Abstract
Background
Prolonged periods of stress may lead to negative health consequences. AlphaWave® l-Theanine was safe and efficacious during an acute stress challenge. However, double-blind, placebo-controlled clinical trials investigating the longer term effects of l-theanine supplementation on stress are warranted.
Methods
Thirty healthy adults (18–65 years) with moderate stress were randomized to AlphaWave® l-Theanine (400 mg l-theanine/day) or placebo (n = 15/group) for 28 days. Stress was assessed by salivary cortisol, Perceived Stress Scale (PSS) and Depression, Anxiety and Stress Scale-21; sleep was assessed by the Healthy People Sleep Quality Index and actigraphy device; cognition was assessed by Computerized Mental Performance Assessment System; mood was assessed by Profile of Mood States. All outcomes were measured at baseline, Days 14 and 28. Safety included vital signs, clinical chemistry, haematology and adverse events (AEs).
Results
All AEs were resolved by the end of the study period or upon subsequent follow up, and out of range laboratory values and changes in vital signs were deemed not clinically relevant following AlphaWave® l-Theanine supplementation. Participants supplemented with AlphaWave® l-Theanine had decreases of 12.92% (p = 0.051) and 17.98% (p = 0.04) in PSS scores after 14 and 28 days, respectively, while those on placebo had respective decreases of 9.74% (p = 0.061) and 17.88% (p = 0.009). There were no significant differences between groups for change in salivary cortisol. The AlphaWave® l-Theanine group demonstrated decreased time asleep after 28 days and significantly reduced light sleep after 14 and 28 days compared to placebo (p ≤ 0.040). The AlphaWave® l-Theanine group significantly improved by 21.79% and 21.33% in Stroop test correct reaction time after 14 and 28 days, respectively, while those on placebo improved after 28 days only (p = 0.005).
Conclusions
AlphaWave® l-Theanine supplementation for 28 days was safe and significantly decreased perceived stress significantly decreased perceived stress and light sleep, improved sleep quality and enhanced cognitive attention in the studied population. Larger, randomized controlled trials with longer duration of AlphaWave® l-Theanine supplementation are warranted to reduce inter-individual variability and the potential placebo effect.
Trial Registration
ClinicalTrials.gov identifier, NCT05808595.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Stress is a normal human reaction when faced with daily challenges; however, prolonged periods of stress can lead to negative health consequences. Safe and effective alternative therapy may present an opportunity to address the burden of stress in healthy populations |
AlphaWave® l-Theanine has been previously shown to significantly increase alpha power, a surrogate marker of relaxation, compared to placebo, when moderately stressed adults were faced with an acute stress challenge |
This study examined the efficacy and safety of AlphaWave® l-Theanine on stress among a moderately stressed, healthy adult population over a period of 28 days |
What was learned from the study? |
AlphaWave® l-Theanine supplementation was safe and well tolerated in a population of healthy adults with moderate stress, further supporting the previously reported safety profile |
Supplementation with AlphaWave® l-Theanine significantly decreased perceived stress and improved cognitive attention from baseline in the studied population. There was no change in salivary cortisol, and a placebo effect was observed |
Future larger randomized controlled trials with longer AlphaWave® l-Theanine supplementation are warranted |
Introduction
Stress is a normal human reaction when faced with daily challenges, which can involve both physiological and psychological responses [1]. While stress responses are normal and can be healthy, prolonged periods of stress can lead to negative health consequences [1] and mental health disorders such as anxiety and depression. In 2020, 21% of Canadian adults reported “most days quite a bit or extremely stressful” [2] while 55% of Americans reported they were stressed in a 2019 survey [3]. Stress negatively affects quality of life as it leads to tiredness, declines in mood, increased anxiety, and reduced sleep quality. Many existing strategies that aim to manage or reduce stress levels promote the formation of healthy habits such as exercising, deep breathing and mindfulness meditation [4]. However, these strategies require sustained compliance to be effective, and it may be challenging to incorporate into daily routine. Currently available drug treatments that may be used for managing the symptoms of stress (e.g., trouble sleeping) can also result in undesirable side effects [5]. Therefore, safe, sustainable and effective natural therapy may present an opportunity to address the burden of stress in healthy populations.
l-Theanine is a water-soluble, non-protein amino acid predominantly found in green tea leaves that has been shown as an emerging option for stress management [6]. The calming effects of l-theanine are believed to be mediated by inhibition of glutamate reuptake, blocking of hippocampal glutamate receptors and increase in gamma aminobutyric acid, dopamine and serotonin in some areas of the brain [7]. AlphaWave® l-Theanine is a dietary supplement containing 200 mg of l-theanine per capsule. Previously, AlphaWave® l-Theanine supplementation demonstrated significantly greater increases in alpha power, a surrogate marker of relaxation, compared to placebo when moderately stressed adults were faced with an acute stress challenge [8]. Other studies examining l-theanine have also shown improvements in markers of stress among healthy populations in acute [9, 10] and chronic [11, 12] settings. However, examination of higher doses for longer durations to better understand the stress-relieving potential of l-theanine is warranted. Therefore, the objective of the current study was to examine the safety and efficacy of AlphaWave® l-Theanine on stress in a moderately stressed, healthy adult population.
Methods
Registration and Regulatory Approvals
This study was conducted at KGK Science Inc. (London, Ontario, Canada) from April 2023 to July 2023 (clinicaltrials.gov ID: NCT05808595). This study was reviewed by the Natural and Non-Prescription Health Product Directorate, Health Canada, with notice of authorization granted on January 31, 2023. Research ethics board approval was granted on March 3, 2023, by Advarra (Aurora, Ontario, Canada; Pro00069946). Consolidated Standards of Reporting Trials (CONSORT) guidelines for randomized controlled trials were followed [13] (Table S1), and written informed consent was obtained from all participants prior to participation in the study. The study was conducted in compliance with the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) Guideline for Good Clinical Practice (GCP) and in accordance with the Declaration of Helsinki guidelines and its subsequent amendments.
Study Design and Population
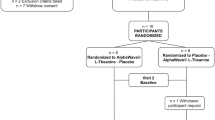
This randomized, double-blind, placebo-controlled, parallel clinical trial consisted of a 7-day run-in period followed by a 28-day supplementation period in which participants were randomized to receive either AlphaWave® l-Theanine or placebo at baseline (Day 0) (Fig. 1). Participants were healthy adults aged 18–65 years with moderate stress as determined by a score of 14–26 on the Perceived Stress Scale (PSS) who agreed to maintain their current lifestyle, avoid high caffeine consumption (e.g., > 2 cups caffeinated coffee or tea per day) throughout the study and avoid vigorous physical activity 24-h prior to study visits.
Individuals were excluded if they were pregnant, breastfeeding or planning to become pregnant during the trial; had allergies to the study products; were employed in rotating shift work or travelled across one or more time zones in the 3 weeks preceding the run-in period and/or during the study that would have disrupted normal circadian rhythm; had a self-reported diagnosis of a neuropsychiatric and/or cognitive impairment or a stress or sleep disorder as assessed by the Qualified Investigator (QI); had self-reported alcohol or drug abuse within the last 12 months; used medications, foods, and/or drinks which may affect stress, sleep, cognition and/or mood; or had any other condition or lifestyle factor that may have adversely affected the participant's ability to complete the study or posed significant risk to the participant.
Investigational Products
Each capsule of AlphaWave® l-Theanine contained 200 mg l-theanine and microcrystalline cellulose, hydroxypropyl methyl cellulose and silicon dioxide (Ethical Naturals, Inc., Redwood City, CA, USA). The placebo contained microcrystalline cellulose and hydroxypropyl methyl cellulose. Participants were instructed to take one capsule twice daily, once in the morning and once in the evening, starting on Day 1. If a dose was missed, participants were instructed to take the missed dose immediately once noticed unless it was noticed within 2 h of taking their next dose, in which case participants were instructed to record the missed dose in the study diary and continue with their regular dosing schedule.
Randomization and Blinding
At the baseline visit, a randomization number was assigned to the participant by a blinded investigator per the order of the randomization list (www.randomization.com). Investigators, site personnel, and participants were blinded to the products. The study products were identical in appearance and sealed in identical bottles to ensure allocation concealment. The bottles were labelled by personnel not involved in study assessments per the requirements of the ICH-GCP and applicable local regulatory guidelines.
Study Outcomes
The primary outcome was the difference in change in stress as assessed by salivary cortisol from baseline at Day 14 and 28 between AlphaWave® l-Theanine and placebo.
Secondary outcomes were differences in change from baseline at days 14 and 28 in the following measures: (1) stress as assessed by the PSS and Depression, Anxiety and Stress Scale-21 (DASS-21), (2) sleep as assessed by Healthy People Sleep Quality Index (HPSQI) and actigraphy device, (3) cognition as assessed by Computerized Mental Performance Assessment System (COMPASS) and (4) mood as assessed by Profile of Moods (POMS).
Study Assessments
Salivary cortisol Participants collected salivary cortisol samples in the evening before and the morning of each study visit using the sample collection kit provided. The morning and evening samples were collected within 30 min of waking and 30 min before going to bed, respectively. Participants were instructed to refrain from brushing their teeth, eating or drinking for 30 min before collecting the samples. Participants were instructed to record the time of the sample collection in the study diary and follow all provided collection and storage instructions.
Perceived Stress Scale The PSS-10 is a validated self-reported tool for the assessment of perceived stress [14]. The questions were scored on a 5-point Likert scale, with the scores of the positive items (items 4, 5, 7, 8) reversed and the total score computed by adding the individual scores for each of the ten questions. Scores ranging from 0–13, 14–26 and 27–40 were considered low stress, moderate stress and high stress, respectively.
Depression, Anxiety and Stress Scale-21 The DASS-21 is a self-reported tool that measures depression, anxiety and stress over the past week and is comprised of three scales (depression, anxiety, stress), each containing seven items [15]. The scores for each item range from 0 to 3, where 0 indicates “Did not apply to me at all” and 3 indicates “Applied to me very much or most of the time”. Scores for each scale were computed by taking the sum of the items and multiplied by two.
Computerized Mental Performance Assessment System COMPASS is used to assess cognitive function and has been reported to be sensitive to nutritional interventions [16, 17]. COMPASS delivers randomly generated tasks for each participant that are selected from a wide range of pre-programmed standard cognitive tests based on the objective of a study. The Stroop Test, Simple Reaction Time and Choice Reaction Time tasks were used to assess attention.
Profile of Mood States The POMS is a self-reported assessment of mood [18]. Responses to questions are used to generate a Total Mood Disturbance score as well as scores for Anger-Hostility, Confusion-Bewilderment, Depression-Dejection, Fatigue-Inertia, Tension-Anxiety, Vigor-Activity and Friendliness subscales.
Actigraphy The BioStrap (Bradbury, CA, USA) provides objective measures of sleep quality. Participants wore the actigraphy device on their non-dominant arm during sleep hours for a total of seven nights leading up to each study visit and brought their device to each visit for a check-in and review of sleep data. Given the potential physical inconveniences participants may have experienced while acclimating to wearing the actigraphy device, the first two nights of each seven-night period were considered an acclimation period and were not included in the statistical analysis. Participants were instructed to record details pertaining to use of the actigraphy device in the study diary.
Healthy Person Sleep Quality Index The HPSQI provides subjective measures of sleep quality [19]. The questionnaire measures sleep efficiency, perceived sleep debt, sleep difficulty and sleep-related quality of life and is comprised of 19 questions. Five questions relate to sleep habits for the previous seven days including five weekdays and two weekend days. The remaining questions were scored on a five-point Likert scale ranging from ‘strongly disagree’ to ‘strongly agree’.
Safety Safety was assessed by adverse events (AE), vital signs, clinical chemistry and haematology. Participants recorded any changes in health in the study diary. The severity of an AE was classified as ‘mild’, ‘moderate’ or ‘severe’, and the degree of relationship between the study product and an AE was categorized as ‘not related’, ‘unlikely’, ‘possibly’, ‘probably’ and ‘most probably’, as determined by the QI.
Study Procedures
Study visits occurred at baseline, Days 14 and 28, with touchpoints (email or phone contact) conducted on Days 7 and 21 to monitor study product compliance and any changes in health. Participants were instructed to complete the study diary daily. At each study visit, participants returned collected saliva samples, completed study diaries and actigraphy device, had vital signs measured, and completed the PSS, DASS-21, POMS, HPSQI, and COMPASS. Urine pregnancy tests for females of child-bearing potential were completed at screening, baseline and end of study. Clinical chemistry and haematology for safety were conducted at screening and end of study.
To assess compliance, participants were instructed to record study product consumption in their study diary daily and to return unused and open product at their next study visit. Compliance was computed by dividing the number of doses consumed by the number of doses expected to have been consumed multiplied by 100. If there was a discrepancy between compliance reporting methods noted above, compliance was based on returned packages unless a reason was given for product loss.
Statistical Analyses
Based on a previous study by Kell et al. [20], a sample size of 24 participants (n = 12 per group) was estimated to detect a difference in mean change in salivary cortisol of 8.5 nmol/l between the IP and placebo groups from baseline to end-of-study with standard deviations of 5.0 nmol/l for the IP group and 7.0 nmol/l for the placebo group. This estimate achieves 90% power at a 5% significance level using two-sample t-test. The sample size was increased to n = 15 participants per group (total n = 30 participants) to account for a 20% attrition rate.
All statistical analyses were performed using the R Statistical Software Package Version 4.2.1. P values ≤ 0.05 were considered statistically significant. Summary statistics including means, medians, standard deviations, minimum, maximum and proportions (if categorical) were obtained for primary and secondary outcomes at baseline, other timepoints and changes from baseline at each timepoint (for continuous outcomes). For continuous study outcomes, between-group differences were evaluated by two-sample t-test or Wilcoxon rank sum test and within-group differences were assessed by paired t-test or Wilcoxon’s signed rank test [8]. For categorical study outcomes, between-group dissimilarities were evaluated using Fisher’s exact test. Results are presented as mean ± standard deviation unless indicated otherwise. Analyses are reported for the intention-to-treat (ITT) and safety population consisting of all participants who received study product and had post-randomization efficacy data available [8].
Results
Study Population
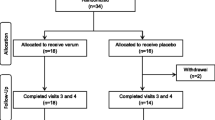
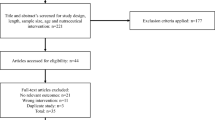
A total of 48 individuals were screened with 30 participants eligible and enrolled in the study (Fig. 2). Fifteen participants were randomly assigned to either AlphaWave® l-Theanine or placebo, and all participants completed the study. The mean age of participants in the AlphaWave® l-Theanine group was 42.47 ± 14.37 years with 80% female participants. The placebo group had a mean age of 39.60 ± 10.22 years with 67% females. There were no significant differences in demographic characteristics between groups (Table 1). Overall study product compliance was 101.78 ± 7.82% for the AlphaWave® l-Theanine group and 101.70 ± 3.04% for the Placebo group with no significant difference between groups.
Safety
A total of nine adverse events (AEs) were reported by six unique participants (Table 2). There were eight AEs reported by five participants supplemented with AlphaWave® l-Theanine and one AE reported by a participant on placebo. Two AEs, metallic taste and dry mouth, reported by two participants in the AlphaWave® l-Theanine group were classified as mild and possibly related to the IP. No other AEs were deemed related to the study products, and all AEs were resolved by the end of the study period or upon subsequent follow up. All out of range laboratory values were deemed not clinically relevant by the QI, and there were no clinically relevant changes in vital signs.
Salivary Cortisol
Mean morning salivary cortisol levels were significantly higher at baseline for the placebo group compared to AlphaWave® l-Theanine (17.05 ± 7.36 vs. 12.64 ± 9.58; p = 0.028). Participants supplemented with AlphaWave® l-Theanine had decreases of 13.84% (− 1.75 ± 9.80 nmol/l) and 7.75% (− 0.98 ± 12.15 nmol/l) in morning salivary cortisol from baseline at Days 14 and 28, respectively (all p > 0.05). Participants on placebo had decreases of 29.85% (− 5.09 ± 10.10 nmol/l) and 29.44% (− 5.02 ± 10.02 nmol/l) at Days 14 and 28, respectively (all p > 0.05). There were no other significant differences in morning or evening salivary cortisol (data not shown).
Perceived Stress Scale
Participants supplemented with AlphaWave® l-Theanine had decreases in PSS scores from baseline at Days 14 (p = 0.051) and 28 (p = 0.04) (Fig. 3). Those on placebo had decreases at Days 14 (p = 0.061) and 28 (p = 0.009). There were no significant differences between groups.
Computerized Mental Performance Assessment System
Participants supplemented with AlphaWave® l-Theanine had decreases in correct reaction time (RT) during the Stroop test from baseline at Days 14 (p = 0.037) and 28 (p = 0.013) (Table 3). Those on placebo had a decrease from baseline at Day 28 (p = 0.005) in Stroop test correct RT with no significant difference at Day 14 (p = 0.108). There were no significant between-group differences in RT outcomes.
For accuracy outcomes, all participants in the placebo group scored 100% for percent correct in the Stroop test at baseline compared to eight participants in the AlphaWave® l-Theanine group (p = 0.039). For incongruent percent correct, all participants on placebo and 62% of those supplemented with AlphaWave® l-Theanine scored 100% at baseline (p = 0.039). There were no other significant between- or within-group differences for Stroop test accuracy outcomes.
There were no significant differences in simple reaction time or choice reaction time outcomes (data not shown).
Depression, Anxiety and Stress Scale
Total DASS-21 score decreased from baseline at Day 28 for those on placebo compared to participants supplemented with AlphaWave® l-Theanine (− 9.20 ± 7.70 vs. 1.07 ± 13.43; p = 0.017). Similarly, stress subscale score decreased in the placebo group compared to the AlphaWave® l-Theanine group from baseline at Day 28 (− 4.67 ± 3.44 vs. 0.53 ± 6.52; p = 0.012). There were no other significant between-group differences in total and stress subscale DASS-21 scores. Participants supplemented with AlphaWave® l-Theanine had a 1.60 ± 8.25 and 1.33 ± 4.82 decrease in total score and stress subscale scores, respectively, from baseline at Day 14 with respective decreases of 5.60 ± 10.70 and 2.53 ± 4.93 for those on placebo (all p > 0.05).
For the anxiety subscale, the AlphaWave® l-Theanine group had a greater score compared to placebo at Day 14 (5.60 ± 4.48 vs. 2.80 ± 3.45; p = 0.020). There were decreases in scores of 0.27 ± 2.37 and 0.13 ± 3.81 in the AlphaWave® l-Theanine group and decreases of 1.73 ± 4.46 and 1.87 ± 4.24 in the placebo group from baseline at Days 14 and 28, respectively (all p > 0.05). There was no difference in the change in depression subscale score for participants supplemented with AlphaWave® l-Theanine, but those on placebo reported a significant decrease from baseline at Day 28 (− 2.67 ± 4.45; p = 0.025).
Actigraphy Device and Healthy People Sleep Quality Index
Mean time asleep was significantly higher at baseline for the AlphaWave® l-Theanine group compared to placebo (Table 4). The AlphaWave® l-Theanine group had a significant decrease in time asleep from baseline at Day 28 and in light sleep from baseline at Days 14 and 28 compared to placebo (Table 4). There were no significant between-group differences in subjective sleep data reported from the HPSQI (data not shown).
Profile of Mood States
There were no significant between-group differences in POMS scores nor were there any significant differences for participants supplemented with AlphaWave® l-Theanine. The placebo group had significant decreases in Total Mood Disturbance, Fatigue-Inertia and Tension-Anxiety from baseline at Days 14 and 28 and in Depression-Dejection from baseline at Day 28 (all p < 0.05; Table S2).
Discussion
This randomized, double-blind, placebo-controlled clinical trial demonstrated that 28-day supplementation with AlphaWave® l-Theanine was safe and well tolerated in a population of healthy adults with moderate stress, further supporting the previously reported safety profile [8]. All reported AEs were resolved by the end of the study or upon subsequent follow up with no clinically relevant changes in vital signs, haematology or clinical chemistry profiles as assessed by the QI. Confirmation of safety and tolerability over a longer period of time is an important step in the investigation of AlphaWave® l-Theanine for improvement of moderate stress in healthy adults.
The current study found AlphaWave® l-Theanine supplementation significantly decreased PSS scores, a validated measure of self-reported stress, in a healthy population of adults who experienced moderate stress on a regular basis. A decrease in stress is important for this population as an inverse association between stress and quality of life has been reported previously [21] suggesting AlphaWave® l-Theanine supplementation may represent a strategy to improve quality of life. This decrease in stress is similar to a previous crossover study that showed supplementation with l-theanine (200 mg/day) for four weeks in healthy adults significantly decreased stress-related outcomes compared to baseline [12]. Moreover, l-theanine supplementation (400 mg/day) for 17 days in healthy pharmacy students resulted in lower stress scores over an examination period compared to placebo [11]. Given the potential benefit of AlphaWave® l-Theanine supplementation on stress and its reported safety profile, future studies in populations with pathological stress who may further benefit from l-theanine supplementation may be warranted.
Although there were improvements in PSS scores from baseline, the lack of statistically significant difference between groups may be due the placebo effect, which is the phenomenon where individuals experience improvements in symptoms based on perceived expectation for change [22]. The placebo effect is commonly reported across human health research and has been specifically reported for stress [23, 24]. Furthermore, given the study population had non-pathological levels of stress, there may have been a greater likelihood of larger natural transient changes in stress over the study period and greater susceptibility to the placebo effect. The findings of the current study suggest this may have been prominent in participants supplemented with AlphaWave® l-Theanine. To account for the potential of the placebo effect, future clinical trials with longer study durations and multiple intra-study visits should be considered to determine if the placebo effect may wane over time in a moderately stressed population.
The decrease in PSS score at the end of study reported by participants in the AlphaWave® l-Theanine group corresponded with a decrease in morning salivary cortisol. This correlation between PSS and cortisol aligns with a previous study that reported higher cortisol levels in those with high stress compared to low stress as assessed by the PSS [25]. The lack of statistical significance in morning salivary cortisol between groups may be due to the large variability in changes in salivary cortisol for participants supplemented with AlphaWave® l-Theanine. There are many factors that may confound salivary cortisol measurements such as time of sampling, vigorous exercise, food intake, light exposure and prior day and/or imminent challenges or stressful events [26, 27]. While the current study was designed specifically to control for the time of sampling upon wakening, vigorous exercise and food intake prior to saliva sample collections, light exposure, brief alterations in sleeping patterns and prior day or unanticipated stressful events may have influenced study results. As participants were instructed to collect saliva samples prior to eating or drinking in the morning, or 30 min after eating or drinking in the evening, consumption of AlphaWave® l-Theanine likely occurred after sample collection. Given a previous randomized controlled trial found a significant decrease in salivary cortisol 1-h post-AlphaWave® l-Theanine supplementation [8], is it possible timing of cortisol collection for the current study might have missed the best timing on collection of cortisol samples for measurement. Future studies should consider the timing of study parameters in relation to AlphaWave® l-Theanine supplementation to fully elucidate the effect AlphaWave® l-Theanine may have on these transient outcomes. The current study suggests the effect of l-theanine might only last a few hours after consumption and does not stay or accumulate in the body for a longer period of time, as it might have efficiently been metabolized by the body [28]. This may have contributed to the safety profile of the product for long-term use, which can be further verified in future studies. Moreover, to minimize variability in participant data, future randomized controlled trials with larger sample sizes should be considered.
Previous research has shown reductions in stress may be associated with improvements in cognitive performance [29]. This finding may be particularly important for individuals who work in high-stress conditions that require higher-order thinking. Results from the current study suggest AlphaWave® l-Theanine supplementation may have a unique ability to improve stress through its calming effects while simultaneously aiding in alertness and focus as demonstrated by decreased reaction time. This study confirmed findings of a previous randomized controlled trial that demonstrated a significant increase in the brain alpha wave power which resulted in a calming effect 3-h post-AlphaWave® l-Theanine supplementation [8]. In the current study, the significant decrease in light sleep for those supplemented with AlphaWave® l-Theanine did not appear to negatively affect cognitive performance, suggesting sleep quality was maintained. l-Theanine may have produced a calming effect without causing drowsiness while also improving alertness and reaction time. These unique benefits of l-theanine make it a desirable product for the fast pace of modern life, as it may reduce stress and improve sleep quality and performance without the need for a longer sleep duration. In contrast to the current study, a previous study assessed l-theanine supplementation (100.6 mg) in healthy adults with self-reported decline in cognitive function, without significant improvements in reaction time during the Stroop test after 12 weeks [30]. However, this study administered a lower dose compared to the current study, which may explain conflicting longer-term results. Further research investigating AlphaWave® l-Theanine in stressed populations with related cognitive performance, or in populations with subjective cognitive decline, may be considered to better understand the relationship among AlphaWave® l-Theanine, stress, sleep and cognition.
The worsening in DASS-21 found for those supplemented with AlphaWave® l-Theanine contrasts with a previous study that administered a dietary supplement containing l-theanine (50 mg/day) to stressed healthy adults for 56 days and found a significant decrease in DASS stress subscale scores compared to placebo after 28 days of supplementation [31]. These contrasting study results may be due to methodological differences between studies including only partial blinding of participants to the study product due to the lack of structural similarity of treatment and placebo products [31]. This may have increased expectancy effects in those receiving the dietary supplement and lowered expectancy effects in those taking placebo. Interestingly, change at the end of study in DASS-21 stress subscale scores for those receiving AlphaWave® l-Theanine did not align with improvement in PSS scores. Differences in findings between the PSS and DASS-21 scores may be explained by what each scale is purported to measure. The PSS is an assessment of how much an individual perceives life to be unpredictable and uncontrollable [32] whereas the DASS-21 stress subscale is more focused on assessing symptoms such as difficulty relaxing, nervous arousal and irritability [15]. Thus, the PSS assesses overall feelings of lack of control rather than specific stressors measured in the DASS-21, possibly contributing to the disagreement between tools. Moreover, participants were instructed to recall the past two weeks when responding to the PSS while the DASS-21 instructed participants to recall the past week. Therefore, a longer recall period may have allowed for a positive experience to dilute stressful events potentially contributing to the incongruent results. The current study suggests there are potential beneficial effects of AlphaWave® l-Theanine supplementation on stress; however, further research is needed to confirm these conflicting findings and better understand this relationship.
The current study had several strengths including the use of an objective stress measure to enroll participants with moderate stress. Moreover, a comprehensive assessment of stress utilizing objective and subjective measures and efforts to minimize controllable confounders in saliva sampling for cortisol analysis were strengths of the study design. While these findings add to the literature examining l-theanine on markers of stress, there were limitations that must be considered. As the study population was comprised primarily of females, the generalizability of study results may be limited, and future studies may consider enrolling equal numbers of males and females. Furthermore, to reduce inter-individual variability observed in the current study, future clinical trials with larger sample sizes should be considered.
In conclusion, 28 days of AlphaWave® l-Theanine supplementation was safe and well tolerated and significantly decreased perceived stress from baseline in a population of moderately stressed healthy adults. Furthermore, the AlphaWave® l-Theanine group demonstrated a significant decrease in light sleep at Days 14 and 28 compared to the placebo group. Moreover, AlphaWave® l-Theanine supplementation resulted in sustained improvement in cognitive attention over the study period. Overall, this study confirmed the safety and benefits of the longer-term use of l-theanine for self-reported stress mitigation, improvement of sleep quality and cognitive attention. Similar improvements in perceived stress were also found for the placebo group at Day 28. Therefore, given the potential for the placebo effect to influence markers of stress combined with its reported safety profile, future study in populations with pathological stress may be considered. Interestingly, there was no change in salivary cortisol despite an improvement in perceived stress, for which the relationship between objective and subjective measures of stress warrants further investigation. Furthermore, based on the large variability observed in the current study, future larger randomized controlled trials with longer AlphaWave® l-Theanine supplementation should be considered.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Medline Plus. Internet: https://medlineplus.gov/ency/article/003211.htm. Accessed 4 Jan 2024.
Statistics Canada. Table 1310-0096-01 Health characteristics, annual estimates. 2018.
The American Institute of Stress. Internet: https://www.stress.org/daily-life. Accessed 4 Jan 2024.
Mayo Clinic. Internet: https://www.mayoclinic.org/healthy-lifestyle/stress-management/in-depth/stress-relievers/art-20047257. Accessed 4 Jan 2024.
Mayo Clinic. Internet: https://www.mayoclinic.org/diseases-conditions/insomnia/in-depth/sleeping-pills/art-20043959. Accessed 4 Jan 2024.
Sakato Y. The chemical constituents of tea; III. A new amide theanine. Nippon Nogeikagaku Kaishi. 1949;23:262–7.
Bryan J. Psychological effects of dietary components of tea: caffeine and l-theanine. Nutr Rev. 2008;66(2):82–90.
Evans M, McDonald AC, Xiong L, Crowley DC, Guthrie N. A randomized, triple-blind, placebo-controlled, crossover study to investigate the efficacy of a single dose of AlphaWave® l-theanine on stress in a healthy adult population. Neurol Ther. 2021;10(2):1061–78.
Kimura K, Ozeki M, Juneja LR, Ohira H. l-Theanine reduces psychological and physiological stress responses. Biol Psychol. 2007;74(1):39–45.
White DJ, De Klerk S, Woods W, Gondalia S, Noonan C, Scholey AB. Anti-stress, behavioural and magnetoencephalography effects of an l-theanine-based nutrient drink: a randomised, double-blind, placebo-controlled, crossover trial. Nutrients. 2016;8(1):53.
Unno K, Tanida N, Ishii N, Yamamoto H, Iguchi K, Hoshino M, Takeda A, Ozawa H, Ohkubo T, Juneja LR. Anti-stress effect of theanine on students during pharmacy practice: positive correlation among salivary α-amylase activity, trait anxiety and subjective stress. Pharmacol Biochem Behav. 2013;111:128–35.
Hidese S, Ogawa S, Ota M, Ishida I, Yasukawa Z, Ozeki M, Kunugi H. Effects of l-theanine administration on stress-related symptoms and cognitive functions in healthy adults: a randomized controlled trial. Nutrients. 2019;11(10):2362.
Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, Elbourne D, Egger M, Altman DG, Consort. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg. 2012;10(1):28–55. https://doi.org/10.1016/j.ijsu.2011.10.001.
Cohen S, Williamson GM. Perceived stress in a probability sample of the United States. In: Spacapan S, Oskamp S, editors. The social psychology of health: Claremont symposium on applied social psychology. Newbury Park: Sage; 1988. p. 31–67.
Psychology Foundation of Australia. Internet: http://www2.psy.unsw.edu.au/dass/over.htm. Accessed 4 Jan 2024.
Wightman EL, Haskell-Ramsay CF, Thompson KG, Blackwell JR, Winyard PG, Forster J, Jones AM, Kennedy DO. Dietary nitrate modulates cerebral blood flow parameters and cognitive performance in humans: a double-blind, placebo-controlled, crossover investigation. Physiol Behav. 2015;149:149–58.
Steiner GZ, Yeung A, Liu J-X, Camfield DA, Blasio FMd, Pipingas A, Scholey AB, Stough C, Chang DH. The effect of Sailuotong (SLT) on neurocognitive and cardiovascular function in healthy adults: a randomised, double-blind, placebo controlled crossover pilot trial. BMC Complement Altern Med. 2015;16(1):1–13.
Heuchert JP, McNair DM. Profile of Mood States, 2nd Edition: POMS 2. Multi-Health Systems Inc., 2012.
Moulin M, Lewis ED, Crowley DC, Langston J, Evans M. A randomized, double-blind, placebo-controlled, cross-over pilot study to investigate the efficacy of Rest-ZZZ formula in healthy participants with occasional sleeplessness. Sleep Biol Rhythms. 2023;21(1):59–68.
Kell G, Rao A, Katsikitis M. A randomised placebo controlled clinical trial on the efficacy of Caralluma fimbriata supplement for reducing anxiety and stress in healthy adults over eight weeks. J Affect Disord. 2019;246:619–26.
Ribeiro ÍJ, Pereira R, Freire IV, de Oliveira BG, Casotti CA, Boery EN. Stress and quality of life among university students: a systematic literature review. Health Prof Educ. 2018;4(2):70–7.
National Institutes of Health. Internet: https://newsinhealth.nih.gov/2023/01/powerful-placebo#:~:text=The%20placebo%20effect%20works%20by,anxiety%2C%20and%20other%20unpleasant%20feelings. Accessed 4 Jan 2024.
Darragh M, Yow B, Kieser A, Booth RJ, Kydd RR, Consedine NS. A take-home placebo treatment can reduce stress, anxiety and symptoms of depression in a non-patient population. Aust N Z J Psychiatry. 2016;50(9):858–65.
Balodis IM, Wynne-Edwards KE, Olmstead MC. The stress–response-dampening effects of placebo. Horm Behav. 2011;59(4):465–72.
Van Eck MM, Nicolson NA. Perceived stress and salivary cortisol in daily life. Ann Behav Med. 1994;16(3):221–7.
Hansen ÅM, Garde AH, Persson R. Sources of biological and methodological variation in salivary cortisol and their impact on measurement among healthy adults: a review. Scand J Clin Lab Investig. 2008;68(6):448–58.
Law R, Hucklebridge F, Thorn L, Evans P, Clow A. State variation in the cortisol awakening response. Stress. 2013;16(5):483–92.
Scheid L, Ellinger S, Alteheld B, Herholz H, Ellinger J, Henn T, Helfrich H-P, Stehle P. Kinetics of l-theanine uptake and metabolism in healthy participants are comparable after ingestion of l-theanine via Capsules and Green Tea, 4. J Nutr. 2012;142(12):2091–6.
Nguyen HT, Quandt SA, Grzywacz JG, Chen H, Galván L, Kitner-Triolo MH, Arcury TA. Stress and cognitive function in Latino farmworkers. Am J Ind Med. 2012;55(8):707–13.
Baba Y, Inagaki S, Nakagawa S, Kaneko T, Kobayashi M, Takihara T. Effects of l-theanine on cognitive function in middle-aged and older subjects: a randomized placebo-controlled study. J Med Food. 2021;24(4):333–41.
Noah L, Morel V, Bertin C, Pouteau E, Macian N, Dualé C, Pereira B, Pickering G. Effect of a combination of magnesium, B vitamins, rhodiola, and green tea (l-theanine) on chronically stressed healthy individuals—a randomized, placebo-controlled study. Nutrients. 2022;14(9):1863.
Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96.
Acknowledgements
The authors thank the participants for their compliance to the conduct of the study.
Medical Writing and Editorial Assistance
The authors did not use any medical writing or editorial assistance for this article.
Funding
This research was funded by Ethical Naturals, Inc. The journal’s Rapid Service Fee was funded by Ethical Naturals, Inc.
Author information
Authors and Affiliations
Contributions
Conceptualization, Marc Moulin, Lora Xiong, Najla Guthrie, Erin D. Lewis; methodology, Marc Moulin, David C. Crowley, Lora Xiong, Erin D. Lewis; investigation, David C. Crowley; data curation, David C. Crowley; formal analysis, Marc Moulin, Erin D. Lewis; writing—original draft preparation, Marc Moulin, Erin D. Lewis; writing—review and editing, Marc Moulin, Erin D. Lewis, Lora Xiong; visualization, Marc Moulin, Erin D. Lewis; supervision, David C. Crowley, Najla Guthrie, Erin D. Lewis; funding acquisition, Lora Xiong. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Lora Xiong is an employee of Ethical Naturals Inc., Marc Moulin, David C. Crowley, Najla Guthrie, and Erin D. Lewis are employees of KGK Science Inc.
Ethical Approval
Research ethics board approval was granted on March 3, 2023, from Advarra (Aurora, Ontario, Canada; Pro00069946). Written informed consent was obtained from all participants prior to any study procedures being initiated. The study was conducted in accordance with the Declaration of Helsinki and its later amendments.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Moulin, M., Crowley, D.C., Xiong, L. et al. Safety and Efficacy of AlphaWave® l-Theanine Supplementation for 28 Days in Healthy Adults with Moderate Stress: A Randomized, Double-Blind, Placebo-Controlled Trial. Neurol Ther (2024). https://doi.org/10.1007/s40120-024-00624-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40120-024-00624-7