in Ped-MS
RCTs in Ped-MS
The effect of some new DMTs on disease activity has been well demonstrated in three randomized clinical trials (RCTs) in which the new medication was tested against an active comparator or a placebo. The results are summarized below:
-
PARADIGMS trial [21]: In this RCT, 107 subjects were randomized to receive fingolimod (Fing) and 107 to receive IFNB-1a 30 μg i.m. once a week. After 2 years of treatment, fingolimod reduced annualized relapse rate (ARR) by 82% compared to IFNB-1a, annualized rate of new or new enlarging T2 lesions by 53% and number of Gd + lesions by 66%; 85.7% vs 38.8% patients were relapse free.
-
TERIKIDS trial [22]: In this RCT, 109 patients were randomized to teriflunomide and 57 to placebo. The median time to first relapse was increased, but not significantly, in patients treated with teriflunomide (75.3 weeks vs 39.1 weeks), and the difference between active treatment vs placebo was significant only when including the finding of MRI activity (72.1 weeks vs 37.0 weeks). Active treatment reduced the number of new or enlarging T2 lesions by 55% and the number of Gd + lesions by 75%.
-
CONNECT trial [23]: Patients were randomized to receive dimethyl fumarate (DMF) (n = 75) or IFNB-1a 30 μg i.m. once a week (n = 72). The number of patients free from new or new enlarging T2 lesions was significantly higher; conversely, the number of new or enlarging T2 lesions was significantly lower in patients treated with DMF compared to IFNB-1a. The number of relapses, ARR and changes in disability were secondary endpoints and were in favor of DMF treatment.
Observational Studies
Many observational studies have been published reporting the results of IFNB and GA, extensively summarized elsewhere [10, 11]; the effect on reduction of ARR during treatment compared to pre-treatment phase is shown in Fig. 1 for studies that have reported this measure [24,25,26,27]. Data of the PARADIGMS trial have also been included [21]. The weighted mean value of ARR before and at the end of the study has also been calculated, the latter being similar to that reported in a meta-analysis (0.69) [20].
DMF became available for treatment of adult MS about 10 years ago and has been tested in ped-MS in two studies including a small number of patients, showing a reduction of ARR and MRI T2 lesions [28, 29]. In a large cohort of 168 patients, ARR decreased from 0.73 to 0.20, and from 1.2 to 0.22 in the subgroup of 100 patients with active MS—at least one relapse or by new or Gd + lesions on MRI in the preceding year; 66% and 67% of cases in the active subgroup were free from relapses and MRI activity [30]. Results of DMF (classified as ME-DMT by most authors—see also “Current use of DMTs in Ped-MS: new or high efficacy vs old or moderate efficacy DMTs”) on ARR are summarized in Fig. 1.
Among HE-DMTs, Nat has been evaluated in ped-MS in many observational studies showing a very strong effect in reducing/suppressing ARR, disability and MRI activity [30,31,32,33,34,35,36,37] and protecting against cognitive deterioration [37]. A pharmacokinetic and pharmacodynamic study has been published, supporting the use of doses administered in adults with MS [38].
Fing strongly reduced both clinical and MRI activity in an observational study including 23 patients (mean age 16 years, follow-up 11 months) who reduced ARR by 75%, new T2 lesions by 81% and Gd + lesions by 93%; the overall effect was slightly lower compared to natalizumab [34]. Fing also reduced clinical and MRI activity in a short-term observational study including 17 patients observed for a mean of 8.6 months [39]. In a cohort of 96 patients [30], ARR decreased from 0.44 to 0.13 and from 0.57 to 0.11 in the subgroup of 61 patients with active MS—at least one relapse or by new or Gd + lesions on MRI in the preceding year; 75% and 77% of cases in the active subgroup were free from relapses and MRI activity.
Rituximab (Rtx) strongly reduced clinical and MRI activity in three observational studies (see [40]) including 14 (no relapses, only 1 new MRI lesion), 11 (only 1 relapse) and 56 patients (62% lower ARR compared with injectable DMTs). A recent study [41] included 61 ped-MS patients with a follow-up of 20.9 months: ARR decreased from 0.6 to 0.03, the rate of MRI new lesions from 1.25 to 0.08 and the rate of new Gd + lesions from 0.86 to 0; 70% of patients remained NEDA-3. In a cohort of 166 patients treated with Rxt, ARR decreased from 1.01 to 0.12 and from 1.14 to 0.15 in 97 patients with active MS, defined by at least 1 relapse or by new or Gd + lesions on MRI in the preceding year. In both groups the proportion of patients without clinical or MRI activity was 84% [30].
Ocrelizumab (Ocr) reduced ARR from 2.0 to 0 and suppressed MRI activity in 10 patients after a median follow-up of 28.3 months [42]. In 37 patients treated with Ocr [30], ARR decreased from 0.71 to 0.05, and the proportion of patients free from relapses or new MRI lesions was 89%. These positive results were confirmed in the subgroup with active MS (at least 1 relapse or new/Gd + lesions on MRI in the preceding year) with a reduction of ARR from 1.5 to 0.08 and 100% all patients were free from MRI activity. The effectiveness of Ocr and Rtx was maintained in 12 patients extending the interval of administration from the standard of 6 months to 18 months [43].
In the whole cohort of 618 patients treated with HE-DMTs [30] (results of Fing, Nat, Rtx, DMF and Ocr have been separately described above), ARR decreased from 0.80 to 0.14 and from 0.99 to 0.15 in the subgroup with active MS. Respectively 77% and 76% of cases were free from relapses and MRI activity.
Alemtuzumab also was effective in some case reports of patients with aggressive MS [44,45,46].
Effective suppression of clinical and MRI activity was observed in 21 patients with ped-MS (median follow-up of 2.8 years) submitted to autologous hematopoietic stem cell transplantation (AHSCT) [47], a procedure that the authors of the study proposed as a therapeutic option for selected cases with aggressive MS not responding to standard care.
The effect of Nat, Fing, Rtx and Ocr on ARR is summarized in Fig. 2.
The weighted mean value of ARR before and during treatment with ME-DMTs and HE-DMTs is shown in Fig. 3. DMF was omitted because of its uncertain position: it was classified as a new and more effective DMT by some authors [30, 48, 49]; it was more effective than injectable DMTs in the CONNECT trial (23), but it was classified as ME-DMT in most studies, in agreement with the classification for adult MS; see “Current use of DMTs in Ped-MS: new or high efficacy vs old or moderate efficacy DMTs”). Of course, studies cannot be directly compared since patients were included and observed in different clinical settings, and probably patients were included many years ago, when only a few DMTs were available, and were differently selected. Despite these limitations, it seems reasonable to conclude that HE-DMTs are superior to injectable DMTs in reducing relapse rates. ARR decreased from 1.56 to 0.46 in patients treated with ME-DMTs and from 1.26 to 0.13 in those treated with HE-DMTs, respectively, from 1.95 to 0.54 and from 1.93 to 0.12 in patients in studies with pretreatment ARR > 1.5.
The direct comparison of clinical and MRI measures of disease activity between ME- and HE-DMTs is provided in the next section.
Current Use of DMTs in Ped-MS: New or High-Efficacy vs Old or Moderate-Efficacy DMTs
As already mentioned, up to 10 years ago the most common therapeutic approach was to initiate treatment of ped-MS patients with IFNB or GA and to move to more aggressive treatments (essentially Nat) in patients with an active and aggressive form of MS [9,10,11].
IFNB and GA continue have an important role in the treatment of ped-MS in many countries [50,51,52,53]; nevertheless, new DMTs are becoming an attractive therapeutic option. An international survey on the attitude toward use of DMTs in ped-MS reported that Fing (94%), IFNB (86%) and Nat (81%) were the most frequently prescribed DMTs, followed by Rtx (69%), GA (63%) and DMF (61%) [54]. The same order was proposed as first choice to initiate the treatment. An increasing number of patients treated with HE-DMTs is now observed in many countries and MS centers [34, 55,56,57,58,59,60]. Data from the Italian MS register showed better prognosis in ped-MS patients treated recently compared to patients treated in the past because of the earlier and more frequent use of DMTs, particularly HF-DMTs [8].
Many studies have compared the effect of new HF-DMTs with ME-DMTs, essentially IFNB and GA. A summary of the most important findings (Table 1) is reported below:
-
Huppke et al. [34], comparing patients treated with IFNB and GA (n = 133, 49% with > 12-month follow-up) with those more recently treated (n 78, 75% with > 12-month follow-up), observed a higher reduction of ARR (by 46%) and disability (by 44%) in the latter cohort because of the more frequent use of Nat (n = 30) or Fing (n = 13) and the earlier treatment initiation (8.9 vs 21 months).
-
Krysko et al. [55], in a survey of US MS centers, found an increasing number of patients treated with new DMTs after 2012 in both patients > and < 12 years of age; patients treated with a new DMT (mainly DMF and Nat, followed by Fing and Rtx, and in a few cases daclizumab, teriflunomide and alemtuzumab) comprised about 42% of the whole cohort of patients treated with DMTs. Two years later [48], data were published on the clinical outcome of 544 patients treated with injectable DMTs (mean age 13.4 years, mean treatment duration 1.8 years) and 197 patients treated with EH-DMTs (mean age 14.3 years, mean treatment duration 1.5 years, DMF n = 56, Nat n = 56, RTX n = 56, Fing n = 26, others n = 3), demonstrating a lower ARR in patients treated with new DMTs (0.17) compared to those treated with injectable DMTs (0.56) and a significant reduction in the number new/enlarging T2 and Gd + lesions.
-
In a cohort of 103 patients observed in UK [49], 89 started with injectable DMTs (IFNB n = 73, GA n = 16), 14 with newer DMTs (DMF n = 7, Fing n = 4, Nat n = 2, alemtuzumab n = 1). Almost all patients treated with new DMTs were relapse free (p < 0.0001), and many were free from new MRI lesions (p < 0.0002), different from those treated with injectable DMTs.
-
De Meo et al. [60], in a study assessing the prognostic role of clinical and MRI variables on the clinical outcome of ped-MS (123 patients) after 9 years, found a significantly higher proportion of patients relapse free in those treated with HE-DMTs (Nat n = 27, others n = 3) compared to those treated with injectable DMTs (n = 90).
-
Solmaz et al. [56], comparing 33 ped-MS patients treated with IFNB or GA (mean follow-up 6.7 years) with 10 patients treated with newer DMTs (mean follow-up 3.9 years), showed that ARR was more markedly reduced in those treated with new DMTs.
-
In a cohort of 64 patients, the probability of being relapse free at 6.5 years was 23.3% in 52 treated with ME-DMTs (IFNB, GA, DMF, teriflunomide) compared to 90.9% in those on HE-DMTs (Nat, Ocr), whereas he cumulative probability of worsening did not differ between the two groups [58].
-
The effect of Nat (111 patients), Fing (104 patients) and injectable DMTs (1003 patients) was compared in patients with a mean age of about 16 years after a follow-up of 3.9, 3.0 and 2.2 years, respectively [61]. Relapse rate in the year before treatment was respectively 1.53, 1.12 and 1.07. It decreased to 0.08, 0.12 and 0.3, respectively. The highest proportion of patients who were relapse free and persisting on DMT was observed in patients treated with Nat, the lowest in those treated with injectable DMT. Nat and Fing were more effective than injectable DMT, but Nat more significantly reduced the risk of relapses compared to injectable DMTs (HR 0.15) or to Fing (HR 0.37).
-
In a cohort of 78 ped-MS patients (median age 14.1 years, median follow-up 4.1 years), the pre-treatment ARR of 1.65 decreased to 0.45 with IFNB, to 0.27 with Fing, to 0.25 with teriflunomide, to 0.14 with DMF and to 0.03 with Nat [59]. The risk of new T2 lesions on MRI was reduced during the active treatment compared to pre-treatment phase with all DMTs but more strongly with newer DMTs.
-
In a study including 530 children with RR-MS (mean age 16.0 years, median follow-up 5.8 years) [62], 108 were treated with HE-DMT (Fing n = 36, Nat n 48) and 422 with ME-DMT (n = 268 with IFNB, n = 58 with GA and n =55 with DMF). Both treatment strategies reduced the risk of first relapse within the first 2 years, but the effect was higher with HE-DMTs compared to ME-DMTs (8% vs 20%), confirmed after a 5-year follow-up (41.3% vs 73.1). The more positive clinical outcome of patients on HE-DMTs was confirmed by measures of MRI activity. The tolerability was lower and the rate of discontinuation six times higher in patients treated with ME-DMTs.
-
In a study including 5224 ped-MS patients, recruited from the international MSBase and the Italian MS Registers (mean age 15.2 years, median observation period 5.05 years) [63], the effect of HE-DMTs (Nat, Fing, Ocr, Rtx, alemtuzumab, cladribine, daclizumab, mitoxantrone, AHSCT) was compared with ME-DMTs (DMF, GA, IFNB, teriflunomide) and with no treatment in relation to the risk of increasing disability. HE-DMTs reduced the hazard of disability worsening, particularly in participants who received the treatment while in the minimal disability state, compared with those remained untreated. Treatment with ME-DMTs also reduced the risk of disability worsening compared to untreated patients, although to a lesser extent compared to HE-DMTs. The benefit of HE-DMTs declined with increasing disability, confirming that ped-MS patients with a relapsing-remitting course should be treated early to prevent disability developing.
In a meta-analysis of studies including patients respectively treated with IFNB, Fing and Nat [20], ARR was more markedly reduced by treatment with Fing (ARR = 0.11) or Nat (ARR = 0.17) regarding IFNB (ARR = 0.69).
The effect of HE-DMT on cognition was addressed in a study showing that, in a cohort of 19 patients initially treated with IFNB, the cognitive outcome was better in those who escalated to HE-DMT (n = 6) compared to those who did not [64].
Conclusions
Only a few data are available from class 1 trials in ped-MS, as summarized in “RCTs in Ped-MS”). Given the difficulty in conducting randomized clinical trials in ped-MS, data on the effects of DMTs can be mainly obtained from observational studies, particularly from national and international registers [65]. The results of observational studies confirm that both ME-DMTs and HE-DMTs are effective and well tolerated in ped-MS patients (data on ARR before and during the treatment are summarized Figs. 1 and 2) but also provide strong and convincing evidence that HE-DMTs are more efficacious than ME-DMTs, with an overall reassuring safety profile. At present, no clear signals of mild/severe adverse events have been reported regarding what is already known in adults [30, 62]. The rate of severe adverse events was not significantly different in patients treated with ME- or HE-DMTs in one study [62]. The results of two cohorts observed > 10 years, one initially treated with IFNB or GA, the other with Nat, did not note negative events regarding safety [66, 67]. Of course, the issue of safety is of crucial importance in young patients, particularly when using new medications; a list of the most important issues was reported in a recent publication [68]. Unfortunately, RCTs do not provide meaningful data because of the low number of patients included and the relatively short duration of the study [65]. Therefore, a more precise assessment of occurrence of adverse events could be provided by national and international registers, collecting a large number of patients and observing them in the long term [65]. A project has been planned in collaboration with EMA to utilize the national register to detect adverse events related to drug exposure with the possibility to gather additional information on demographic and clinical findings, comorbidities, number and duration of previous treatments (Trojano, personal communication).
Tolerability significantly influences adherence to treatment. Tolerability was largely higher and the rate of discontinuation lower when using HE-DMTs than ME-DMTs [57, 62]. Finally, when addressing the issue of adverse events, and the balance between risks and benefit, not only the negative effects caused by medications but also those caused by the demyelinating process (occurrence of relapses, severe in many cases, risk of disability, occurrence of cognitive impairment) should be considered. Preserving quality of life is a key point in MS treatment. A study has shown an improvement of all quality of life scores in ped-MS patients treated with Fing, contrary to the deterioration in those treated with IFNB [69].
In the treatment of ped-MS patients, some findings have been supported by increasing evidence and should be considered when deciding to initiate therapy in ped-MS patients.
-
Better results are obtained when treating patients early rather than late. In ped-MS patients, at their first episode of MS, the best prognostic factor among clinical and demographic findings [70] was early treatment with DMTs. According to data of the Danish register [71], early treatment did not modify the risk of subsequent relapses, which was equally reduced in patients treated early or late (this is not surprisingly since DMTs are active on relapses anyway) but reduced the risk of disability because of their effect on pathophysiological mechanisms of brain damage. A clear effect of milder evolution in patients treated in an early phase, with a low level of disability, has been demonstrated in a large cohort of MS patients by a recent international cooperative study [63]. Data of the Italian Register have shown that early and more frequent use of DMTs, particularly those with high efficacy, leads to a reduction of mild and severe disability (by 50%–70%), supporting the concept of early and active treatment of ped-MS [8].
-
Relapses are more frequent in ped- than adult-MS [7, 72]. In young patients, relapses present a higher capability to recover after the acute phase compared to older patients [73], a factor explaining the longer interval to reach mild and severe disability in ped-MS [7, 74, 75]. Medications targeting the inflammatory phase of the disease, and consequently reducing the risk of relapses, are expected to have a greater effect in young than old patients. Data from clinical trials support this finding [76, 77].
-
The ped-MS patients reach mild and severe disability at a younger age, about 10 years earlier, than adult MS-patients [7, 74, 75]. The objective of a treatment is to delay the development of disability, promoting compensatory mechanisms, per se more active in young patients [78, 79].
-
Cognitive impairment is present in about 1/3 of ped-MS patients [79, 80], increasing with time and affecting social outcome [81]. Some preliminary data show an effect of DMTs, particularly HE-DMTs, protecting against cognitive impairment [27, 37, 64].
Finally, many patients switch to more aggressive treatments after initiation with ME-DMT [66], so why not start immediately with HF-DMTs and limit the use of ME-DMTs in selected mild cases, with favorable prognostic factors, or if problems of costs and medication availability are present or if HE-DMTs cannot be used because of clinical contraindications?
To conclude, the scenario has changed in recent years:
-
Many new medications have been introduced in the armamentarium of MS treatment, with different mechanisms of action, targeting different pathogenetic mechanisms, with a different spectrum of efficacy and tolerability and different routes of administration, offering many alternatives to injectable DMTs and making it possible to personalize MS treatment [65]. Adverse events are well known from data of RCTs and post-marketing studies and should be carefully considered in the final choice of a medication together with the evaluation of the effectiveness.
-
Changes have occurred in the prescription of medications in ped-MS, with increasing use of HE-DMTs in both children and adolescents, with a better effect on clinical outcome.
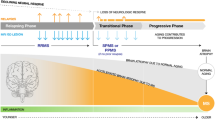
As summarized in Fig. 4, the “old” approach that suggested initiating treatment of ped-MS patients with ME-DMTs, carefully assessing their effectiveness, safety and tolerability during follow-up and switching to more aggressive treatments if evidence of disease activity or concerns about tolerability, should be modified in favor of using new and more active DMTs because of their superiority in measures of clinical and MRI outcome and their better tolerability, with a lower rate of discontinuation.
Change history
30 June 2024
The original online version of this article was revised to remove the red lines from the figure text
References
Montalban X, Gold R, Thompson AJ, et al. ECTRIMS/EAN Guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler. 2018;24:96–120.
Rae-Grant A, Day GS, Marrie RA, et al. Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90:777–88.
Cristiano E, Alonso R, Alvez Pinheiro A, et al. Argentinean recommendations on the identification of treatment failure in relapsing remitting multiple sclerosis patients. J Neurol Sci. 2018;385:217–24.
Marques VD, Passos GRD, Mendes MF, et al. Brazilian Consensus for the Treatment of Multiple Sclerosis: Brazilian Academy of Neurology and Brazilian Committee on Treatment and Research in Multiple Sclerosis. Arq Neuropsiquiatr. 2018;76:539–54.
Yamout B, Sahraian M, Bohlega S, et al. Consensus recommendations for the diagnosis and treatment of multiple sclerosis: 2019 revisions to the MENACTRIMS guidelines. Mult Scler Relat Disord. 2020;37: 101459.
Freedman MS, Devonshire V, Duquette P, the Canadian MS Working Group, et al. Can treatment optimization in multiple sclerosis: Canadian MS Working Group Recommendations. J Neurol Sci. 2020; 47:437–455.
McKay KA, Hillert J, Manouchehrinia A. Long-term disability progression of pediatric-onset multiple sclerosis. Neurology. 2019;92:e2764–73.
Baroncini D, Simone M, Iaffaldano P, Italian MS registry, et al. Risk of persistent disability in patients with pediatric-onset multiple sclerosis. JAMA Neurol. 2021; 78:726–735.
Ghezzi A, Banwell B, Boyko A, et al. The management of multiple sclerosis in children: a European view. Mult Scler. 2010;16:1258–67.
Ghezzi A, Amato MP, Makhani N, et al. Pediatric multiple sclerosis: conventional first-line treatment and general management. Neurology. 2016;87(9 Suppl 2):S97–102.
Chitnis T, Ghezzi A, Bajer-Kornek B, et al. Pediatric multiple sclerosis: escalation and emerging treatments. Neurology. 2016;87(9 Suppl 2):S103–9.
Waldman A, Ghezzi A, Bar-Or A, et al. Multiple sclerosis in children: an update on clinical diagnosis, therapeutic strategies, and research. Lancet Neurol. 2014;13:936–48.
Chitnis T, Tenembaum S, Banwell B, International Pediatric Multiple Sclerosis Study Group, et al. Consensus statement: evaluation of new and existing therapeutics for pediatric multiple sclerosis. Mult Scler. 2012;18:116–27.
Bunyan RF, AbdulSalam AM, Albarakati RG, et al. Saudi consensus recommendations on the management of multiple sclerosis: MS management in children and adolescents. Mult Scler Relat Disord. 2022;66: 104061.
Krupp LB, Vieira MC, Toledano H, Peneva D, Druyts E, Wu P, Boulos FC. A review of available treatments, clinical evidence, and guidelines for diagnosis and treatment of pediatric multiple sclerosis in the United States. J Child Neurol. 2019;34:612–20.
Alroughani R, Boyko A. Pediatric multiple sclerosis: a review. BMC Neurol. 2018;18(1):27.
Margoni M, Rinaldi F, Perini P, Gallo P. Therapy of pediatric-onset multiple sclerosis: state of the art, challenges, and opportunities. Front Neurol. 2021;12: 676095.
Jakimovski D, Awan S, Eckert SP, et al. Multiple sclerosis in children: differential diagnosis, prognosis, and disease-modifying treatment. CNS Drugs. 2022;36:45–59.
Nicotera AG, Spoto G, Saia MC, et al. Treatment of multiple sclerosis in children: a brief overview. Clin Immunol. 2022;237: 108947.
Graves JS, Thomas M, Li J, et al. Improving pediatric multiple sclerosis interventional phase III study design: a meta-analysis. Ther Adv Neurol Disord. 2022;15:1–13.
Chitnis T, Arnold DL, Banwell BW, PARADIGMS Study Group, et al. Trial of fingolimod versus interferon β-1a in pediatric multiple sclerosis. N Engl J Med. 2018;379:1017–27.
Chitnis T, Banwell B, Kappos L, TERIKIDS Investigators, et al. Safety and efficacy of teriflunomide in paediatric multiple sclerosis (TERIKIDS): a multicentre, double-blind, phase 3, randomised, placebo-controlled trial. Lancet Neurol. 2021;20:1001–11.
Vermersch P, Scaramozza M, Levin S, et al. Effect of dimethyl fumarate vs interferon β-1a in patients with pediatric-onset multiple sclerosis: the CONNECT randomized clinical trial. JAMA Netw Open. 2022;5(9): e2230439.
Pohl D, Rostasy K, Gartner J, et al. Treatment of early onset multiple sclerosis with subcutaneous interferon β-1a. Neurology. 2005;65:888–90.
Ghezzi A, Amato MP, Annovazzi P, et al. Long-term results of immunomodulatory treatment in children and adolescents with multiple sclerosis: the Italian experience. Neurol Sci. 2009;30:193–9.
Tenembaum S, Banwell B, Pohl D, et al. Subcutaneous interferon β-1a in pediatric multiple sclerosis: a retrospective study. J Child Neurol. 2013;28:849–56.
Gärtner J, Brück W, Weddige A, et al. Interferon β-1b in treatment-naïve paediatric patients with relapsing-remitting multiple sclerosis: two-year results from the BETAPAEDIC study. Mult Scler J Exp Transl Clin. 2017;26:3.
Alroughani R, Huppke P, Mazurkiewicz-Beldzinska M, et al. Delayed-release dimethyl fumarate safety and efficacy in pediatric patients with relapsing-remitting multiple sclerosis. Front Neurol. 2021;11: 606418.
Makhani N, Schreiner T. Oral dimethyl fumarate in children with multiple sclerosis: a dual-center study. Pediatr Neurol. 2016;57:101–4.
Shukla N, Casper TC, Ness J, et al. Demographic features and clinical course of patients with pediatric-onset multiple sclerosis on newer disease-modifying treatments. Pediatr Neurol. 2023;145:125–31.
Kornek B, Aboul-Enein F, Rostasy K, et al. Natalizumab therapy for highly active pediatric multiple sclerosis. JAMA Neurol. 2013;70:469–75.
Ghezzi A, Moiola L, Pozzilli C, et al. Natalizumab in the pediatric MS population: results of the Italian registry. BMC Neurol. 2015;2515:174.
Arnal-Garcia C, García-Montero MR, Málaga I, et al. Natalizumab use in pediatric patients with relapsingremitting multiple sclerosis. Eur J Paediatr Neurol. 2013;17:50–4.
Huppke P, Huppke B, Ellenberger D, et al. Therapy of highly active pediatric multiple sclerosis. Mult Scler. 2019;25:72–80.
Alroughani R, Ahmed SF, Behbehani R, et al. The use of natalizumab in pediatric patients with active relapsing multiple sclerosis: a prospective study. Pediatr Neurol. 2017;70:56–60.
Palavra F, Figueiroa S, Correia AS, et al. TyPed study: natalizumab for the treatment of pediatric-onset multiple sclerosis in Portugal. Mult Scler Relat Disord. 2021;51: 102865.
Margoni M, Rinaldi F, Riccardi A, et al. No evidence of disease activity including cognition (NEDA-3 plus) in naive pediatric multiple sclerosis patients treated with natalizumab. J Neurol. 2020;267:100–5.
Ghezzi A, Comi G, Grimaldi LM, et al. Pharmacokinetics and pharmacodynamics of natalizumab in pediatric patients with RRMS. Neurol Neuroimmunol Neuroinflamm. 2019;6(5): e591.
Fragoso YD, Alves-Leon SV, Barreira AA, et al. Fingolimod prescribed for the treatment of multiple sclerosis. Pediatr Neurol. 2015;53:166–8.
Ghezzi A, Banwell B, Bar-Or A, et al. Rituximab in patients with pediatric multiple sclerosis and other demyelinating disorders of the CNS: practical considerations. Mult Scler. 2021;27:1814–22.
Breu M, Sandesjö F, Milos RI, et al. Rituximab treatment in pediatric-onset multiple sclerosis. Eur J Neurol. 2024;00: e16228.
Bibinoglu Amirov CB, Saltık S, Cengiz YC, et al. Ocrelizumab in pediatric multiple sclerosis. Eur J Paediatr Neurol. 2023;43:1–5.
Venet M, Lepine A, Maarouf A, et al. Control of disease activity with large extended-interval dosing of rituximab/ocrelizumab in highly active pediatric multiple sclerosis. Mult Scler. 2024;30:261–5.
Margoni M, Rinaldi F, Miante S, et al. Alemtuzumab following natalizumab in highly active paediatric-onset multiple sclerosis. Mult Scler J Exp Transl Clin. 2019;5(3):205521731987547.
Baroncini D, Annovazzi P, Guaschino C, et al. Long-term remission of tumefactive relapsing multiple sclerosis after alemtuzumab rescue treatment in an adolescent patient. Mult Scler Relat Disord. 2020;41: 102061.
Jure Hunt D, Traboulsee A. Short-term outcomes of pediatric multiple sclerosis patients treated with alemtuzumab at a Canadian University multiple sclerosis clinic. Mult Scler J. 2020;6:2.
Burman J, Kirgizov K, Carlson K, et al. Autologous hematopoietic stem cell transplantation for pediatric multiple sclerosis: a registry-based study of the Autoimmune Diseases Working Party (ADWP) and Pediatric Diseases Working Party (PDWP) of the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant. 2017;52:1133–7.
Krysko KM, Graves JS, Rensel M, US Network of Pediatric MS Centers, et al. Real-world effectiveness of initial disease-modifying therapies in pediatric multiple sclerosis. Ann Neurol. 2020;88:42–55.
Abdel-Mannan OA, Manchoon C, Rossor T, UK-Childhood Inflammatory Disease Network, et al. Use of disease-modifying therapies in pediatric relapsing remitting multiple sclerosis in the United Kingdom. Neurol Neuroimmunol Neuroinflamm. 2021;8(4): e1008.
Frahm N, Peters M, Bätzing J, et al. Treatment patterns in pediatric patients with multiple sclerosis in Germany—a nationwide claim-based analysis. Ther Adv Neurol Disord. 2021;14:1–12.
Greenberg B, Kolodny S, Wang M. Disease-modifying therapy in pediatric patients with multiple sclerosis in the United States. Int J MS Care. 2021;23:101–5.
Vališ M, Zbyšek Pavelek Z, Novotný M, et al. Analysis of the group of pediatric patients with relapsing-remitting multiple sclerosis: data from the Czech National Registry. Front Neurol. 2022;13: 851426.
Henderson M, Horton DB, Bhise V, et al. Initiation patterns of disease-modifying therapies for multiple sclerosis among US adults and children, 2001 through 2020. JAMA Neurol. 2023;80:860–7.
Sandesjo F, Wassmer E, Deiva K, et al. Current international trends in the treatment of multiple sclerosis in children—impact of the COVID-19 pandemic. Mult Scler Relat Disord. 2021;56: 103277.
Krysko KM, Graves J, Rensel M, US Network of Pediatric MS Centers, et al. Use of newer disease-modifying therapies in pediatric multiple sclerosis in the US. Neurology. 2018;91:e1778–87.
Solmaz I, Acar Ozen P, Parlak S, et al. Newer disease modifying treatments in pediatric onset multiple sclerosis: experience from a single center. Eur J Paediatr Neurol. 2022;39:110–5.
Erdal JL, Kopp TI, Blinkenberg M, et al. Clinical characteristics and use of disease modifying therapy in the nationwide Danish cohort of paediatric onset multiple sclerosis. Mult Scler Relat Disord. 2020;37: 101431.
Moreau A, Kolitsi I, Kremer L, et al. Early use of high efficacy therapies in pediatric forms of relapsing-remitting multiple sclerosis: a real-life observational study. Mult Scler Relat Disord. 2023;79: 104942.
Saponaro A, Tully T, Maillart E, et al. Treatments of paediatric multiple sclerosis: efficacy and tolerance in a longitudinal follow-up study. Eur J Paediatr Neurol. 2023;45:22–8.
De Meo E, Bonacchi R, Moiola L, et al. Early predictors of 9-year disability in pediatric multiple sclerosis. Ann Neurol. 2021;00:1–12.
Spelman T, Simoneau G, Hyde R, et al. Comparative effectiveness of natalizumab, fingolimod, and injectable therapies in pediatric-onset multiple sclerosis. A registry-based study. Neurology. 2024;102: e208114.
Benallegue N, Rollot F, Wiertlewski S, et al. Highly effective therapies as first-line treatment for pediatric-onset multiple sclerosis. JAMA Neurol. 2024;81:273–82.
Sharmin S, Roos I, Malpas CB et al. Disease-modifying therapies in managing disability worsening in paediatric-onset multiple sclerosis: a longitudinal analysis of global and national registries. Lancet Child Adolesc Health. 2024;8:348–357
Johnen A, Elpers C, Riepl E, et al. Early effective treatment may protect from cognitive decline in paediatric multiple sclerosis. Eur J Paediatr Neurol. 2019;23:783–91.
Ghezzi A, Amato MP, Edan G, et al. The introduction of new medications in pediatric multiple sclerosis: open issues and challenges. Mult Scler. 2021;27:479–82.
Hacohen Y, Banwell B, Ciccarelli O. What does first-line therapy mean for paediatric multiple sclerosis in the current era? Mult Scler. 2021;27:1970–6.
Baroncini D, Zaffaroni M, Moiola L, et al. Long-term follow-up of pediatric MS patients starting treatment with injectable first-line agents: a multicentre, Italian, retrospective, observational study. Mult Scler. 2019;25:399–407.
Baroncini D, Ghezzi A, Guaschino C, MS Study Group of the Italian Neurological Society, et al. Long-term follow-up (up to 11 years) of an Italian pediatric MS cohort treated with Natalizumab: a multicenter, observational study. Neurol Sci. 2022;43:6415–23.
Krupp L, Banwell B, Chitnis T, et al. Effect of fingolimod on health-related quality of life in paediatric patients with multiple sclerosis: results from the phase 3 PARADIGMS Study. BMJ Neurol Open. 2022;4(1): e000215.
Iaffaldano P, Simone M, Lucisano G, Italian iMedWeb Registry and the MSBase Registry, et al. Prognostic indicators in pediatric clinically isolated syndrome. Ann Neurol. 2017;81:729–39.
Kopp TI, Blinkenberg M, Chalmer TA, et al. Predictors of treatment outcome in patients with paediatric onset multiple sclerosis. M Mult Scler. 2020;26:964–75.
Gorman MP, Healy BC, Polgar-Turcsanyi M, et al. Increased relapse rate in pediatric-onset compared with adult-onset multiple sclerosis. Arch Neurol. 2009;66:54.
Chitnis T, Aaen G, Belman A, US Network of Paediatric Multiple Sclerosis Centers, et al. Improved relapse recovery in paediatric compared to adult multiple sclerosis. Brain. 2020;143(9):2733–41.
Renoux C, Vukusic S, Mikaeloff Y, Adult Neurology Departments KIDMUS Study Group, et al. Natural history of multiple sclerosis with childhood onset. N Engl J Med. 2007;356:2603–13.
Harding KE, Liang K, Cossburn MD, et al. Neurol long-term outcome of paediatric-onset multiple sclerosis: a population-based study. J Neurol Neurosurg Psych. 2013;84:141.
Gärtner J, Chitnis T, Ghezzi A, et al. Relapse rate and MRI activity in young adult patients with multiple sclerosis: a post hoc analysis of phase 3 fingolimod trials. Mult Scler J Exp Transl Clin. 2018;4(2):2055217318778610.
Ghezzi A, Chitnis T, K-Laflamme A, et al. Long-term effect of immediate versus delayed fingolimod treatment in young adult patients with relapsing-remitting multiple sclerosis: pooled analysis from the FREEDOMS/FREEDOMSII trials. Neurol Ther. 2020;9:193–5.
Ghezzi A, Baroncini D, Zaffaroni M, et al. Pediatric versus adult MS: similar or different? Multi Scler Demyelinating Disord. 2017;2:5.
Graves JS, Krysko KM, Hua LH, et al. Ageing and multiple sclerosis. Lancet Neurol. 2023;22:66–77.
Amato MP, Krupp LB, Charvet LE, et al. Pediatric multiple sclerosis: cognition and mood. Neurology. 2016;87(9 Suppl 2):S82–7.
Portaccio E, Bellinvia A, Razzolini L, MS Study Group of the Italian Neurological Society, et al. Long-term cognitive outcomes and socioprofessional attainment in people with multiple sclerosis with childhood onset. Neurology. 2022;98:e1626–36.
Acknowledgements
Medical Writing and Editorial Assistance
The author did not receive any medical writing or editorial assistance for this article.
Funding
No funding or sponsorship was received for this study or publication of this article.
Author information
Authors and Affiliations
Contributions
Angelo Ghezzi is the corresponding author for this article and prepared this article for publication.
Corresponding author
Ethics declarations
Conflict of Interest
Angelo Ghezzi has nothing to disclose. Angelo Ghezzi is an Editorial Board member of Neurology and Therapy. Angelo Ghezzi was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Additional information
The original online version of this article was revised to remove the red lines from the figure text.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ghezzi, A. Old and New Strategies in the Treatment of Pediatric Multiple Sclerosis: A Personal View for a New Treatment Approach. Neurol Ther (2024). https://doi.org/10.1007/s40120-024-00633-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40120-024-00633-6