Abstract
Introduction
This study aimed to explore influencing factors and clinical significance of ultra-long-term microischemia following intracranial aneurysm (IA) embolization and establish a theoretical foundation for reducing both the incidence of ultra-long-term microischemia and cognitive dysfunction in patients post embolization.
Methods
A retrospective analysis was conducted on data from 147 patients who received endovascular treatment for IAs. Patients were categorized into microischemic and control (non-microischemic) groups on the based on the findings of high-resolution magnetic resonance vessel wall imaging (HR-VWI) examinations performed 3 days postoperatively and 6 months postoperatively. Risk factors for the occurrence of ultra-long-term microischemia were determined by univariate analysis and multivariate logistic regression analysis.
Results
Out of 147 patients included in the study, 51 (34.69%) developed microischemia while the remaining 96 (65.31%) did not experience this condition. Analysis revealed that factors such as sex, age, history of underlying diseases (hypertension, diabetes mellitus), aneurysmal site characteristics, the presence or absence of stenosis in the aneurysm-bearing artery, modified Fisher score at admission, Barthel’s index at discharge, immunoinflammatory index at 3 days postoperatively and at the 6-month follow-up, the presence or absence of aneurysmal wall enhancement, and the presence or absence of aneurysmal lumen showed no statistically significant differences between the two groups (all P > 0.05). By contrast, variables like in operative time, rupture status of the aneurysm before surgery according to World Federation of Neurologic Surgeons (WFNS) grade, aneurysm size, number of stents used, number of guidewires and catheters used, and Evans index between the two groups were found to have statistically significant disparities between those who developed microischemia and those who did not (P < 0.05). A subsequent multivariate analysis revealed that aneurysm size, Evans index, and the number of stents used were independent risk factors for the occurrence of ultra-long-term microischemia after surgical intervention of aneurysms (P < 0.05). The receiver operating characteristic (ROC) curves of the patients were constructed on the basis of risk factors determined through multivariate logistic regression analysis. Results indicated that aneurysm size (area under ROC curve (AUC) 0.619, sensitivity 94.7%, specificity 17.1%, P = 0.049), Evans index (AUC 0.670, sensitivity 96.4%, specificity 26.8%, P = 0.004), and number of stents (AUC 0.639, sensitivity 44.6%, specificity 90.2%, P < 0.001) effectively predicted the occurrence of microischemia. The incidence of cognitive dysfunction was higher in the microischemic group than in the control group (P < 0.05), and a greater number of microischemic foci was associated with a higher incidence of cognitive dysfunction. The proportion of microschemia foci in the thalamus and basal ganglia in patients with cognitive dysfunction (60.87%) was significantly higher than that in patients without cognitive dysfunction (34.55%) (P < 0.05).
Conclusion
Aneurysm size, Evans index > 0.3, and the quantity of stents were independent risk factors for the occurrence of ultra-long-term microischemia after aneurysm embolization and provided good predictive performance. Cognitive dysfunction was closely associated with microischemia, with its severity increasing with an increase in the number of ischemic foci.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? | |
The stratified management of patients with aneurysmal subarachnoid hemorrhage (aSAH) should not be merely limited to the hospitalization period but should also involve comprehensive assessment of long-term patient prognosis. | |
There remains a lack of convincing research evidence of the association between the occurrence of cerebral microischemia during ultra-long-term follow-up (≥ 6 months) of aSAH and cognitive dysfunction. | |
What was learned from the study? | |
A large aneurysm size, Evans index > 0.3, and an increase in the number of stents lead to an increased incidence of ischemic events after embolization and are independent risk factors for the occurrence of long-term microischemia after aneurysm embolization. | |
The occurrence of long-term microischemia is closely associated with cognitive dysfunction. |
Introduction
Aneurysmal subarachnoid hemorrhage (aSAH) is a severe type of stroke resulting from the rupture of an intracranial aneurysm (IA) [1]. It not only has high morbidity and mortality rates but also leads to dependence on daily care for about one-third of survivors [2]. Long-term disability and mortality following aSAH can result in up to 27% loss of potential productive life years [3]. Given that most patients with aSAH develop the condition during the relatively young middle age range, survivors inevitably place greater demands on their return to the workforce, improved quality of life, and number of productive life years [4].
With advances in medical imaging technology and improvements in endovascular intervention techniques and materials, endovascular intervention has gradually replaced traditional aneurysm clipping surgery as the first-choice and mainstream treatment modality for ruptured or unruptured IAs [5,6,7]. However, with the continuous emergence of new interventional methods and materials and the use of a wide variety of novel materials, some patients continue to exhibit a poor prognosis despite recent improvements in the success rate of aneurysm embolization [8]. Thus, whether such novel methods and materials increase the incidence of complications or even introduce new complications after interventional embolization remains unclear.
In addition, much attention on cerebral ischemia has focused on the occurrence or non-occurrence of delayed cerebral ischemia (DCI) [9]. Researchers have acknowledged that delayed DCI is a complex process that develops over time and plays a crucial role in the diagnosis and treatment of IAs. Studies have shown that DCI persists not only during the entire clinical trajectory but also can impact patients with aSAH even beyond the perioperative phase, extending into long-term prognosis [10]. However, there is a current lack of clinical research related to microischemia during ultra-long-term follow-up (≥ 6 months), like imaging studies on microischemia, and investigations into the significance of ultra-long-term microischemia in patient recovery. This study employs high-resolution magnetic resonance vessel wall imaging (HR-VWI) to observe the clinical imaging data of patients with aSAH in the perioperative period and within 180 days after surgical intervention. Risk factors for the occurrence of ultra-long-term microischemia were subsequently analyzed to determine the probability and risk of occurrence of cerebral microischemia after aneurysm embolization. These results can serve as a theoretical basis for the reduction of microischemia occurrence in patients after IA embolization.
Methods
General Data
A total of 147 patients diagnosed with IA were included in this study (Fig. 1), conducted at the Department of Neurosurgery, The Second Affiliated Hospital of Chongqing Medical University and Chongqing University Three Gorges Hospital from June 2022 to June 2023. We analyzed the data from the patients’ 6-month follow-up after surgery and their previous clinical data. The group comprised 52 men and 95 women, ranging in age from 38 to 79 years, with a mean age of 56.54 ± 9.30 years.
The inclusion criteria were as follows: (1) patients who were diagnosed as having IA by computed tomography angiography (CTA) or digital subtraction angiography (DSA); (2) patients who underwent endovascular embolization within 3 days of onset; (3) patients who received multimodal cranial HR-VWI at 3 days postoperatively; (4) patients who received blood biochemical indicator testing and multimodal cranial HR-VWI during the 6-month follow-up after discharge. This study was reviewed and approved by the ethics committee of Chongqing University Three Gorges Hospital under project number [2024] 16. All subjects or their legal representatives were informed of the nature of the study, fully understood the provisions in the protocol, and were able to ensure compliance. Informed consent was acquired from each patient and/or their legal surrogates according to the Declaration of Helsinki. All the patients agreed to participate in the study and gave consent for their identifiable data to be used for the study and publication. The exclusion criteria were as follows: (1) patients with concurrent systemic infectious diseases, severe organ dysfunction, or other contraindications to surgery; (2) patients with dissecting aneurysms, pseudoaneurysms, or multiple IAs; (3) pregnant women with IA complications; (4) cases of intraoperative acute thrombosis or aneurysm rupture during surgery; (5) possible non-compliance with study protocol (including the follow-up period), such as patients with mental abnormalities, alcoholism, or drug abuse; (6) patients with missing 6-month multimodal cranial HR-VWI data.
Multimodal Cranial HR-VWI Examination
The surgical procedures of all patients were performed by the same treatment team, and pertinent data of the patients were recorded. Magnetic resonance imaging (MRI) sequences used included the T1- and T2-weighted imaging, time-of-flight magnetic resonance angiography (TOF MRA), T2-weighted fluid-attenuated inversion recovery (T2-FLAIR), diffusion-weighted imaging (DWI), and high-resolution sequences. High-resolution vessel wall imaging (HR-VWI) is an imaging method that utilizes the principle of magnetic resonance to suppress blood flow signals within blood vessels in order to obtain images of static tissues, such as vascular walls, it allows for the clear visualization of the vessel lumen, wall, and perivascular structures, and can be used for assessing vascular wall lesions, measuring vessel diameter, and assessing the extent of vascular spasmodic contractions [11,12,13]. The scanning parameters for the T2-FLAIR sequence were TR/TI/TE = 10,000/2800/14010, 000/2800/120 ms, voxel sizes of 0.7 × 0.7 × 5 mm3 and 0.4 × 0.4 × 4 mm3, and layer spacing of 1 mm. The scanning parameters for the DWI sequence were TR/TE = 3348/98 and 3015/68 ms, and b-factor = 2 (0 and 1000 s/mm2). Indicator measurements were made through simultaneous assessment of T2-FLAIR and DWI image quality, number of microischemic foci, and locations of the foci by two physicians with associate or higher titles in radiology and neurosurgery. Two assessors independently evaluated the number and location of microvascular lesions. In case of disagreement, a more experienced radiologist made the final judgment.
Surgical Procedures
All interventional surgical procedures were performed under general anesthesia via the femoral arterial approach by neurosurgeons of the same interventional team. Patients with ruptured aneurysms received dual antiplatelet therapy (aspirin 300 mg and clopidogrel 225 mg) at 2 h preoperatively. Unruptured aneurysms were treated with dual antiplatelet therapy (aspirin 100 mg/day and clopidogrel 75 mg/day) for at least 3 days preoperatively. During surgery, the right femoral artery was first punctured for the insertion of an introducer sheath and introducer catheter by the Seldinger method. After systemic heparinization, whole-brain angiography was performed using an angiographic pigtail catheter. The microcatheter was shaped at the optimal working angle and subsequently implanted into the aneurysmal cavity. Dense packing was performed with appropriate coils selected on the basis of intraoperative situation, and patients underwent adjunctive embolization on an as-needed basis with appropriate stents. An angiographic re-examination was conducted to determine the effect of embolization. Upon confirming that the aneurysm-bearing artery was unobstructed and the contrast agent had not been retained, the catheter and arterial sheath were slowly withdrawn, and a compression bandage was applied to the puncture site of the femoral artery.
Patients who underwent simple aneurysm embolization did not receive postoperative antiplatelet therapy, while patients treated with stent placement received postoperative dual antiplatelet therapy (aspirin 100 mg qd combined with ticagrelor 90 mg bid or clopidogrel 75 mg qd) for 3 months and single antiplatelet therapy (aspirin 100 mg qd, or ticagrelor 90 mg qd, or clopidogrel 75 mg qd) for 1 year. The effectiveness of dual antiplatelet therapy was determined by platelet aggregation rate, with an arachidonic acid (AA) inhibition rate within 50–75% and an ADP inhibition rate within 30–75% used as benchmarks. Patients with aspirin resistance and/or the CYP2C19 slow metabolizer genotype received antiplatelet therapy with ticagrelor, and platelet aggregation rate was monitored to ensure that the standard had been met.
Patient Grouping, Definitions, and Observation Indicators
Definitions
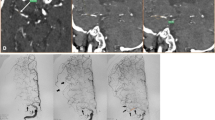
Criteria for microischemia were (i) multimodal HR-VWI was performed in all patients at 3 days postoperatively and at the 6-month follow-up after discharge. Microischemia was defined as lesions ≤ 15 mm in diameter with high signals on high-resolution T2-FLAIR that had appeared during follow-up (Fig. 2a, b); (ii) Evans index (EI), calculated as the maximum frontal horn width divided by the maximum biparietal diameter at an equivalent level (Fig. 2c) was used to diagnose ventricular enlargement; an EI value > 0.3 indicated ventricular enlargement [14].
Patient Grouping
The patients were divided into microischemic and control (non-microischemic) groups based on the findings of HR-VWI examinations: (i) the microischemic group comprised patients in whom microischemic foci were detected by HR-VWI at the 6-month follow-up after discharge; (ii) the control (non-microischemic) group comprised patients in whom microischemic foci were not found by HR-VWI at the 6-month follow-up after discharge.
Observation Indicators
Cognitive dysfunction diagnosis at the 6-month follow-up post discharge involved assessing patients using the Clinical Dementia Rating (CDR) scale. A CDR global score (CDR-GS) of 0 indicates normal; 0.5 indicates suspected dementia; 1 indicates mild dementia; 2 indicates moderate dementia; 3 indicates severe dementia. The CDR scale was independently evaluated by two neurologists. In case of disagreement, a more experienced neurologists made the final judgment. Patients were categorized into five grades: normal, questionable, mild, moderate, and severe. Patients assigned the normal grade were defined as having no cognitive dysfunction, while patients assigned the other four grades (questionable, mild, moderate, and severe) were defined as having cognitive dysfunction.
The clinical indicators considered included a history of hypertension or diabetes mellitus; aneurysmal site: anterior circulation (anterior cerebral artery aneurysm, anterior communicating artery aneurysm, middle cerebral artery aneurysm) and non-anterior circulation aneurysm, aneurysm size (calculated as the product of longest length and width); presence or absence of rupture and hemorrhage; World Federation of Neurologic Surgeons (WFNS) grade; modified Fisher score; Barthel’s index at discharge; immunoinflammatory index [peripheral blood platelet count (× 109 g/L) × peripheral blood neutrophil count (× 109 g/L) × peripheral blood lymphocyte count (× 109 g/L)] at 3 days postoperatively and at the 6-month follow-up; number of stents used; number of guidewires and catheters used intraoperatively; and operative time. The Barthel’s index at discharge was independently evaluated by two neurologists. In case of disagreement, a more experienced neurologists made the final judgment.
HR-VWI MRI indices at the 6-month follow-up after discharge included the Evans index, the presence or absence of aneurysmal wall enhancement, the presence or absence of stenosis in the aneurysm-bearing artery, and the presence or absence of aneurysmal lumen contrast.
Statistical Methods
Statistical analyses were performed using SPSS 22.0. General patient status at enrollment and degree of balance in patient condition at baseline were compared between groups. Measurement data that fulfilled the assumptions for parametric tests were compared by analysis of variance (ANOVA), while measurement data that did not fulfill the assumptions for parametric tests and ordinal data were analyzed by the rank sum test for multiple groups. Count data that met the conditions for the χ2 test were compared using the χ2 test, whereas those that did not meet the χ2 test conditions were analyzed by Fisher’s exact test. Multivariate analysis was conducted by logistic regression analysis. Two-sided tests were used for all statistical analyses, and tested differences were considered statistically significant when P ≤ 0.05.
Results
Comparison of Baseline Data and Laboratory Indicators Between Groups
Statistically, there were no significant differences between the two groups concerning sex, age, history of underlying diseases (hypertensive disease and diabetes mellitus), aneurysmal site, the presence or absence of stenosis in the aneurysm-bearing artery, modified Fisher score, Barthel’s index at discharge, immunoinflammatory index at 3 days postoperatively and at the 6-month follow-up, the presence or absence of aneurysmal wall enhancement, and the presence or absence of aneurysmal lumen (P > 0.05) (Table 1). Differences in operative time, rupture or non-rupture of the aneurysm, WFNS grade, aneurysm size, number of stents used, number of guidewires and catheters used, and Evans index between the two groups were statistically significant (P < 0.05) (Table 1).
In this study, we included a total of 147 subjects, of whom 27 had an Evan’s index > 0.3. Six of these patients underwent VP shunt surgery due to symptoms such as high intracranial pressure, unstable gait, delayed response, and urinary incontinence.
Regression Analysis of Factors Influencing the Occurrence of Long-Term Microischemia After Intervention in Patients with Aneurysm
Variables with P < 0.05 in univariate analysis were screened for multivariate logistic regression analysis by stepwise regression. The results indicated that aneurysm size, Evans index, and number of stents were independent influencing factors for the occurrence of microischemia after surgical intervention (P < 0.05) (Table 2).
The performance of each factor in predicting the occurrence of microischemia was further validated by establishment of the receiver operating characteristic (ROC) curves. The results showed that aneurysm size (area under ROC curve (AUC) 0.619, sensitivity 94.7%, specificity 17.1%, P = 0.049), Evans index (AUC 0.670, sensitivity 96.4%, specificity 26.8%, P = 0.004), and number of stents (AUC 0.639, sensitivity 44.6%, specificity 90.2%, P < 0.001) effectively predicted the occurrence of long-term microischemia (Table 3, Fig. 3). We calculated the power of three independent risk factors for long-term microischemia in this study using JMP software: the power of aneurysm size, number of stents used, and Evans index was 73.01%, 99.91%, and 88.10%, respectively.
Correlation Analysis of Cognitive Dysfunction and Occurrence of Microischemia During Follow-up
The incidence of cognitive dysfunction in the microischemic group was 21.6%, which was significantly higher than the incidence of 9.3% in the control group (P < 0.05). A greater number of microischemic foci was associated with a higher incidence of cognitive dysfunction (P < 0.05) (Table 4).
Differences in Distribution of Aneurysm Locations and Microischemic Foci Between Groups
Among the patients with microschemia, there were a total of 18 cases of intracranial segment aneurysms of the internal carotid artery, 16 cases of anterior and anterior communicating artery aneurysms, 10 cases of middle cerebral artery aneurysms, 4 cases of basilar artery aneurysms, 2 cases of vertebral artery aneurysms, and 1 case of posterior inferior cerebellar artery aneurysm. In the control group of patients, there were a total of 43 cases of intracranial carotid artery aneurysms, 22 cases of anterior and anterior communicating artery aneurysms, 18 cases of middle cerebral artery aneurysms, 7 cases of basilar artery aneurysms, 4 cases of vertebral artery aneurysms, and 2 cases of posterior inferior cerebellar artery aneurysms.
The distribution of microschemia foci in the two groups of patients is shown in Table 5. In the cognitive dysfunction group, there were a total of 23 microschemia foci and 16 in the left hemisphere. In the group without cognitive dysfunction, there were a total of 55 microschemia foci and 24 in the left hemisphere of the brain. The proportion of microschemia foci in the thalamus and basal ganglia in patients with cognitive dysfunction (60.87%) was significantly higher than that in patients without cognitive dysfunction (34.55%) (P < 0.05). There was no statistically significant difference in the proportion of microschemia foci in the frontal lobe, temporal lobe, parietal lobe, occipital lobe, cerebellum, and brainstem between the two groups of patients (P > 0.05). The proportion of microschemia foci in the left hemisphere in the cognitive dysfunction group (69.57%) was significantly higher than that in the non-cognitive dysfunction group (43.64%), and the difference was statistically significant (P < 0.05).
Discussion
The results of this study showed that a large aneurysm size, Evans index > 0.3, and an increase in the number of stents lead to an increased incidence of ischemic events after embolization and are independent risk factors for the occurrence of long-term microischemia after aneurysm embolization. The occurrence of long-term microischemia has also been found to be closely associated with cognitive dysfunction. The results presented in this study provide a theoretical basis for reducing the occurrence of microischemia and cognitive dysfunction in patients after IA embolization.
Cerebral vasospasm (CVS) has been recognized as an important factor of poor prognosis in patients with aSAH in recent decades [15, 16]. Recent studies have identified early brain injury (EBI) and delayed cerebral ischemia (DCI) as two key post-aSAH pathophysiological processes, and understanding the complex mechanisms underlying these processes is essential [17,18,19]. Of the two, DCI has garnered significant research attention recently. DCI usually occurs 3–14 days after aSAH and in approximately 30% of patients with aSAH, it is also a key influencing factor of delayed or secondary neurologic deterioration [20, 21]. Existing studies have found that cerebral edema, blood–brain barrier (BBB) damage, vascular endothelial cell dysfunction, sympathetic nervous system activation, autoregulatory failure, microthrombosis, and spreading depolarization are involved in the entire pathophysiological process of DCI occurrence and development [22,23,24]. The effects of these pathophysiological changes are usually not fully attenuated at the end of acute-phase treatment [25]. Therefore, the stratified management of patients with aSAH should not be merely limited to the hospitalization period, but should also involve comprehensive assessment of long-term patient prognosis [26]. However, there remains a lack of convincing research evidence of the association between the occurrence of cerebral microischemia during ultra-long-term follow-up (≥ 6 months) of aSAH and cognitive dysfunction. Previous measurements of patients with aSAH outcomes have relied primarily on the evaluation of neurologic function and rehabilitation. This has led to neglect of an extremely important factor, cognitive function (including cognitive dysfunction and cognitive decline). The risk factors and mechanisms of ultra-long-term physical and cognitive dysfunction in patients with aSAH have not yet been fully elucidated. Therefore, it is difficult to predict between the outcomes of patients with aSAH that are related to secondary injuries and ischemia and those which require early intervention.
Stent-assisted interventional embolization has been widely used in the clinical treatment of IAs and it is characterized by low rebleeding rates and high occlusion rates [27,28,29]. However, following the use of stent-assisted interventional embolization for aneurysm treatment in clinical practice, studies conducted at various institutions have revealed that patients were at risk of developing microthrombosis after undergoing stent-assisted interventional aneurysm embolization [30,31,32]. The cumulative burden of ischemic brain injury may exacerbate vascular dysfunction and cause transient ischemic attacks, especially in elderly patients with small brain volumes and reduced neuroplasticity, and this may consequently result in cognitive decline [33, 34]. Studies have also highlighted the occurrence of cognitive decline in certain patients with asymptomatic cerebral infarction, which leads to an increased risk of dementia [35]. Even small cerebral infarcts can damage neural circuits in the brain and lead to a poor long-term prognosis [36]. Therefore, the early detection of microischemic foci and timely intervention exert an important influence on patient prognosis. The application of multimodal HR-VWI not only allows for the early and timely assessment of aneurysms and aneurysm-bearing vessels and the prediction of postoperative cerebral blood flow improvement but also aids in identifying the risk factors of postoperative microischemia. This facilitates early intervention and treatment, thereby improving the post-IA embolization prognosis of patients.
In the present study, we found that aneurysm size and volume in the patients in the microischemic group were significantly larger than in those of the control group. This difference highlights that aneurysm size played a crucial role as an independent risk factor for the occurrence of long-term microischemia [37, 38]. Patients with large aneurysms are more susceptible to intra-aneurysmal thrombosis [39, 40]. Following detachment, thrombi may dislodge and travel to the artery with an aneurysm, disrupting blood flow and causing ischemic complications [41, 42]. Aneurysms with larger diameters are more susceptible to the detachment of intraluminal mural thrombi following coil placement, heightening the risk of thromboembolism and subsequent ischemic issues [43, 44]. Additionally, larger aneurysms require a longer operative time for the interventional embolization procedure, which may contribute to intraoperative thrombosis [45]. From the hemodynamic perspective, aneurysms with larger diameters are also more prone to intra-aneurysmal turbulence, which may increase the risk of thrombosis [46, 47]. Upon the occurrence of thrombus detachment during coil packing, the generated microemboli may result in microinfarction through arterial-arterial embolization of distal vessels. Aneurysms with a large diameter also have a larger volume, which increases the volume of coil packing [48]. This raises the difficulty of achieving dense embolization, and the resultant packed aneurysm will cause significant hemodynamic changes, resulting in an increased tendency for thrombosis.
Stent-assisted coil embolization is one of the main methods for the treatment of intracranial aneurysms. It increases the degree of dense embolization, improves coil basket formation, and reduces long-term disease recurrence rate in patients with aneurysm [49]. Recent studies suggest that an increase in the number of stents will lead to an increase in the probability of occurrence of microischemic foci [50]. Hahnemann et al. reported that stent-assisted embolization was an independent risk factor for post-interventional microischemia [51]. A study that performed transcranial Doppler monitoring of the incidence of microembolic signals after interventional IA embolization found that the rate of microemboli positivity was significantly higher in patients who underwent stent-assisted embolization than that of patients who underwent coil embolization [52]. Such a finding suggests that stent-assisted embolization is a major risk factor for microemboli formation after IA embolization. Stents serve as attachment points for platelets in the vascular lumen, and metal stent wires may damage the vascular endothelium, leading to the exposure of subendothelial collagen fibers and activation of the endogenous coagulation system [53]. This promotes microemboli formation and leads to the occurrence of microischemia. Studies have also found that stent type affects the incidence of microischemia, which may be caused by differences in the degree of lumen apposition and metal coverage among different types of stents [54, 55].
Previous research suggests that the timely management of acute hydrocephalus is directly related to prognosis [56]. Studies in other countries have found that hydrocephalus is a predictor of cerebral ischemia [57]. Dong et al. analyzed the effects of certain factors in predicting cerebral ischemia by computed tomography perfusion (CTP), and the results revealed that hydrocephalus was a predictor of ischemia [58]. The correlation can be attributed to acute hydrocephalus elevating intracranial pressure while reducing cerebral perfusion pressure substantially, potentially leading to cerebral hypoxia and subsequent ischemic manifestations [59]. In the present study, we observed that hydrocephalus was significantly associated with the occurrence of long-term microischemia (P < 0.001). Therefore, upon encountering complications of hydrocephalus in clinical practice, it is advisable to actively perform cerebrospinal fluid drainage to prevent progressive hydrocephalus and the occurrence of cerebral ischemic lesions.
Our results further revealed that the occurrence of long-term microischemia increased the risk of cognitive dysfunction. Recent studies have found that silent lacunar infarction is an independent risk factor for post-stroke cognitive dysfunction [60]. The mechanism is possibly related to damage to cognitive function-related circuits, such as the frontal-subcortical circuits associated with executive function, and the limbic system and basal ganglia circuits associated with memory [61]. Inflammatory responses may also be involved in the development of cognitive impairment. Recurrent silent lacunar infarction leads to widespread and persistent inflammatory responses, and the damage caused by oxygen free radicals and inflammatory mediators in turn leads to cognitive impairment [62]. A 2-year follow-up study demonstrated that comprehensive lifestyle interventions encompassing aspects like a Mediterranean diet, regular exercise regimen, engaging in cognitive exercises, and social interactions along with managing vascular risks (e.g., blood pressure control) can stabilize or enhance cognitive function among at-risk populations [63]. Currently, behavioral intervention therapy is considered the mainstay of non-pharmacological treatment for cognitive dysfunction [64]. This includes exercise therapy, music therapy, cognitive interventions, physical therapy, and lifestyle changes [65, 66]. These methods improve cognitive function by reducing the expression of harmful inflammatory cytokines, balancing oxygen free radicals, improving neural plasticity, and increasing the supply of oxygen to the brain, cerebral blood perfusion, and glucose utilization [67]. In the field of pharmacotherapy, drug trials for the improvement of vascular cognitive impairment (VCI) have mainly been targeted towards patients with vascular or mixed dementia [68]. These studies have also involved an extensive range of drugs, including cholinesterase inhibitors, excitatory amino acid receptor antagonists, ergot alkaloids, and calcium antagonists. Among the high-risk population for microischemia, we advocate early identification and intervention to improve clinical prognosis and quality of life in patients.
This study found that the proportion of microischemic foci in the thalamus and basal ganglia was higher in the cognitive dysfunction group (P < 0.05). As an intermediate station of the limbic system, the thalamus has extensive connections with the cerebral cortex and subcortical structures, and is involved in the activities of higher nerves, psychiatric and internal organs, and is an important subcortical information transmission center [69]. The ischemia of the thalamus is manifested as the impairment of cognitive function in the whole brain [70], and the reduction of motor ability and executive function in the basal ganglia region is mainly manifested by the decline of memory, executive, and speech ability [71], and can also destroy the integrity of the white matter of the brain and further impair cognitive function [72]. In this study, the proportion of left cerebral ischemia in the cognitive dysfunction group was significantly increased (P < 0.05). Previous studies have shown that patients with dominant hemisphere lesions are at greater risk of cognitive dysfunction, which may be because the left cerebral hemisphere is mainly involved in memory, language, and cognitive activities, while the right cerebral hemisphere is mainly involved in non-verbal cognitive functions, and cognitive functions often need to be manifested through language, thinking, and other forms.
Previous studies have identified a myriad of risk factors for the occurrence of microischemic lesions after IA embolization. Factors such as preoperative hypothalamic hamartoma (HH) classification, aneurysm site, age, sex, hypertension, diabetes mellitus, and antiplatelet resistance have been found to be closely associated with the occurrence of ischemic events after interventional aneurysm embolization. However, we did not observe any correlations between these factors and the occurrence of ischemic events in the present study. Since Dasenbrock’s study, several studies have reported that smoking is an independent predictor of symptomatic vasospasm and cerebral ischemia after aSAH [73, 74]. Krishnamurthy et al. have shown that smoking increases the risk of delayed cerebral ischemia, and that the longer the smoking history, the worse the prognosis [75]. Diabetes mellitus is a condition that accelerates the aging of blood vessels. Hyperglycemia leads to vascular endothelial dysfunction and atherosclerosis, which increases the risk of ischemic events [76]. Patients with diabetes mellitus are at significantly increased risk of cerebral ischemia compared with patients without diabetes [77]. Liu et al. showed that a history of diabetes mellitus is an independent early risk factor for the development of DCI [78]. Previous studies have found that high levels of triglycerides are significantly correlated with DCI after aSAH [79], which can not only cause endothelial dysfunction but also promotes cell adhesion and smooth muscle cell proliferation after deposition of blood vessel walls, accompanied by impaired plasma coagulation fibrinolysis mechanism, and the formation of atheroma plaques that block or even occlude the lumen [80]. Previous studies mainly focused on the perioperative period, and the relevant studies mainly focused on the analysis of risk factors for ischemic events in the short period after surgery, with few confounding factors. In the process of long-term follow-up, more uncontrollable confounding factors were added, including the use of bispecific antibody drugs in postoperative patients, the compliance of drug use, and the change of personal lifestyle habits of postoperative patients, and the more standardized blood pressure and blood glucose control compared with preoperative control; we believe that these factors may have an impact on the results of the study, and we will further expand the scale of follow-up in future studies.
This study has limitations, namely the limited sample size and its single-center nature, which may have contributed to flaws in statistical analysis and findings. Therefore, future multicenter studies with larger sample sizes will be needed to verify the results of the present study and determine the potential underlying mechanisms.
Conclusion
The aneurysm size, Evans index, and number of stents are closely associated with the occurrence of long-term microischemia after aneurysm embolization. The occurrence of long-term microischemia is also closely associated with cognitive dysfunction. Therefore, particular attention should be paid to patients with risk factors in clinical practice, and early identification and intervention are advisable to reduce the incidence of cognitive dysfunction.
Data Availability
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
References
Kane SF, Butler E, Sindelar BD. Nontraumatic subarachnoid hemorrhage and ruptured intracranial aneurysm: recognition and evaluation. Am Fam Phys. 2023;108(4):386–95.
Petridis AK, Kamp MA, Cornelius JF, et al. Aneurysmal subarachnoid hemorrhage. Deutsches Arzteblatt Int. 2017;114(13):226–36.
D’Souza S. Aneurysmal subarachnoid hemorrhage. J Neurosurg Anesthesiol. 2015;27(3):222–40.
Lee WJ, Kim MK, Lim YC. Clinical analysis of young adult patients with ruptured intracranial aneurysms: a single-center study of 113 consecutive patients. J Cerebrovasc Endovasc Neurosurg. 2020;22(3):127–33.
Pierot L, Gawlitza M, Soize S. Unruptured intracranial aneurysms: management strategy and current endovascular treatment options. Expert Rev Neurother. 2017;17(10):977–86.
Lindgren A, Vergouwen MD, van der Schaaf I, et al. Endovascular coiling versus neurosurgical clipping for people with aneurysmal subarachnoid haemorrhage. Cochrane Datab Syst Rev. 2018;8(8):CD003085.
Deshmukh AS, Priola SM, Katsanos AH, et al. The management of intracranial aneurysms: current trends and future directions. Neurol Int. 2024;16(1):74–94.
Campos JK, Cheaney Ii B, Lien BV, et al. Advances in endovascular aneurysm management: flow modulation techniques with braided mesh devices. Stroke Vasc Neurol. 2020;5(1):1–13.
Chen HY, Elmer J, Zafar SF, et al. Combining transcranial Doppler and EEG data to predict delayed cerebral ischemia after subarachnoid hemorrhage. Neurology. 2022;98(5):e459–69.
Alsbrook DL, Di Napoli M, Bhatia K, et al. Pathophysiology of early brain injury and its association with delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage: a review of current literature. J Clin Med. 2023;12(3)
Vranic JE, Hartman JB, Mossa-Basha M. High-resolution magnetic resonance vessel wall imaging for the evaluation of intracranial vascular pathology. Neuroimaging Clin N Am. 2021;31(2):223–33.
Samaniego EA, Roa JA, Hasan D. Vessel wall imaging in intracranial aneurysms. J Neurointervent Surg. 2019;11(11):1105–12.
Alexander MD, Yuan C, Rutman A, et al. High-resolution intracranial vessel wall imaging: imaging beyond the lumen. J Neurol Neurosurg Psychiatry. 2016;87(6):589–97.
Regenhardt RW, Das AS, Lo EH, Caplan LR. Advances in understanding the pathophysiology of lacunar stroke: a review. JAMA Neurol. 2018;75(10):1273–81.
Maruhashi T, Higashi Y. An overview of pharmacotherapy for cerebral vasospasm and delayed cerebral ischemia after subarachnoid hemorrhage. Expert Opin Pharmacother. 2021;22(12):1601–14.
Keyrouz SG, Diringer MN. Clinical review: prevention and therapy of vasospasm in subarachnoid hemorrhage. Crit Care. 2007;11(4):220.
Ciurea AV, Palade C, Voinescu D, Nica DA. Subarachnoid hemorrhage and cerebral vasospasm—literature review. J Med Life. 2013;6(2):120–5.
Sanicola HW, Stewart CE, Luther P, et al. Pathophysiology, management, and therapeutics in subarachnoid hemorrhage and delayed cerebral ischemia: an overview. Pathophysiol. 2023;30(3):420–42.
Dodd WS, Noda I, Martinez M, Hosaka K, Hoh BL. NLRP3 inhibition attenuates early brain injury and delayed cerebral vasospasm after subarachnoid hemorrhage. J Neuroinflam. 2021;18(1):163.
Janardhan V, Biondi A, Riina HA, Sanelli PC, Stieg PE, Gobin YP. Vasospasm in aneurysmal subarachnoid hemorrhage: diagnosis, prevention, and management. Neuroimaging Clin North Am. 2006;16(3):483–496, viii-ix.
Velat GJ, Kimball MM, Mocco JD, Hoh BL. Vasospasm after aneurysmal subarachnoid hemorrhage: review of randomized controlled trials and meta-analyses in the literature. World Neurosurg. 2011;76(5):446–54.
Dodd WS, Laurent D, Dumont AS, et al. Pathophysiology of delayed cerebral ischemia after subarachnoid hemorrhage: a review. J Am Heart Assoc. 2021;10(15):e021845.
Geraghty JR, Testai FD. Delayed cerebral ischemia after subarachnoid hemorrhage: beyond vasospasm and towards a multifactorial pathophysiology. Curr Atheroscler Rep. 2017;19(12):50.
Suzuki H, Kanamaru H, Kawakita F, Asada R, Fujimoto M, Shiba M. Cerebrovascular pathophysiology of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Histol Histopathol. 2021;36(2):143–58.
Wenneberg SB, Block L, Sörbo A, et al. Long-term outcomes after aneurysmal subarachnoid hemorrhage: a prospective observational cohort study. Acta Neurol Scand. 2022;146(5):525–36.
Al-Khindi T, Macdonald RL, Schweizer TA. Cognitive and functional outcome after aneurysmal subarachnoid hemorrhage. Stroke. 2010;41(8):e519–536.
Campos JK, Lien BV, Wang AS, Lin LM. Advances in endovascular aneurysm management: coiling and adjunctive devices. Stroke Vasc Neurol. 2020;5(1):14–21.
Jin Y, Guo X, Quan T, Chen Z, Liu C, Guan S. Safety and efficacy of endovascular treatment for tiny ruptured intracranial aneurysms with low-profile visualized intraluminal support stents. Intervent Neuroradioleurosci. 2023;29(2):141–7.
Gordhan A, Invergo D. Stent-assisted aneurysm coil embolization: safety and efficacy at a low-volume center. Neurol Res. 2011;33(9):942–6.
Pikis S, Mantziaris G, Mamalis V, et al. Diffusion weighted image documented cerebral ischemia in the postprocedural period following pipeline embolization device with shield technology treatment of unruptured intracranial aneurysms: a prospective, single center study. J Neurointervent Surg. 2020;12(4):407–11.
Grüter BE, Wanderer S, Andereggen L, et al. Incidence and outcome of peri-interventional vasospasm during endovascular or microsurgical treatment of unruptured intracranial aneurysms. Neurosurgery. 2023;92(3):599–606.
Heit JJ, Ball RL, Telischak NA, et al. Patient outcomes and cerebral infarction after ruptured anterior communicating artery aneurysm treatment. AJNR Am J Neuroradiol. 2017;38(11):2119–25.
Li J, You SJ, Xu YN, et al. Cognitive impairment and sleep disturbances after minor ischemic stroke. Sleep Breath. 2019;23(2):455–62.
Suda S, Nishimura T, Ishiwata A, et al. Early cognitive impairment after minor stroke: associated factors and functional outcome. J Stroke Cerebrovasc Dis. 2020;29(5):104749.
Yang T, Zhang L, Xiang M, et al. Cognitive impairment and gray matter volume abnormalities in silent cerebral infarction. Neuroreport. 2015;26(15):890–5.
Lin H, Jin T, Chen L, et al. Longitudinal tracing of neurochemical metabolic disorders in working memory neural circuit and optogenetics modulation in rats with vascular cognitive impairment. Brain Res Bull. 2021;170:174–86.
Shiue I, Arima H, Hankey GJ, Anderson CS. Location and size of ruptured intracranial aneurysm and serious clinical outcomes early after subarachnoid hemorrhage: a population-based study in Australasia. Cerebrovasc Dis. 2011;31(6):573–9.
Khanna RK, Malik GM, Qureshi N. Predicting outcome following surgical treatment of unruptured intracranial aneurysms: a proposed grading system. J Neurosurg. 1996;84(1):49–54.
Sluzewski M, Menovsky T, van Rooij WJ, Wijnalda D. Coiling of very large or giant cerebral aneurysms: long-term clinical and serial angiographic results. AJNR Am J Neuroradiol. 2003;24(2):257–62.
Gu Y, Miao C, Li A, Zhang Y, Xu J. High-resolution magnetic resonance imaging (HR-MRI) evaluation of the distribution and characteristics of intra-aneurysm thrombosis to improve clinical diagnosis of thrombotic intracranial aneurysm. Med Sci Monit. 2022;28:e935613.
Fomenko A, Kaufmann AM. Spontaneous thrombosis of an unruptured saccular aneurysm causing MCA infarction. Can J Neurol Sci. 2016;43(6):856–8.
Cohen JE, Itshayek E, Gomori JM, et al. Spontaneous thrombosis of cerebral aneurysms presenting with ischemic stroke. J Neurol Sci. 2007;254(1–2):95–8.
Park JC, Lee DH, Kim JK, et al. Microembolism after endovascular coiling of unruptured cerebral aneurysms: incidence and risk factors. J Neurosurg. 2016;124(3):777–83.
Derdeyn CP, Cross DT 3rd, Moran CJ, et al. Postprocedure ischemic events after treatment of intracranial aneurysms with Guglielmi detachable coils. J Neurosurg. 2002;96(5):837–43.
Eliava S, Pilipenko Y, Shekhtman O, Konovalov A. Reversal of intraoperative arterial thrombosis with a fibrinolytic agent when treating large and giant partially thrombosed aneurysms of the middle cerebral artery. J Neurosurg. 2016;124(4):1114–22.
Salimi Ashkezari SF, Mut F, Chung BJ, et al. Hemodynamics in aneurysm blebs with different wall characteristics. J Neurointervent Surg. 2021;13(7):642–6.
Berge J, Blanco P, Rooryck C, et al. Understanding flow patterns and inflammatory status in intracranial aneurysms: towards a personalized medicine. J Neuroradiol. 2016;43(2):141–147.
Zhang Y, Wang C, Tian Z, et al. Risk factors for periprocedural ischemic stroke following endovascular treatment of intracranial aneurysms. Chin Neurosurg J. 2021;7(1):38.
Chalouhi N, Tjoumakaris S, Gonzalez LF, et al. Coiling of large and giant aneurysms: complications and long-term results of 334 cases. AJNR Am J Neuroradiol. 2014;35(3):546–52.
Phan K, Huo YR, Jia F, et al. Meta-analysis of stent-assisted coiling versus coiling-only for the treatment of intracranial aneurysms. J Clin Neurosci. 2016;31:15–22.
Hahnemann ML, Ringelstein A, Sandalcioglu IE, et al. Silent embolism after stent-assisted coiling of cerebral aneurysms: diffusion-weighted MRI study of 75 cases. J Neurointervent Surg. 2014;6(6):461–5.
Das AS, Regenhardt RW, LaRose S, et al. Microembolic signals detected by transcranial Doppler predict future stroke and poor outcomes. J Neuroimaging. 2020;30(6):882–9.
Casa LDC, Ku DN. Thrombus formation at high shear rates. Annu Rev Biomed Eng. 2017;19:415–33.
Chen YA, Hussain M, Zhang JY, Zhang KP, Pang Q. Stent-assisted coiling of cerebral aneurysms using the Enterprise and the Solitaire devices. Neurol Res. 2014;36(5):461–7.
Han JT, Zhang YX, Jia ZC, et al. Clinical application of Neuroform Atlas stent-assisted coiling in the treatment of unruptured wide-neck intracranial aneurysms. Beijing Da Xue Xue Bao Yi Xue Ban. 2023;55(1):139–143.
Bhattacharjee S, Rakesh D, Ramnadha R, Manas P. Subarachnoid hemorrhage and hydrocephalus. Neurol India. 2021;69(Suppl):S429–S433.
Bakker AM, Dorhout Mees SM, Algra A, Rinkel GJ. Extent of acute hydrocephalus after aneurysmal subarachnoid hemorrhage as a risk factor for delayed cerebral infarction. Stroke. 2007;38(9):2496–9.
Dong L, Zhou Y, Wang M, Yang C, Yuan Q, Fang X. Whole-brain CT perfusion on admission predicts delayed cerebral ischemia following aneurysmal subarachnoid hemorrhage. Eur J Radiol. 2019;11(6):165–173
van Asch CJ, van der Schaaf IC, Rinkel GJ. Acute hydrocephalus and cerebral perfusion after aneurysmal subarachnoid hemorrhage. AJNR Am J Neuroradiol. 2010;31(1):67–70.
Wang Z, Wong A, Liu W, et al. Cerebral microbleeds and cognitive function in ischemic stroke or transient ischemic attack patients. Dement Geriatr Cogn Disord. 2015;40(3–4):130–6.
Kaur M, Sharma S. Molecular mechanisms of cognitive impairment associated with stroke. Metab Brain Dis. 2022;37(2):279–87.
Woodruff TM, Thundyil J, Tang SC, Sobey CG, Taylor SM, Arumugam TV. Pathophysiology, treatment, and animal and cellular models of human ischemic stroke. Mol Neurodegener. 2011;6(1):11.
Scarmeas N, Stern Y, Mayeux R, Manly JJ, Schupf N, Luchsinger JA. Mediterranean diet and mild cognitive impairment. Arch Neurol. 2009;66(2):216–25.
Custodero C, Ciavarella A, Panza F, et al. Role of inflammatory markers in the diagnosis of vascular contributions to cognitive impairment and dementia: a systematic review and meta-analysis. Geroscience. 2022;44(3):1373–92.
Law CK, Lam FM, Chung RC, Pang MY. Physical exercise attenuates cognitive decline and reduces behavioural problems in people with mild cognitive impairment and dementia: a systematic review. J Physiother. 2020;66(1):9–18.
Kim Y, Oh W, You JSH. Immediate effects of multimodal cognitive therapy in mild cognitive impairment. NeuroRehabilit. 2023;53(3):297–308.
Tao J, Liu J, Chen X, et al. Mind-body exercise improves cognitive function and modulates the function and structure of the hippocampus and anterior cingulate cortex in patients with mild cognitive impairment. NeuroImage Clin. 2019;23:101834.
Farooq MU, Min J, Goshgarian C, Gorelick PB. Pharmacotherapy for vascular cognitive impairment. CNS Drugs. 2017;31(9):759–76.
Weaver NA, Kuijf HJ, Aben HP, et al. Strategic infarct locations for post-stroke cognitive impairment: a pooled analysis of individual patient data from 12 acute ischaemic stroke cohorts. Lancet Neurol. 2021;20(6):448–59.
Savage LM, Nunes PT, Gursky ZH, Milbocker KA, Klintsova AY. Midline thalamic damage associated with alcohol-use disorders: disruption of distinct thalamocortical pathways and function. Neuropsychol Rev. 2021;31(3):447–71.
Hellmuth J, Casaletto K, Cuneo R, Possin KL, Dillon W, Geschwind MD. Bilateral basal ganglia infarcts presenting as rapid onset cognitive and behavioral disturbance. Neurocase. 2020;26(2):115–9.
Benisty S, Gouw AA, Porcher R, et al. Location of lacunar infarcts correlates with cognition in a sample of non-disabled subjects with age-related white-matter changes: the LADIS study. J Neurol Neurosurg Psychiatry. 2009;80(5):478–83.
Dasenbrock HH, Rudy RF, Rosalind Lai PM, et al. Cigarette smoking and outcomes after aneurysmal subarachnoid hemorrhage: a nationwide analysis. J Neurosurg. 2018;129(2):446–57.
Ya X, Zhang C, Zhang S, et al. The relationship between smoking and delayed cerebral ischemia after intracranial aneurysm rupture: a systematic review and meta-analysis. Front Neurol. 2021;12:625087.
Krishnamurthy S, Kelleher JP, Lehman EB, Cockroft KM. Effects of tobacco dose and length of exposure on delayed neurological deterioration and overall clinical outcome after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2007;61(3):475–480; discussion 480–471
Arboix A, Rivas A, García-Eroles L, de Marcos L, Massons J, Oliveres M. Cerebral infarction in diabetes: clinical pattern, stroke subtypes, and predictors of in-hospital mortality. BMC Neurol. 2005;5(1):9.
Hill MD. Stroke and diabetes mellitus. Handb Clin Neurol. 2014;126:167–74.
Duan W, Pan Y, Wang C, et al. Risk factors and clinical impact of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage: analysis from the China National Stroke Registry. Neuroepidemiology. 2018;50(3–4):128–36.
Dhandapani S, Aggarwal A, Srinivasan A, . Serum lipid profile spectrum and delayed cerebral ischemia following subarachnoid hemorrhage: is there a relation? Surg Neurol Int. 2015;6(Suppl 21):S543–548.
Badjatia N, Seres D, Carpenter A, et al. Free fatty acids and delayed cerebral ischemia after subarachnoid hemorrhage. Stroke. 2012;43(3):691–6.
Acknowledgements
We thank the participants of the study.
Medical Writing and Editorial Assistance
We thank editage editorial team (www.editage.com) for language editing service.
Funding
Sponsorship for this study and Rapid Service Fee were funded by the project of Central University Basic Research Business Fee Project Medical and Industrial Integration Special Project (Project No. 2023CDJYGRH-ZD05).
Author information
Authors and Affiliations
Contributions
Yuan Cheng contributed to designed and reviewed research; Yi Song wrote and modified of the manuscript. Jianxin Zhou translated and performed the statistical analysis; Yun Tan, Yao Wu and Mingdong Liu responsible for submission and format modification. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Yi Song, Jianxin Zhou, Yun Tan, Yao Wu, Mingdong Liu and Yuan Cheng certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical Approval
This study was reviewed and approved by the ethics committee of Chongqing University Three Gorges Hospital under project number [2024] 16. All subjects or their legal representatives were informed of the nature of the study, fully understood the provisions in the protocol, and were able to ensure compliance. Informed consent was acquired from each patient and/or their legal surrogates according to the Declaration of Helsinki. All the patients agreed to participate in the study and gave consent for their identifiable data to be used for the study and publication.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Song, Y., Zhou, J., Tan, Y. et al. Risk Factors and Clinical Significance of Ultra-Long-Term Microischemia After Intracranial Aneurysm Embolization. Neurol Ther (2024). https://doi.org/10.1007/s40120-024-00630-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40120-024-00630-9