Abstract
The number of ageing people with relapsing multiple sclerosis (RMS) is increasing. The efficacy of disease-modifying therapies (DMTs) for RMS declines with age. Also, older persons with MS may be more susceptible to infections, hospitalisations and malignancy. Aging people with MS have higher rates of comorbidities versus aged-matched controls, increasing the individual risk of disability. We review the therapeutic properties of cladribine tablets (CladT) in ageing people with RMS, with regard to their utility for allowing these individuals to cease continuous administration of a DMT (i.e. to act as an “exit therapy”). CladT is thought to be an immune reconstitution therapy, in that two short courses of oral treatment 1 year apart provide suppression of MS disease activity in responders that far outlasts the duration of treatment and post-treatment reductions in lymphocyte counts. Post hoc analyses, long-term follow-up of populations with RMS in randomised trials, and real-world evidence suggest that the efficacy of CladT is probably independent of age, although more data in the elderly are still needed. No clear adverse signals for lymphopenia or other adverse safety signals have emerged with increasing age, although immunosenescence in the setting of age-related “inflammaging” may predispose elderly patients to a higher risk of infections. Updating vaccination status is recommended, especially against pneumococci and herpes zoster for older patients, to minimise the risk of these infections. CladT may be a useful alternative treatment for ageing people with MS who often bear a burden of multiple comorbidities and polypharmacy and who are more exposed to the adverse effects of continuous immunosuppressive therapy.
Similar content being viewed by others
The number of ageing people with relapsing multiple sclerosis (RMS) is increasing. |
As people with RMS age, the efficacy of disease-modifying therapies (DMTs) for RMS may decline while safety and adherence issues may increase. |
Data from randomised trials and real-world evidence suggest that the effectiveness of cladribine tablets does not decrease with increasing age. |
No clear adverse signals for lymphopenia or other adverse safety signals have emerged in older people with MS. |
Cladribine tablets appear to be a rational therapeutic option as an exit therapy for ageing people with RMS who have difficulty with tolerating or adhering to continuous immunosuppressive DMT regimens. |
Introduction
Important demographic shifts are occurring in the population of people with relapsing multiple sclerosis (RMS). These include an increase in the overall age of this population, which is of potential importance as the efficacy of disease-modifying therapies (DMTs) has been reported to decrease, and the risk of infections has been reported to increase, as people with MS grow older [1, 2]. It is therefore important to study the effects of individual DMTs in older and younger people with RMS separately.
Most high-efficacy DMTs used in the management of RMS act by continuously applied immunosuppression [3]. Immune reconstitution therapy (IRT), by contrast, is given as a small number of short courses of treatment, as the efficacy of the treatment in a responder to therapy far outlasts both the administration period of the drug and its effects on immune cell counts [3]. Cladribine tablets (CladT) and alemtuzumab are selective IRTs that are available currently for the management of RMS [4].
This narrative review considers the changing demographic background of RMS, the challenges associated with managing the ageing people with RMS, and the efficacy, tolerability and safety of pharmacologic IRT in this population. In particular, we consider the extent to which CladT as an IRT may represent a potential “exit therapy”, i.e. when a prolonged period of freedom from MS disease activity following a successful response to IRT provides an opportunity for cessation of DMT for selected cases.
The therapeutic use of alemtuzumab has been restricted in some countries because of concerns over the potential for serious cardio-cerebrovascular adverse events and new-onset secondary autoimmune conditions such as thyroid disease (in as many as half of recipients of this treatment [5]), haematological and renal autoimmune diseases [6,7,8,9]. In addition, we do not review the use other treatments which may demonstrate some IRT-like properties, such as autologous haematopoietic stem cell therapy (aHSCT), which is based on different protocols and requires specialized centres [10], mitoxantrone which is less used in the management of RMS as a result of concerns over serious side effects including cardiotoxicity and increased risk of leukaemia [11, 12], or anti-CD20 agents which (like mitoxantrone) are administered as continuous immunosuppressants [4]. Accordingly, this review will focus mainly on the effects of CladT according to age.
This review article is based on previously conducted studies and the clinical expertise of the authors in treating patients with RMS. No new clinical studies were performed by the authors. No patient-specific efficacy or safety data were reported; therefore, institutional review board (IRB)/ethics approval was not required.
The MS Population is Getting Older
Demographic Shifts in the Global Population with MS
The population with MS is becoming older, on average. In Manitoba, Canada, the age range for the peak prevalence of MS moved from 35 to 39 years (with a prevalence of about 80/100,000) in 1984 to 55–59 years (with a prevalence of about 470/100,000) in 2006 [13]. A study in Genoa, Italy, found an increasing proportion of older people with MS comparing the years 2007 with 1997; at the later time point, 18% of their MS population were aged > 65 years [14]. The median age at last clinical contact was 48 years in the Observatoire Français de la Sclérose en Plaques (OFSEP) cohort, with a quarter of the population aged ≥ 57 years, in a survey published in 2018 [15]. In the USA 55% of people with MS are over 50 years of age and the prevalence of people with MS aged ≥ 66 years in Switzerland in 2015 was 60/100,000 for women and 20/100,000 for men [16].
Improvements in life expectancy are also contributing to the ageing of the MS population: data from the Global Burden of Disease Study showed that the age-standardised mortality rate for people with MS vs. the general population declined by 11.5% (uncertainty interval − 35.4 to − 4.7) between 1990 and 2016 [17] and estimated the annual percentage change in mortality in people with MS to be − 0.62 (95% CI − 0.67 to − 0.56) between 1990 and 2019 [18]. In Norway, the standardised mortality ratio (SMR) for people with MS vs. the general population was 3.0 for the years 1953–1974, 3.1 for 1975–1996, and 0.8 for 1997–2012 [19]. More data from Norway reported SMRs for people with vs. without MS of 2.2–3.2 for time periods between < 1950 and 1989, followed by SMRs of 1.9 for 1990–1999 and 1.1 for 2000–2015 (Fig. 1) [20].
Changes in the standardised mortality ratio (SMR) and excess death rates (EDR) for people with multiple sclerosis compared with the general population from a registry-based observational study in Norway. Bars are 95% confidence intervals. Drawn from data presented in ref. [20]
The timings of these improvements in outcome among the population with RMS coincide broadly with the era of DMT use in RMS management, which can be considered to begin with the introduction of the interferons in the 1990s. A publication in 2012 reported increased life expectancy for people with MS treated early and continuously with interferon-β in randomised clinical trials [21], and this finding was supported by real-world data in 2019 [22]. Non-receipt of DMT was a significant predictor of excess mortality in US veterans in the outpatient setting [23] and other observational studies have reported lower mortality rates in people with MS with vs. without (or with lower exposure to) DMTs [24, 25]. It is likely, therefore, that the availability of modern DMT has contributed to the increase in longevity of people with MS. However, mortality rates were decreasing in this population before the availability of DMTs, so other factors are likely to play some role [26]. More data with longer follow-up with high-efficacy DMTs will be required to quantify the effect of modern DMT on mortality rates in people with MS. Moreover, new diagnostic criteria play an important role in the increasing rate of diagnosis of late onset of MS.
Increasing Age and the Phenotype and Pathology of RMS
Retrospective studies suggest that younger vs. older people with MS tend to have a higher frequency of relapses, with a secondary progressive component to MS often apparent during and after their fifties [27,28,29,30]. One study of 11,722 relapses in 2477 people with MS showed that the frequency of relapses decreased by 17% during each 5-year period post-MS onset, with about three in four reporting a 5-year relapse-free period at some time before the onset of secondary MS progression [30]. A model derived within a post hoc analysis of the placebo arms of trials that evaluated fingolimod showed that even comparing individuals aged 20 vs. 30 years was associated with a 1.7–1.8-fold increase in the absolute relapse rate at the younger age [31]. These changes are consistent with a shift from a relapsing form of MS, characterised by relapses and focal inflammation in central nervous tissues, to a more exclusively progressive form as the patient ages. Overall, it has been reported that a substantial minority of patients diagnosed with RMS (about one-third) still have RMS as they become elderly [32].
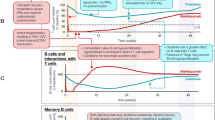
Natural ageing involves modifications of the immune system (immunosenescence), which may be accelerated in people with MS [33,34,35]. Increased levels of chronic, low-grade inflammation and increased secretion of inflammatory cytokines, partly in response to infections, leads to a more pro-inflammatory overall phenotype in older subjects, which has been termed “inflammaging” [36, 37]. These adverse changes are exacerbated by the effects of substances secreted by already senescent cells and can promote the onset of senescence in previously healthy cells, setting up a vicious circle of inflammation and immunosenescence [36, 37]. Senescence of T cells, coupled with thymic involution (age-related atrophy of the thymus) and inflammaging, has been described as a key drive of age-related diseases [36, 38]. Neuronal repair mechanisms are attenuated in older people, partially related to abnormal function of innate immune cells (macrophages and microglia). Oxidative stress (partly caused by increased iron deposition), dysfunction of mitochondria and increased levels of pro-inflammatory cytokines also contribute to accelerated ageing of the brain in MS [32]. The toxic milieu of inflammaging, oxidative stress and immunosenescence accelerates the neuronal damage in MS that leads to the progression of disability [38]. Figure 2 summarises these deficiencies, which likely underpin the observations of diminished the efficacy of DMTs and increased susceptibility to infections in older people described above [38, 39].

Reproduced from ref. [38] in accordance with the Creative Commons Attribution License CC BY (http://creativecommons.org/licenses/by/4.0/). The figure has been redrawn for clarity only. Abs antibodies, NK natural killer (immune cells)
Overview of mechanisms of immune senescence and diminished efficacy of disease-modifying therapies (DMT) in the setting of inflammaging in a person with relapsing multiple sclerosis.
Ageing also increases the risk of significant comorbidities, such as cardiovascular disease, cancer, diabetes, respiratory disease, and psychiatric disorders, which increase the risk of premature mortality in people with MS and may also impact negatively on MS disability [40, 41]. Increased comorbidity is likely to mean additional pharmacologic treatments and increasing age increases the likelihood of polypharmacy in people with MS [42, 43], and higher burden of medication or comorbidities leads in turn to an impaired adherence to treatment and an increased risk of hospitalisation in this population [44]. Cognitive impairment in people with MS can also impair ability to self-care [45, 46].
Changes in the Efficacy of Disease-Modifying Therapy with Age
Age and the Effectiveness of Therapies Based on Continuous Immunosuppression
A meta-analysis published in 2017 evaluated the effect of age on the efficacy of DMTs used in the management of RMS using the ability of the DMTs to prevent the progression of disability (“inhibition of disability progression”; IDP) as a marker of their efficacy in randomised controlled trials [47]. Estimates of IDP in the pooled data for all included trials fell from about 60% at age 30 years to essentially 0% at age 50 years. This was true for both platform and high-efficacy DMTs, although the rate efficacy loss with age was higher for the high-efficacy DMTs, having started from a higher value at age 30 years. Increasing age was the strongest predictor of efficacy loss of DMTs in this analysis, with the influence of the Expanded Disability Status Scale (EDSS) becoming non-significant in higher-order multivariate models.
The results of a small, retrospective study in 35 people with MS aged > 60 years supported the findings of this meta-analysis, in that discontinuation of a DMT did not predict a higher frequency of MS relapses or increased progression of disability during a subsequent, 2-year follow-up period [48]. The post hoc analysis of randomised evaluations of fingolimod, also showed greater effects of the DMT in younger than in older individuals with regard to suppression of relapses and new MRI lesions and achievement of no evidence of disease activity [31]. A meta-analysis quantified the efficacy of DMT treatment in older and younger subgroups, compared with the overall population, and found a relative effectiveness of 0.83 vs. 1.30, respectively (p < 0.001) for relapse rate and 0.82 vs. 1.28, respectively (p = 0.017) for disability progression [49].
A post hoc analysis of data from the randomised, OPERA I and II studies (comparisons of ocrelizumab with interferon-β1a) reported significant interactions between age and the effect of ocrelizumab on the annual relapse rate (p = 0.0055) and the proportion with no evidence of disease activity (NEDA; p = 0.0006), with smaller effects for age > 40 years, although no significant interaction was seen for other treatment outcomes [50]. The 2017 meta-analysis, described above, included a limited range of ages, as the average ages of subjects in the included trials ranged from about 32 years to about 45 years [47], as older individuals have largely been excluded from randomised, phase III trials [51]. In addition, IRT was not well represented in this meta-analysis. Three trials on alemtuzumab were included, which had mean ages at baseline of 32–35 years, limiting the scope for examining the effects of this treatment according to age within the design of the analysis (although other data on this aspect of alemtuzumab are presented later in the article) [52,53,54]. CladT was not included at all in this analysis, which therefore seems to relate mainly to the interactions between efficacy and age of either immunomodulators, such as the interferons, or DMTs acting via continuous immunosuppression, including other high-efficacy DMTs. Data on the therapeutic profile of CladT in older people with RMS is discussed separately below. Importantly, the authors of this meta-analysis did not conclude that all older people with MS should be denied treatment with a DMT, as their pooled data concealed variation in the response of individuals to DMTs.
Age and the Effectiveness of Cladribine Tablets
The randomised, placebo-controlled Cladribine Tablets Treating Multiple Sclerosis Orally (CLARITY) study was the pivotal phase III clinical trial that underpinned the approval of CladT for therapeutic use in RMS [55]. A total of 1326 people with RMS and at least one relapse in the previous year, who had received no more than one DMT previously (no immunosuppressants or cytokine-based treatments) were randomly assigned to treatment with placebo or one of two doses of CladT (cumulative total doses of 3.5 or 5.25 mg/kg, given as two 4–5-day courses 1 month apart at the start of the first and second years of treatment). The efficacy response was similar for the lower and higher doses (e.g. annualised relapse rate (ARR) at 2 years was 0.14 and 0.15, respectively, vs. 0.33 for placebo and similar benefits for the two doses were seen relative to placebo for reduced risk of confirmed EDSS progression and reduced MRI lesions in the CNS). As the risk of severe lymphopenia was higher for the 5.25 mg/kg arm, only the cumulative 3.5 mg/kg dose is used clinically, and all data on CladT in this review refer to this dose. Long-term (10.9 years) post-trial follow-up of 435 enrolees from the CLARITY study demonstrated long-term benefits from treatment with CladT 3.5 mg/kg vs. placebo, including a higher proportion never needing a wheelchair (88.2% vs. 77.8%), never needing additional DMT (66.3% vs. 36.6%), and with no evidence of disease reactivation (50% vs. 28%) [56]. A post hoc analysis of CLARITY stratified the trial population by age ≤ 45 years (N = 649) and > 45 years (N = 221) [57]. The risk of MS relapses was reduced significantly in both age groups (Fig. 3). In addition, there were significant (p < 0.0001) reductions in the mean [95% CI] number (per subject per scan) of active T2 MRI lesions in those aged ≤ 45 years (− 0.667 [− 0.67 to − 0.5]) and > 45 years (− 0.167 [− 0.33 to 0.00]), as well as significant (p < 0.0001) reductions in the number of combined unique lesions in each group (− 0.667 [− 1.00 to − 0.67] and − 0.333 [− 0.33 to 0.00], respectively).
Effect of cladribine tablets 3.5 mg/kg on annualised relapse rates in a post hoc analysis of the CLARITY randomised trial. aAdjudicated relapses counted as events in the original analysis. CI confidence intervals. Drawn from data presented in ref. [57]
Real-world studies facilitate the study of the effects of interventions in a broader population outside the restrictive inclusion/exclusion criteria of randomised clinical trials. Follow-up from the Australian MSBase registry cohort included 60 patients with RMS who had a mean age of 45 years, which was somewhat higher than the mean age at baseline of 39 years in the CLARITY study [55, 58]. ARR was reduced from 1.8 at baseline to 0.31 at 2 years after treatment with CladT. Another real-world study evaluated CladT in subgroups aged ≥ 50 years (N = 31) and aged < 50 years (N = 62), with average follow-up of 13 months. After receiving CladT, high and similar proportions of the < 50 years and ≥ 50 years groups were relapse-free (97.1% and 95.2%, respectively) and without progression of disability (91.4% vs. 88.7%, respectively). There was no difference between age groups for the risk of MS disease activity (hazard ratio [HR] 0.73 [95% CI 0.18–2.91, p = 0.657), consistent with this finding, or of adverse events (odds ratio 0.84 [95% CI 0.24–2.93], p = 0.791) [59] (Fig. 4).

Reproduced from ref. [64] according to the terms of the Creative Commons Attribution License (CC BY) (http://creativecommons.org/licenses/by/4.0/)
Time to first episode of grade 3 or 4 lymphopenia according to age during treatment of patients with relapsing multiple sclerosis with cladribine tablets.
A similar lack of effect of age on the efficacy of alemtuzumab was seen in a post hoc, pooled analysis of long-term extensions to the randomised CARE-MS I and CARE-MS II phase III studies [60]. The median number of relapses in the year before randomisation was one [52, 53], and the ARRs during 3–8 years post-randomisation in the alemtuzumab group were 0.19 for those aged 18–25 years, 0.16 for 26–35 years, 0.19 for 36–45 years and 0.15 for 46–55 years.
Lymphopenia and Infections
CladT induces a profound reduction in B lymphocytes, with a lesser reduction in T lymphocytes, with nadirs occurring several months after dosing with CladT, followed by a gradual recovery [61]. Recovery of these cell types occurs over approximately 30 and 43 weeks, respectively, after the second course of CladT [61]. Accordingly, lymphopenia is a recognised side effect of CladT [62]. Severe (grade 3 or 4) lymphopenia is uncommon, especially if the absolute lymphocyte count (ALC) is allowed to recover to at least 800/mm3 before the second annual course of treatment with CladT, as per the product label.
A real-world analysis comprising data from spontaneous adverse event reporting and post-marketing studies estimated the incidence of severe lymphopenia as 0.12 events/100,000 person-years of treatment [63]. A pooled analysis estimated the incidence of lymphopenia in those who received CladT according to age [64] using data from the CLARITY study [55] and its extension [65] and the ORACLE study in clinically isolated syndrome (total N = 1564) [66]. The incidence of grade 3 lymphopenia during year 1 of treatment with CladT was 8.3% (age < 50 years) and 10.0% (age ≥ 50 years) and no grade 4 lymphopenia occurred at this time. During year 2 of treatment, the incidences of grade 3 lymphopenia for the age < 50 years and ≥ 50 years age groups were 18.7% and 20.0%, respectively, with corresponding incidences of grade 4 lymphopenia of 0.3% and 1.0%, respectively. Figure 4 shows the time to the first occurrence of grade 3 or 4 lymphopenia in this population. The median times to recovery from grade 3–4 to grade ≤ 2 lymphopenia were 5.7 weeks for age < 50 years and 6.4 weeks for age ≥ 50 years.
It should be noted that the randomised evaluations of CladT included in this analysis employed a fixed dosing schedule, where the second-year course was given 1 year after the first course irrespective of the lymphocyte count. Now, the CladT label allows the year 2 course to be delayed for up to 6 months to allow the ALC to rise to ≥ 800/mm3. Real-world data suggest that this change has reduced the potential for severe lymphopenia with CladT [59, 62].
The same integrated analysis also included data on infections [64]. The overall incidence of any treatment-emergent viral or bacterial infection was 19.71/100 person-years (95% CI 16.28–23.87) for age < 50 years and 23.78/100 person-years (95% CI 14.78–38.25) for age ≥ 50 years. The incidences of viral upper respiratory tract infections (URTI) and influenza were generally comparable between age groups; however, there was an apparent trend towards an excess incidence of herpes zoster in the older age group versus the younger age group (3.37/100 person-years [1.27–8.99] vs. 0.76/100 person-years [0.36–1.59]). The appearance of herpes zoster during treatment with CladT is usually associated with ALC < 500/mm3 [62, 67]. Importantly, a specific non-live vaccine is now available in several countries that prevents such infectious complications [68]. This vaccination should be recommended prior to CladT treatment if the patient is seronegative for varicella zoster virus.
Patient-Reported Outcomes
An uncontrolled, prospective observational study in 58 CladT-treated people with RMS evaluated satisfaction with treatment 6 months following administration of the first course of CladT using the validated Treatment Satisfaction Questionnaire for Medication (TSQM) instrument [69]. TSQM scores (a score of 100 signifies total satisfaction with treatment) were similar following stratification for age < 45 years (mean score 76.4 ± 17.0) or ≥ 45 years (mean score 73.5 ± 15.2).
Practical Considerations for Prescribing Cladribine Tablets as Exit Therapy in the Ageing Person with RMS
The evidence summarised above suggests that the efficacy of some DMTs in preventing relapses and/or disability decreases with increasing age, while the risk of infection increases with age. These trends imply a less positive risk-to-benefit ratio for the ageing vs. younger person with MS. Whether to withdraw DMT in ageing people with MS needs careful consideration of the potential risks and benefits for the individual [27, 51, 70]. However, prescribing an intervention to protect an ageing person with MS from future MS disease activity is often reasonable: a recent randomised trial has shown that there is some risk of reactivation of clinical or radiologic MS disease activity during the 2 years after withdrawing DMT at age > 55 years with no relapse in the last 5 years or new MRI lesions in the last 3 years; 12.2% of the DMT discontinuation group had a new or expanding brain MRI lesion, compared with 4.2% in the DMT continuation group [71, 72]. The randomised DOT MS trial evaluation of treatment discontinuation in people with a mean age of 53 years and stable MS (absence of clinical and radiological inflammatory activity for > 5 years on first-line treatment) was prematurely stopped because of significant inflammation observed in the discontinuation group (6/45 vs. 0/45 in the continuation group at the time the study was terminated) [73]. Currently, another similar study (STOP-I-SEP) is ongoing in France.
Observational data also support this concept. A recent (2024) observational study from the OFSEP registry in France was conducted in people with MS aged > 50 years who had demonstrated no inflammatory MS disease activity for a mean of 5.6 years while receiving a high-efficacy DMT [74]. There was an increased risk of MS relapse between propensity score-matched cohorts of 168 participants who discontinued the DMT vs. 1452 who did not (the hazard ratio [HR] for time to first relapse was 4.1 [95% CI 2.0–8.5], p < 0.001). The risk of MS disease reactivation differed according to the DMT, with HRs (95% CI) of 7.2 (2.14–24.5, p = 0.001) for natalizumab, 4.5 (1.3–15.5, p = 0.02) for fingolimod and 1.1 (0.3–4.8, p = 0.8) for anti-CD20 agents. Similar findings are available from a large retrospective study of 14,213 people with MS enrolled in the MS-BASE and OFSEP registries published in 2022 [75]. These studies did not evaluate CladT, as comparing an IRT such as CladT with continuously applied DMTs is difficult as CladT is given as two annual courses without expectation of further treatment unless MS disease activity recurs [3, 4, 76].
Available evidence suggests that the efficacy of CladT is not markedly diminished in ageing people with MS. Moreover, CladT is generally well tolerated with no safety issues that relate especially to older age, including issues related to lymphocytopenia. CladT is therefore a candidate therapy for selected older persons with MS who need or wish to discontinue active treatment with a DMT. Table 1 shows our expert recommendations on the use of CladT as an exit therapy for people with stable MS, rather than in its more usual role earlier in the course of MS. CladT may be considered for those aged ≥ 45 years previously receiving a platform/first-line DMT (interferons, glatiramer acetate, dimethyl fumarate or teriflunomide [74]) or for age ≥ 55 years after a high-efficacy DMT (S1P inhibitors, natalizumab, anti-CD20 agents, alemtuzumab [77]); 3-point no evidence of disease activity (NEDA-3; no new MRI activity, no clinical relapses, no change in EDSS status [78]) should have persisted for ≥ 5 years in each scenario.
For patients previously receiving a high-efficacy DMT, it is important to consider the severity of MS disease before treatment, and the potential for rebound MS disease activity after withdrawal of the previous DMT. This applies especially (but not exclusively) to natalizumab or S1P inhibitors [75]. It should also be noted that reduced levels of gamma globulins have been observed during treatment with anti-CD20 DMTs [79]. An observational study in 45 subjects with MS who had experienced ≥ 10% reduction in IgG or IgM or in recurrent infections during ≥ 18 months of anti-CD20 therapy showed that the time course of levels of IgG or IgM were similar during 1 year of follow-up after a switch to CladT or continued anti-CD20 treatment, with low MS disease activity and adverse event rates [80]. Switching from anti-CD20 to CladT is therefore feasible, even where levels of antibodies have been depressed, although the potential benefits and risks for each patient will need to be considered.
Effective, evidence-led multidisciplinary care to address comorbidities and other medical or social concerns continues to be important for the older individual with RMS as it is for other conditions, irrespective of whether they continue to receive a DMT or not [81]. Older people with MS may be generally more prone to infections and special care should be given to vaccination if a decision is made to initiate or switch to CladT especially with the non-live vaccine against herpes zoster where available. There is evidence that older age (> 50 years) and the presence of comorbidity reduce the likelihood of an older person with highly active MS receiving a high-efficacy DMT, which may leave them exposed to an unnecessary risk of MS disease activity [82].
A 1-year observational study in MS (N = 503) reported an increased risk of infections with orally administered DMTs (incidence rate ratio [IRR] 2.04 [95% CI 1.19–3.49]) and monoclonal antibody DMTs (IRR 2.32 [95% CI 1.39–3.89) vs. a control group who received interferons, glatiramer acetate or no DMT [1]. The effect of treatment with monoclonal antibody DMTs (vs. other treatments) on infections was stronger in younger (IRR 5.90) vs. older (IRR 1.95) subjects in this analysis. While CladT was included in the oral treatments studied, the analysis was unfortunately not powered to provide the risk of infection with individual DMTs. Careful attention to the ALC following the first course of CladT, ensuring that the second course of CladT is not given until lymphocytes have recovered sufficiently, appears to hold the key to preventing severe lymphopenia associated with increased risk of opportunistic infections.
Older people with MS are more likely to have significant comorbidity and polypharmacy. The lack of need for continuously applied treatment is an advantage of the IRT approach in this setting: as long as adherence to the 8–10 days of treatment required for each of the year 1 and year 2 courses is high (this is usually the case [83] and a support programme is available to assist with this in a number of countries [84, 85]). The question of adherence then becomes effectively moot for responders to treatment, as there is no continued treatment burden [86]. Expert opinion supports the use of CladT as a rational therapeutic option for those with problems adhering to their current regimen, based on the convenience of (lack of continuous) administration and the durability of the treatment benefit seen in responders to treatment [86,87,88]. Importantly, short-term treatment limits drug–drug interactions that may occur particularly in case of polypharmacotherapy.
Conclusions
Reports of declining efficacy and increased risk of infection with increasing age with some DMTs suggest a less positive risk-to-benefit ratio for the ageing vs. younger person with MS. Evidence from randomised trials and real-world studies indicates that the efficacy of CladT in suppressing markers of MS disease activity is similar in older and younger age groups, although more data from the elderly would be welcome. There is also no indication of an increased risk of serious safety issues in older people receiving CladT, though this also requires further study. In addition to its more usual role earlier in the MS disease course, CladT appears to be a rational therapeutic option for selected older people with RMS, including as an “exit therapy” for those with low and stable clinical and radiological MS disease activity on previous continuous DMT.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Jacober SLS, Disanto G, Sacco R, et al. Interplay between age and disease-modifying treatments in influencing infection risk in multiple sclerosis. Mult Scler. 2023. https://doi.org/10.1177/13524585231199820.
Knapp R, Hardtstock F, Krieger J, et al. Serious infections in patients with relapsing and progressive forms of multiple sclerosis: a German claims data study. Mult Scler Relat Disord. 2022;68:104245.
AlSharoqi IA, Aljumah M, Bohlega S, et al. Immune reconstitution therapy or continuous immunosuppression for the management of active relapsing-remitting multiple sclerosis patients? A narrative review. Neurol Ther. 2020;9:55–66.
Sorensen PS, Sellebjerg F. Pulsed immune reconstitution therapy in multiple sclerosis. Ther Adv Neurol Disord. 2019;12:1756286419836913.
Kazakou P, Tzanetakos D, Vakrakou AG, et al. Thyroid autoimmunity following alemtuzumab treatment in multiple sclerosis patients: a prospective study. Clin Exp Med. 2023;23:2885–94.
Sellner J, Rommer PS. Immunological consequences of “immune reconstitution therapy” in multiple sclerosis: a systematic review. Autoimmun Rev. 2020;19:102492.
European Medicines Agency. Lemtrada. Measures to minimise risk of serious side effects of multiple sclerosis medicine Lemtrada. https://www.ema.europa.eu/en/medicines/human/referrals/lemtrada. Accessed Jul 2023.
Richter S, Wagner B, Celius EG. Two cases of diabetes mellitus type 1 after alemtuzumab treatment for multiple sclerosis: another probable secondary autoimmune disease. J Neurol. 2019;266:1270–1.
Costa GD, Comi G. A safety review of current monoclonal antibodies used to treat multiple sclerosis. Expert Opin Drug Saf. 2023. https://doi.org/10.1080/14740338.2023.2224556.
Sharrack B, Saccardi R, Alexander T, et al. Autologous haematopoietic stem cell transplantation and other cellular therapy in multiple sclerosis and immune-mediated neurological diseases: updated guidelines and recommendations from the EBMT Autoimmune Diseases Working Party (ADWP) and the Joint Accreditation Committee of EBMT and ISCT (JACIE). Bone Marrow Transplant. 2020;55:283–306.
Buttmann M, Seuffert L, Mäder U, Toyka KV. Malignancies after mitoxantrone for multiple sclerosis: a retrospective cohort study. Neurology. 2016;86:2203–7.
Kingwell E, Koch M, Leung B, et al. Cardiotoxicity and other adverse events associated with mitoxantrone treatment for MS. Neurology. 2010;74:1822–6.
Marrie RA, Yu N, Blanchard J, Leung S, Elliott L. The rising prevalence and changing age distribution of multiple sclerosis in Manitoba. Neurology. 2010;74:465–71.
Solaro C, Ponzio M, Moran E, et al. The changing face of multiple sclerosis: prevalence and incidence in an aging population. Mult Scler. 2015;21:1244–50.
Vukusic S, Casey R, Rollot F, et al. Observatoire Français de la Sclérose en Plaques (OFSEP): a unique multimodal nationwide MS registry in France. Mult Scler. 2020;26:118–22.
Blozik E, Rapold R, Eichler K, Reich O. Epidemiology and costs of multiple sclerosis in Switzerland: an analysis of health-care claims data, 2011–2015. Neuropsychiatr Dis Treat. 2017;13:2737–45.
GBD 2016 Multiple Sclerosis Collaborators. Global, regional, and national burden of multiple sclerosis 1990–2016: a systematic analysis for the global burden of disease study. Lancet Neurol. 2016;2019(18):269–85.
Qian Z, Li Y, Guan Z, et al. Global, regional, and national burden of multiple sclerosis from 1990 to 2019: findings of global burden of disease study 2019. Front Public Health. 2023;11:1073278.
Lunde HMB, Assmus J, Myhr KM, Bø L, Grytten N. Survival and cause of death in multiple sclerosis: a 60-year longitudinal population study. J Neurol Neurosurg Psychiatry. 2017;88:621–5.
Willumsen JS, Grytten N, Aarseth J, Myklebust TÅ, Myhr KM, Midgard R. Mortality and cause of death in multiple sclerosis in western Norway 1950–2021: a registry-based linkage study. J Neurol Neurosurg Psychiatry. 2022;93(11):1154–61.
Goodin DS, Reder AT, Ebers GC, et al. Survival in MS: a randomized cohort study 21 years after the start of the pivotal IFNβ-1b trial. Neurology. 2012;78(17):1315–22.
Kingwell E, Leray E, Zhu F, et al. Multiple sclerosis: effect of beta interferon treatment on survival. Brain. 2019;142:1324–33.
Rabadi MH, Aston CE. Predictors of mortality in veterans with multiple sclerosis in an outpatient clinic setting. Int J MS Care. 2017;19:265–73.
Ng HS, Zhu F, Kingwell E, et al. Disease-modifying drugs for multiple sclerosis and association with survival. Neurol Neuroimmunol Neuroinflamm. 2022;9: e200005.
Tsai CP, Lee CT. Impact of disease-modifying therapies on the survival of patients with multiple sclerosis in Taiwan, 1997–2008. Clin Drug Investig. 2013;33:647–52.
Koch-Henriksen N, Laursen B, Stenager E, Magyari M. Excess mortality among patients with multiple sclerosis in Denmark has dropped significantly over the past six decades: a population based study. J Neurol Neurosurg Psychiatry. 2017;88:626–31.
Riley CS, Vargas W. Multiple sclerosis in the elderly: considerations in the geriatric population for diagnosis and management. Curr Geriatr Rep. 2015;4:131–41.
Scalfari A, Lederer C, Daumer M, Nicholas R, Ebers GC, Muraro PA. The relationship of age with the clinical phenotype in multiple sclerosis. Mult Scler. 2016;22:1750–8.
Zeydan B, Kantarci OH. Impact of age on multiple sclerosis disease activity and progression. Curr Neurol Neurosci Rep. 2020;20:24.
Tremlett H, Zhao Y, Joseph J, Devonshire V, UBCMS Clinic Neurologists. Relapses in multiple sclerosis are age- and time-dependent. J Neurol Neurosurg Psychiatry. 2008;79:1368–74.
Gärtner J, Chitnis T, Ghezzi A, et al. Relapse rate and MRI activity in young adult patients with multiple sclerosis: a post hoc analysis of phase 3 fingolimod trials. Mult Scler J Exp Transl Clin. 2018;4:2055217318778610.
Sanai SA, Saini V, Benedict RHB, et al. Aging and multiple sclerosis. Mult Scler J. 2016;22:717–25.
Eschborn M, Pawlitzki M, Wirth T, et al. Evaluation of age-dependent immune signatures in patients with multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2021;8: e1094.
Rawji KS, Mishra MK, Michaels NJ, Rivest S, Stys PK, Yong VW. Immunosenescence of microglia and macrophages: impact on the ageing central nervous system. Brain. 2016;139:653–61.
Balusha AAK, Morrow SA. Multiple sclerosis in people over age 55. Pract Neurol. 2021;41:41–3.
Li X, Li C, Zhang W, Wang Y, Qian P, Huang H. Inflammation and aging: signaling pathways and intervention therapies. Signal Transduct Target Ther. 2023;8:239.
Fulop T, Larbi A, Pawelec G, et al. Immunology of aging: the birth of inflammaging. Clin Rev Allergy Immunol. 2023;64:109–22.
Thakolwiboon S, Mills EA, Yang J, et al. Immunosenescence and multiple sclerosis: inflammaging for prognosis and therapeutic consideration. Front Aging. 2023;4:1234572.
Buscarinu MC, Reniè R, Morena E, et al. Late-onset MS: disease course and safety-efficacy of DMTS. Front Neurol. 2022;13: 829331.
Marrie RA, Elliott L, Marriott J, et al. Effect of comorbidity on mortality in multiple sclerosis. Neurology. 2015;85:240–7.
Ostolaza A, Corroza J, Ayuso T. Multiple sclerosis and aging: comorbidity and treatment challenges. Mult Scler Relat Disord. 2021;50: 102815.
Patti F, Penaherrera JN, Zieger L, Wicklein EM. Clinical characteristics of middle-aged and older patients with MS treated with interferon beta-1b: post-hoc analysis of a 2-year, prospective, international, observational study. BMC Neurol. 2021;21:324.
Frahm N, Hecker M, Zettl UK. Polypharmacy in outpatients with relapsing-remitting multiple sclerosis: a single-center study. PLoS ONE. 2019;14:e0211120.
Evans C, Marrie RA, Zhu F, et al. Adherence to disease-modifying therapies for multiple sclerosis and subsequent hospitalizations. Pharmacoepidemiol Drug Saf. 2017;26:702–11.
Gullo HL, Fleming J, Bennett S, Shum DHK. Cognitive and physical fatigue are associated with distinct problems in daily functioning, role fulfilment, and quality of life in multiple sclerosis. Mult Scler Relat Disord. 2019;31:118–23.
Sabanagic-Hajric S, Suljic E, Memic-Serdarevic A, Sulejmanpasic G, Mahmutbegovic N. Quality of life in multiple sclerosis patients: influence of gender, age and marital status. Mater Sociomed. 2022;34:19–24.
Weideman AM, Tapia-Maltos MA, Johnson K, Greenwood M, Bielekova B. Meta-analysis of the age-dependent efficacy of multiple sclerosis treatments. Front Neurol. 2017;8:577.
Salavisa M, Serrazina F, Ladeira AF, Correia AS. Discontinuation of disease-modifying therapy in MS patients over 60 years old and its impact on relapse rate and disease progression. Clin Neurol Neurosurg. 2023;225: 107612.
Signori A, Schiavetti I, Gallo F, Sormani MP. Subgroups of multiple sclerosis patients with larger treatment benefits: a meta-analysis of randomized trials. Eur J Neurol. 2015;22:960–6.
Turner B, Cree BAC, Kappos L, et al. Ocrelizumab efficacy in subgroups of patients with relapsing multiple sclerosis. J Neurol. 2019;266:1182–93.
Strijbis EMM, Kerbrat A, Corboy JR. Discontinuation of disease-modifying therapy in multiple sclerosis: should we stay or should we go? JAMA Neurol. 2021;78:787–8.
Cohen JA, Coles AJ, Arnold DL, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet. 2012;380(9856):1819–28.
Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet. 2012;380(9856):1829–39.
CAMMS223 Trial Investigators, Coles AJ, Compston DA, et al. Alemtuzumab vs interferon beta-1a in early multiple sclerosis. N Engl J Med. 2008;359:1786–801.
Giovannoni G, Comi G, Cook S, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med. 2010;362:416–26.
Giovannoni G, Boyko A, Correale J, et al. Long-term follow-up of patients with relapsing multiple sclerosis from the CLARITY/CLARITY Extension cohort of CLASSIC-MS: an ambispective study. Mult Scler. 2023;29:719–30.
Giovanonni G, Rammohan K, Cook S, et al. Cladribine tablets 35 mg/kg is efficacious in patients aged above and below 45 years with relapsing multiple sclerosis in the clarity study. Mult Scler Relat Disord. 2018;26:P262.
Lizak N, Hodgkinson S, Butler E, et al. Real-world effectiveness of cladribine for Australian patients with multiple sclerosis: an MSBase registry substudy. Mult Scler. 2021;27:465–74.
Disanto G, Moccia M, Sacco R, et al. Monitoring of safety and effectiveness of cladribine in multiple sclerosis patients over 50 years. Mult Scler Relat Disord. 2022;58:103490.
Bass AD, Arroyo R, Boster AL, et al. Alemtuzumab outcomes by age: post hoc analysis from the randomized CARE-MS studies over 8 years. Mult Scler Relat Disord. 2021;49:102717.
Comi G, Cook S, Giovannoni G, et al. Effect of cladribine tablets on lymphocyte reduction and repopulation dynamics in patients with relapsing multiple sclerosis. Mult Scler Relat Disord. 2019;29:168–74.
Clavelou P, Castelnovo G, Pourcher V, et al. Expert narrative review of the safety of cladribine tablets for the management of relapsing multiple sclerosis. Neurol Ther. 2023;12:1457–76.
Giovannoni G, Leist T, Jack D, Galazka A. Updated post-approval safety of cladribine tablets in the treatment of multiple sclerosis, with particular reference to liver safety. Abstract 341 at the 2022 meeting of ECTRIMS, October 26–28 2022. https://doi.org/10.1177/13524585221123687.
Giovannoni G, Coyle PK, Vermersch P, et al. Integrated lymphopenia analysis in younger and older patients with multiple sclerosis treated with cladribine tablets. Front Immunol. 2021;12:763433.
Giovannoni G, Soelberg Sorensen P, Cook S, et al. Safety and efficacy of cladribine tablets in patients with relapsing-remitting multiple sclerosis: results from the randomized extension trial of the CLARITY study. Mult Scler. 2018;24:1594–604.
Freedman MS, Leist TP, Comi G, et al. The efficacy of cladribine tablets in CIS patients retrospectively assigned the diagnosis of MS using modern criteria: results from the ORACLE-MS study. Mult Scler J Exp Transl Clin. 2017;3:2055217317732802.
Cook S, Leist T, Comi G, et al. Safety of cladribine tablets in the treatment of patients with multiple sclerosis: an integrated analysis. Mult Scler Relat Disord. 2019;29:157–67.
Herpes zoster (shingles) vaccination update. Aust Prescr. 2023;46:91.
Inshasi J, Farouk S, Shatila A, et al. Multicentre observational study of treatment satisfaction with cladribine tablets in the management of relapsing multiple sclerosis in the Arabian Gulf: the CLUE study. Neurol Ther. 2023;12:1309–18.
Olival GS, Cavenaghi VB, Serafim V, Thomaz RB, Tilbery CP. Medication withdrawal may be an option for a select group of patients in relapsing-remitting multiple sclerosis. Arq Neuropsiquiatr. 2013;71:516–20.
Corboy JR, Fox RJ, Kister I, et al. Risk of new disease activity in patients with multiple sclerosis who continue or discontinue disease-modifying therapies (DISCOMS): a multicentre, randomised, single-blind, phase 4, non-inferiority trial. Lancet Neurol. 2023;22:568–77.
Corboy J, Engebretson E, Cutter G, et al. DISCOntinuation of disease-modifying therapies in multiple sclerosis (DISCOMS): primary results of the extension trial. Mult Scler J. 2023;29(3S):356.
Coerver E, Fung WH, De Beukelaar J, et al. Discontinuation of first-line disease-modifying therapy in stable multiple sclerosis (DOT-MS): an early terminated multicenter randomized controlled trial. Mult Scler J. 2023;29(3S):76.
Jouvenot G, Courbon G, Lefort M, et al. Effects of high-efficacy therapy discontinuation vs continuation in patients with non-active multiple sclerosis aged over 50. JAMA Neurol 2024. https://doi.org/10.1001/jamaneurol.2024.0395
Roos I, Malpas C, Leray E, et al. Disease reactivation after cessation of disease-modifying therapy in patients with relapsing-remitting multiple sclerosis. Neurology. 2022;99:e1926–44.
De Sèze J, Suchet L, Mekies C, et al. The place of immune reconstitution therapy in the management of relapsing multiple sclerosis in France: an expert consensus. Neurol Ther. 2023;12:351–69.
Alroughani R, Inshasi JS, Deleu D, et al. An overview of high-efficacy drugs for multiple sclerosis: gulf region expert opinion. Neurol Ther. 2019;8:13–23.
Multiple Sclerosis Trust. NEDA (no evidence of disease activity). https://mstrust.org.uk/a-z/neda-no-evidence-disease-activity. Accessed Nov 2023.
Kelly H, Vishnevetsky A, Chibnik LB, Levy M. Hypogammaglobulinemia secondary to B cell depleting therapies in neuroimmunology: comparing management strategies. Mult Scler J Exp Transl Clin. 2023;9:20552173231182536.
Sacco R, Disanto G, Pravatà E, Gobbi C, Zecca C. Safety and efficacy of cladribine therapy following a treatment with anti-CD20 compounds in relapsing multiple sclerosis patients: a pilot study. Mult Scler J. 2022;28(3S):852.
Ellis G, Sevdalis N. Understanding and improving multidisciplinary team working in geriatric medicine. Age Ageing. 2019;48:498–505.
Ysrraelit MC, Piedrabuena MA, Fiol M, et al. Selection of high efficacy disease modifying therapies in multiple sclerosis patients older than 50 years. Mult Scler J. 2023;29(3S):633 (Abstract).
Brownlee W, Amin A, Ashton L, Herbert A. Real-world use of cladribine tablets (completion rates and treatment persistence) in patients with multiple sclerosis in England: the CLARENCE study. Mult Scler Relat Disord. 2023;79:104951.
Lenz F, Harms L. The impact of patient support programs on adherence to disease-modifying therapies of patients with relapsing-remitting multiple sclerosis in Germany: a non-interventional, prospective study. Adv Ther. 2020;37:2999–3009.
Evans C, Marrie RA, Yao S, et al. Medication adherence in multiple sclerosis as a potential model for other chronic diseases: a population-based cohort study. BMJ Open. 2021;11:e043930.
Giovannoni G, Mathews J. Cladribine tablets for relapsing-remitting multiple sclerosis: a clinician’s review. Neurol Ther. 2022;11:571–95.
Meca-Lallana V, García Domínguez JM, López Ruiz R, et al. Expert-agreed practical recommendations on the use of cladribine. Neurol Ther. 2022;11:1475–88.
Inshasi JS, Alfahad S, Alsaadi T, et al. Position of cladribine tablets in the management of relapsing-remitting multiple sclerosis: an expert narrative review from the United Arab Emirates. Neurol Ther. 2021;10:435–54.
Acknowledgements
Medical Writing and/or Editorial Assistance
A medical writer (Dr Mike Gwilt, GT Communications) provided editorial support, funded by Merck Serono S.A.S.
Funding
Merck Serono S.A.S., Lyon, France, an affiliate of Merck KGaA, Darmstadt, Germany (CrossRef Funder ID: https://doi.org/10.13039/100009945) funded editorial support (see below) and the journal’s Rapid Service Fee.
Author information
Authors and Affiliations
Contributions
Jerome de Seze, Dominique Dive, Xavier Ayrignac, Giovanni Castelnovo, Marianne Payet, Amel Rayah, Claudio Gobbi, Patrick Vermersch, and Chiara Zecca contributed equally to the conception, design, content and interpretation of data in the article, participated in its drafting and critical revision for intellectual content, approved the final version for submission, and acknowledge that they are accountable for all aspects of the article.
Corresponding author
Ethics declarations
Conflict of Interest
Jerome de Seze has received fees for consultancy, advisory board and clinical trials from UCB, Novartis, Biogen, Merck, Teva, Genzyme/ Sanofi, Roche, Alexion, BMS/Celegene, Janssen, Horizon Therapeutics. Dominique Dive has received fees for consultancy, advisory board and clinical trials from Novartis, Biogen, Merck, Teva, Genzyme/ Sanofi, Roche, Alexion, BMS/Celegene, Janssen. Xavier Ayrignac has received consulting and lecturing fees, travel grants, and unconditional research support from Alexion, Biogen, Genzyme, Janssen, Novartis, Merck Serono, Roche, and Teva Pharma. Giovanni Castelnovo received fees for consulting and speaking from Biogen, Abbvie, Merck, Novartis, Roche, Sanofi Genzyme, Merz, Celgene BMS. Marianne Payet and Amel Rayah are employees of Merck Santé S.A.S., Lyon, France, an affiliate of Merck KGaA. Ente Ospedaliero Cantonale (employer) received compensation for Chiara Zecca’s speaking activities, consulting fees, or grants from Abbvie, Almirall, Biogen Idec, Bristol Meyer Squibb, Lundbeck, Merck, Novartis, Sandoz, Sanofi, Teva Pharma, Roche. CZ is recipient of a grant for senior researchers provided by AFRI (Area Formazione accademica, Ricerca e Innovazione), EOC. Patrick Vermersch received honoraria for contributions to meetings from Biogen, Sanofi-Genzyme, Novartis, Teva, Merck, Roche, Imcyse, AB Science, Ad scientiam and BMS-Celgene, and research support from Novartis, Sanofi-Genzyme and Merck. Ente Ospedaliero Cantonale (employer) received compensation for Claudio Gobbi’s speaking activities, consulting fees, or grants from Almirall, Biogen Idec, Bristol Meyer Squibb, Merck, Novartis, Sandoz, Sanofi, Teva Pharma, Roche.
Ethical Approval
This narrative review is based on previously conducted studies and the clinical expertise of the authors in treating patients with RMS. No new clinical studies were performed by the authors. No patient-specific efficacy or safety data were reported. Therefore, institutional review board (IRB)/ethics approval was not required.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
de Seze, J., Dive, D., Ayrignac, X. et al. Narrative Review on the Use of Cladribine Tablets as Exit Therapy for Stable Elderly Patients with Multiple Sclerosis. Neurol Ther (2024). https://doi.org/10.1007/s40120-024-00603-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40120-024-00603-y