Abstract
Introduction
The DYSCOVER study was a phase 3b, open-label, randomized trial (NCT02799381) that evaluated levodopa-carbidopa intestinal gel (LCIG) versus optimized medical treatment (OMT) in patients with Parkinson’s disease (PD) and a high burden of dyskinesia at baseline (defined as Unified Dyskinesia Rating Scale [UDysRS] total score ≥ 30). At week 12, patients receiving LCIG versus OMT experienced significant improvements in dyskinesia, pain, and health-related outcomes. The objective of this analysis was to examine correlations between dyskinesia, pain, and health-related outcomes in PD.
Methods
This post hoc analysis assessed correlations between UDysRS, King’s Parkinson’s Disease Pain Scale (KPPS), 8-item Parkinson’s Disease Questionnaire (PDQ-8), Unified Parkinson’s Disease Rating Scale part II, Clinical Global Impression of Severity (CGI-S) or Change (CGI-C), and “On” time without troublesome dyskinesia at baseline and after 12 weeks of LCIG or OMT. Correlations were assessed by Pearson correlation coefficients (categorization: weak, r = 0.20–0.39; moderate, r = 0.40–0.59; strong, r ≥ 0.60).
Results
Among 61 patients, moderate-to-strong positive and significant correlations were observed between UDysRS and KPPS scores (baseline, r = 0.47; week 12 change from baseline [CFB], r = 0.63; all p < 0.001). UDysRS and KPPS scores had moderate-to-strong positive and highly significant correlations with PDQ-8 scores (baseline, r = 0.45 and 0.46, respectively; CFB, r = 0.54 and 0.64, respectively; all p < 0.001). Moderate positive and significant correlations were observed between UDysRS and CGI-S/CGI-C scores (baseline, r = 0.41; CFB, r = 0.47; all p < 0.001).
Conclusions
In patients with high dyskinesia burden, positive correlations were observed between dyskinesia, pain, and health-related quality of life (HRQoL) at baseline. Improvements in dyskinesia and pain were associated with improvements in HRQoL, demonstrating the clinical burden of troublesome dyskinesia.
Trial Registration Number
Clinicaltrials.gov identifier NCT02799381.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Why carry out this study? |
Levodopa-carbidopa intestinal gel (LCIG) is a stable gel suspension of levodopa carbidopa (20 mg/mL and 5 mg/mL, respectively) approved for continuous daytime infusion in patients with advanced Parkinson’s disease (PD). |
In the 12-week, phase 3b, open-label, randomized DYSkinesia COmparative interventional trial on Duodopa VERsus oral medication (DYSCOVER) study, patients treated with LCIG experienced significant improvements in dyskinesia, pain, health related quality of life (HRQoL), activities of daily living (ADL), and clinical impression versus patients treated with optimized medical treatment (OMT). |
To better understand the relationship between dyskinesia, pain, HRQoL, ADL, clinical impression, and “On” time without troublesome dyskinesia, this post hoc analysis of the DYSCOVER study examined correlations between PD outcomes at baseline and after 12 weeks of treatment with LCIG or OMT. |
What was learned from the study? |
Dyskinesia and pain were both associated with HRQoL at baseline, and improvements in dyskinesia and pain correlated with improvements in HRQoL. |
Improvements in dyskinesia also correlated with improvements in ADL and clinical impression, and improvements in “On” time without troublesome dyskinesia correlated with improvements in HRQoL, ADL, and clinical impression. |
Introduction
Levodopa remains the gold standard for the treatment of Parkinson’s disease (PD) [1]; however, the progression of disease presents challenges in controlling motor fluctuations and other symptoms despite optimized medical treatment (OMT) [2]. Dyskinesias are among the most prominent and distressing symptoms for patients and can cause pain, impair quality of life, and reduce a patient’s ability to engage in daily activities [3, 4]. Dyskinesias affect approximately 30% of patients within just 2–3 years of levodopa treatment initiation and nearly 90% of patients after 9 years or more [5].
Levodopa-carbidopa intestinal gel (LCIG; also known as carbidopa-levodopa enteral suspension [CLES]) is a stable gel suspension of levodopa-carbidopa (20 mg/mL and 5 mg/mL, respectively) approved for continuous daytime infusion in patients with advanced PD [6, 7]. Continuous delivery of levodopa with LCIG results in stable levodopa plasma levels and avoids the effects of pulsatile gastric emptying [8,9,10]. Results from multiple clinical trials have demonstrated that LCIG therapy improves dyskinesia, pain, health-related quality of life (HRQoL), and other clinically relevant outcomes in PD [6, 11,12,13]. Furthermore, results from observational studies have shown the effectiveness of LCIG in improving health-related outcomes in routine patient-care settings [14,15,16]. In particular, patients treated with LCIG in the long-term, prospective DUOdopa/Duopa in Patients with Advanced Parkinson’s Disease–a GLobal OBservational Study Evaluating Long-Term Effectiveness (DUOGLOBE) study experienced significant improvements from baseline to month 36 in dyskinesia, “Off” time, and non-motor symptoms [16]. The reduction in dyskinesia observed in the DUOGLOBE study is particularly noteworthy because DUOGLOBE was the first fully prospective, observational study to assess dyskinesia using the newly developed Unified Dyskinesia Rating Scale (UDysRS), which is a validated, comprehensive scale that includes both subjective and objective assessments of dyskinesia [17].
The DYSkinesia COmparative interventional trial on Duodopa VERsus oral medication (DYSCOVER) study was the first randomized trial to evaluate the efficacy of LCIG versus OMT on symptoms of dyskinesia as assessed by the UDysRS [11]. In addition, the DYSCOVER study utilized the recently developed King’s Parkinson’s disease Pain Scale (KPPS), which is the only validated scale that characterizes the various types of pain in PD [18].
In the DYSCOVER study, the primary endpoint (change from baseline to week 12 in UDysRS score) was met with LCIG versus OMT (least squares mean [standard error] difference [based on mixed effect for repeated measures model] − 15.1 [3.2]; p < 0.001). At week 12, patients who received LCIG also experienced significant improvements from baseline in pain, HRQoL, activities of daily living (ADL), and clinical impression versus OMT [11]. However, the exact relationship between dyskinesia and pain and how improvements in one or both measures can impact health-related outcomes are not fully understood. Our post hoc analysis of data from the DYSCOVER study examines the correlations between dyskinesia, pain, HRQoL, ADL, overall condition/clinical impression, and “On” time without troublesome dyskinesia at baseline and after 12 weeks of treatment with LCIG or OMT.
Methods
Study Design and Patients
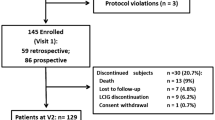
This is a post hoc analysis of data from female and male patients who participated in the DYSCOVER study (NCT02799381). The methodology of the study is presented in detail elsewhere [11]. Briefly, the DYSCOVER study was a phase 3b, open-label, randomized, 12-week study conducted in 23 movement disorder specialist sites across the USA, Finland, Greece, Hungary, Italy, Slovakia, and Spain (Table S1 in the Supplementary Material). Patients aged ≥ 30 years with advanced levodopa-responsive PD, persistent motor fluctuations, and significant burden of dyskinesia at baseline (UDysRS total score ≥ 30) that were not controlled with OMT were randomized 1:1 to receive open-label LCIG or OMT for 12 weeks. Patients with predominantly diphasic dyskinesia, previous surgery for PD (e.g., deep brain stimulation), or a Mini-Mental State Examination score < 24 were excluded.
Assessments
This analysis assessed correlations between dyskinesia (UDysRS) and PD-specific pain (KPPS total score, KPPS fluctuation-related domain score, and KPPS dyskinesia item score), HRQoL (8-item Parkinson’s Disease Questionnaire [PDQ-8]), ADL (Unified Parkinson’s Disease Rating Scale part II [UPDRS II]), symptomatology and impact of illness on functioning (Clinical Global Impression of Severity [CGI-S]), and overall improvement in clinical impression (Clinical Global Impression of Change [CGI-C]) at baseline and week 12 change from baseline. Correlations of KPPS scores with PDQ-8 and UPDRS II scores were evaluated at baseline and week 12 change from baseline. Further assessments included correlations between “On” time without troublesome dyskinesia (Parkinson’s disease diary) and PDQ-8, UPDRS II, and CGI-S/CGI-C scores at baseline and week 12 change from baseline.
Statistics
We combined data from the LCIG and OMT arms into a single group in all analyses presented here. Patient demographics and disease characteristics at baseline were summarized using descriptive statistics. Categorical variables were summarized by the number and percentage of patients in each category; continuous variables were summarized by mean and standard deviation. Pearson correlation coefficients (r) were used to analyze combined data from both treatment groups at baseline and changes from baseline at week 12. Each correlation was evaluated separately as a marginal correlation without adjustment for any other variables; multivariate regression analyses were not performed. Correlation strength was defined with the following cutoffs: weak, r = 0.20–0.39; moderate, r = 0.40–0.59; strong, r ≥ 0.60. Statistical analyses were conducted using SAS® version 9.4 (SAS Institute Inc., Cary, NC, USA).
Compliance with Ethics Guidelines
Patients provided written informed consent before enrollment. Institutional review boards and ethics committees approved the DYSCOVER study protocol at all study sites in participating countries (Table S2 in the Supplementary Material). This clinical study was conducted in accordance with the Good Clinical Practice guideline as defined by the International Council for Harmonisation guidelines, Declaration of Helsinki of 1964 and its later amendments, institutional review boards, and all applicable regional regulations. Where applicable, permissions were obtained to use the scales and assessment measures employed in this clinical trial (UDysRS, KPPS, PDQ-8, UPDRS II, CGI-S, CGI-C, and PD diary).
Results
Patients
A total of 61 patients were included in this analysis. The mean age was 69.0 years, and 32 (52.5%) patients were female. At baseline, patients had a high dyskinesia burden (mean UDysRS total score 52.1), with a mean KPPS total score of 29.3. Patients had a mean UPDRS II score of 18.3 and a mean PDQ-8 total score of 44.2. Detailed patient demographics and disease characteristics at baseline have been presented previously [11]; baseline values relevant to this analysis are summarized in Table 1.
Correlation of Dyskinesia with Pain
At baseline, there were moderate positive and significant correlations between UDysRS and KPPS total and fluctuation-related domain scores (r = 0.47 and 0.42, respectively; p < 0.001; Fig. 1a). Correlation of baseline UDysRS and KPPS dyskinesia item scores were weakly positive but not statistically significant (r = 0.21; p = 0.114).
KPPS score correlations. a KPPS score correlations with UDysRS total scores at baseline and week 12 CFB. CFB in b KPPS total score versus CFB in UDysRS total score, c PDQ-8 score, and d UPDRS II score at week 12. CFB change from baseline, KPPS King’s Parkinson’s Disease Pain Scale, PDQ-8 8-item Parkinson’s Disease Questionnaire, UDysRS Unified Dyskinesia Rating Scale, UPDRS II Unified Parkinson’s Disease Rating Scale part II. Shaded areas on scatterplots represent 95% confidence limits for regression line. ***p < 0.001, *p < 0.05
At week 12, there was a strong positive and significant correlation between the change from baseline in UDysRS and KPPS total scores (r = 0.63; p < 0.001; Fig. 1a, b). At week 12, change from baseline in UDysRS score was weakly positively and significantly correlated with change in KPPS fluctuation-related domain score (r = 0.36; p = 0.011) and did not correlate with change in KPPS dyskinesia item score (r = − 0.10; p = 0.484; Fig. 1a).
Correlations of Pain, HRQoL, and ADL
At baseline, there was a moderate positive and significant correlation between KPPS total score and PDQ-8 score (r = 0.46; p < 0.001), and at week 12, there was a strong positive and significant correlation between change from baseline in KPPS total score and change in PDQ-8 score (r = 0.64; p < 0.001; Fig. 1c).
The correlation between baseline KPPS total score and UPDRS II score was weakly positive and statistically significant (r = 0.38; p = 0.003). At week 12, there was a strong positive and significant correlation between the change from baseline in KPPS total score and change in UPDRS II score (r = 0.73; p < 0.001; Fig. 1d).
Correlation of Dyskinesia with HRQoL, ADL, Current Severity of Illness, and Overall Improvement in Clinical Impression
At baseline, there were moderate positive and significant correlations between UDysRS and PDQ-8 scores (r = 0.45), UPDRS II scores (r = 0.45), and CGI-S scores (r = 0.41); all p ≤ 0.001 (Fig. 2a).
UDysRS total score correlations. a UDysRS total score correlations with PDQ-8, UPDRS II, and CGI-S/CGI-C scores at baseline and week 12 CFB. CFB in b UDysRS total score versus CFB in PDQ-8 score, c UPDRS II score, and d CGI-C score at week 12. CFB change from baseline, CGI-C Clinical Global Impression of Change, CGI-S Clinical Global Impression of Severity, PDQ-8 8-item Parkinson’s Disease Questionnaire, UDysRS Unified Dyskinesia Rating Scale, UPDRS II Unified Parkinson’s Disease Rating Scale part II. Shaded areas on scatterplots represent 95% confidence limits for regression line. ***p ≤ 0.001
At week 12, there were moderate positive and significant correlations between UDysRS and PDQ-8 scores (r = 0.54; Fig. 2b), UPDRS II scores (r = 0.57; Fig. 2c), and CGI-C scores (r = 0.47; Fig. 2d); all p < 0.001.
Correlations of “On” Time Without Troublesome Dyskinesia with HRQoL, ADL, Current Severity of Illness, and Overall Improvement in Clinical Impression
At baseline, “On” time without troublesome dyskinesia was weakly negatively and significantly correlated with PDQ-8 score (r = − 0.32; p = 0.016) and was not correlated with UPDRS II and CGI-S scores (r = 0.01 and − 0.06, respectively; Fig. 3a).
“On” time without TSD correlations. a “On” time without TSD correlations with PDQ-8, UPDRS II, and CGI-S/CGI-C scores at baseline and week 12 CFB. CFB in b “On” time without TSD versus CFB in PDQ-8 score, c UPDRS II score, and d CGI-C score at week 12. CFB change from baseline, CGI-C Clinical Global Impression of Change, CGI-S Clinical Global Impression of Severity, PDQ-8 8-item Parkinson’s Disease Questionnaire, TSD troublesome dyskinesia, UPDRS II Unified Parkinson’s Disease Rating Scale part II. Shaded areas on scatterplots represent 95% confidence limits for regression line. ***p < 0.001, *p < 0.05
At week 12, “On” time without troublesome dyskinesia was moderately negatively and significantly correlated with PDQ-8 score (r = − 0.50; p < 0.001; Fig. 3b), UPDRS II score (r = − 0.45; p < 0.001; Fig. 3c), and CGI-C score (r = − 0.52; p < 0.001; Fig. 3d), meaning that longer “On” times without troublesome dyskinesia were associated with improvements in HRQoL, ADL, and clinical impression.
Discussion
Findings from this post hoc analysis of data from the DYSCOVER study demonstrated that both dyskinesia and pain are associated with HRQoL, and that improvements in dyskinesia and pain correlated with improvements in HRQoL. Similarly, both dyskinesia and pain were positively associated with ADL at baseline, and improvements in dyskinesia and pain corresponded to improvements in ADL.
Results from this present analysis also expand on findings from the randomized trial and affirm that treatment-related improvements in dyskinesia and pain are positively associated with corresponding changes in HRQoL. These results are consistent with findings from a long-term observational study that showed that after 12 months of treatment with LCIG, changes in HRQoL were positively correlated with changes in non-motor symptoms and dyskinesia. The correlation with dyskinesia was weak (r = 0.23; p = 0.011), which may be due to the substantially lower burden of dyskinesia at baseline (mean [standard deviation] UDysRS score 33.7 [21.1] vs. 52.1 [11.8] in the DYSCOVER study) [19]. Furthermore, results from the GLORIA registry also support the findings that non-motor symptoms are associated with HRQoL in patients with PD treated with LCIG [20]. These findings from the aforementioned studies support our findings in the current analysis by providing additional evidence that the impact of treatment on HRQoL may extend beyond improvements solely attributed to changes in motor symptoms [19, 20].
The findings from this analysis also showed that dyskinesia was moderately positively and significantly correlated with pain and severity of illness at baseline, with stronger correlations observed at week 12 change from baseline. Similarly, at baseline, “On” time without troublesome dyskinesia showed weakly negative to no correlations with HRQoL, ADL, and severity of illness; however, at week 12, the change from baseline in “On” time without troublesome dyskinesia was moderately negatively and significantly correlated with changes in HRQoL, ADL, and clinical impression. In nearly all correlations presented here, except for correlations of UDysRS with KPPS subscale scores, stronger correlations were observed at week 12 change from baseline than at baseline.
A strength of this analysis was the use of the UDysRS to assess dyskinesia. Previous studies used PD diaries or the motor section of the UPDRS to assess dyskinesia [12, 21, 22], but these tools have limitations in effectively quantifying the full spectrum of dyskinesia symptoms [11]. The UDysRS provides a valid and reliable assessment of dyskinesia, encompassing the patient’s subjective perception of historical disability and an objective assessment of impairment [17]. The UDysRS is also particularly sensitive to change [23].
The study was limited by the post hoc design, small sample size, and short treatment duration (12 weeks), which may reduce the external validity of the results. Furthermore, although randomized, the DYSCOVER study was an open-label study, and while dyskinesia was assessed by blinded raters, a bias affecting other observed outcomes cannot be fully excluded. Lastly, this analysis indicates a positive correlation between dyskinesia, pain, and HRQoL, but it is not clear if these variables are independently related or if causal relationships exist. Further multivariate assessments exploring the relationship between dyskinesia, pain, and HRQoL are warranted. Nonetheless, results from this analysis are consistent with results from previous studies in that improvements in motor and non-motor symptoms are positively associated with HRQoL [19, 20, 24].
Conclusion
This post hoc analysis of data from the open-label DYSCOVER study demonstrated that dyskinesia and pain are both associated with HRQoL. Improvements in dyskinesia and pain due to LCIG or OMT were moderately positively and significantly correlated with improvements in HRQoL. The results of this analysis, coupled with findings reported in studies in the existing literature, highlight the importance of addressing both motor fluctuations and dyskinesia in PD, as they are closely associated with HRQoL, ADL, and overall condition.
Data Availability
The datasets generated and analyzed in this study are available from the corresponding author on reasonable request. AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized individual and trial-level data (analysis data sets), as well as other information (e.g., protocols, clinical study reports, or analysis plans), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent, scientific research, and will be provided following review and approval of a research proposal, statistical analysis plan (SAP), and execution of a data sharing agreement (DSA). Data requests can be submitted at any time after approval in the United States and Europe and after acceptance of this manuscript for publication. Data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://vivli.org/ourmember/abbvie/, then select “Home”.
References
Tambasco N, Romoli M, Calabresi P. Levodopa in Parkinson’s disease: current status and future developments. Curr Neuropharmacol. 2018;16(8):1239–52.
Aradi SD, Hauser RA. Medical management and prevention of motor complications in Parkinson’s disease. Neurotherapeutics. 2020;17(4):1339–65.
Péchevis M, Clarke CE, Vieregge P, et al. Effects of dyskinesias in Parkinson’s disease on quality of life and health-related costs: a prospective European study. Eur J Neurol. 2005;12(12):956–63.
Thanvi B, Lo N, Robinson T. Levodopa-induced dyskinesia in Parkinson’s disease: clinical features, pathogenesis, prevention and treatment. Postgrad Med J. 2007;83(980):384–8.
Ahlskog JE, Muenter MD. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord. 2001;16(3):448–58.
Antonini A, Odin P, Pahwa R, et al. The long-term impact of levodopa/carbidopa intestinal gel on ‘Off’-time in patients with advanced Parkinson’s disease: a systematic review. Adv Ther. 2021;38(6):2854–90.
DUOPA. Prescribing information. AbbVie Inc; 2022. https://www.rxabbvie.com/pdf/duopa_pi.pdf. Accessed May, 2023.
Nyholm D. The rationale for continuous dopaminergic stimulation in advanced Parkinson’s disease. Parkinsonism Relat Disord. 2007;13(Suppl):S13–7.
Nyholm D, Odin P, Johansson A, et al. Pharmacokinetics of levodopa, carbidopa, and 3-O-methyldopa following 16-hour jejunal infusion of levodopa-carbidopa intestinal gel in advanced Parkinson’s disease patients. AAPS J. 2013;15(2):316–23.
Othman AA, Rosebraugh M, Chatamra K, Locke C, Dutta S. Levodopa-carbidopa intestinal gel pharmacokinetics: lower variability than oral levodopa-carbidopa. J Parkinsons Dis. 2017;7(2):275–8.
Freire-Alvarez E, Kurča E, Lopez Manzanares L, et al. Levodopa-carbidopa intestinal gel reduces dyskinesia in Parkinson’s disease in a randomized trial. Mov Disord. 2021;36(11):2615–23.
Fernandez HH, Standaert DG, Hauser RA, et al. Levodopa-carbidopa intestinal gel in advanced Parkinson’s disease: final 12-month, open-label results. Mov Disord. 2015;30(4):500–9.
Standaert DG, Rodriguez RL, Slevin JT, et al. Effect of levodopa-carbidopa intestinal gel on non-motor symptoms in patients with advanced Parkinson’s disease. Mov Disord Clin Pract. 2017;4(6):829–37.
Antonini A, Poewe W, Chaudhuri KR, et al. Levodopa-carbidopa intestinal gel in advanced Parkinson’s: final results of the GLORIA registry. Parkinsonism Relat Disord. 2017;45:13–20.
Lopiano L, Modugno N, Marano P, et al. Motor and non-motor outcomes in patients with advanced Parkinson’s disease treated with levodopa/carbidopa intestinal gel: final results of the GREENFIELD observational study. J Neurol. 2019;266(9):2164–76.
Chaudhuri KR, Kovács N, Pontieri FE, et al. Levodopa carbidopa intestinal gel in advanced Parkinson’s disease: DUOGLOBE final 3-year results. J Parkinsons Dis. 2023;5(13):769–83.
Goetz CG, Nutt JG, Stebbins GT. The Unified Dyskinesia Rating Scale: presentation and clinimetric profile. Mov Disord. 2008;23(16):2398–403.
Chaudhuri KR, Rizos A, Trenkwalder C, et al. King’s Parkinson’s disease pain scale, the first scale for pain in PD: an international validation. Mov Disord. 2015;30(12):1623–31.
Kovács N, Bergmann L, Anca-Herschkovitsch M, et al. Outcomes impacting quality of life in advanced Parkinson’s disease patients treated with levodopa-carbidopa intestinal gel. J Parkinsons Dis. 2022;12(3):917–26.
Chaudhuri KR, Antonini A, Robieson WZ, Sanchez-Soliño O, Bergmann L, Poewe W. Burden of non-motor symptoms in Parkinson’s disease patients predicts improvement in quality of life during treatment with levodopa-carbidopa intestinal gel. Eur J Neurol. 2019;26(4):581-e43.
Olanow CW, Kieburtz K, Odin P, et al. Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson’s disease: a randomised, controlled, double-blind, double-dummy study. Lancet Neurol. 2014;13(2):141–9.
Slevin JT, Fernandez HH, Zadikoff C, et al. Long-term safety and maintenance of efficacy of levodopa-carbidopa intestinal gel: an open-label extension of the double-blind pivotal study in advanced Parkinson’s disease patients. J Parkinsons Dis. 2015;5(1):165–74.
Goetz CG, Stebbins GT, Chung KA, et al. Which dyskinesia scale best detects treatment response? Mov Disord. 2013;28(3):341–6.
Candel-Parra E, Córcoles-Jiménez MP, Delicado-Useros V, Hernández-Martínez A, Molina-Alarcón M. Relationship between motor and nonmotor symptoms and quality of life in patients with Parkinson’s disease. Nurs Rep. 2021;12(1):1–12.
Guy W. ECDEU Assessment Manual for Psychopharmacology, Revised. Rockville, MD: National Institute of Mental Health: US Department of Heath, Education, and Welfare Public Health Service Alcohol, Drug Abuse, and Mental Health Administration; 1976.
Acknowledgements
AbbVie and authors thank all the trial investigators and the patients who participated in this clinical trial. Investigators have been listed in the supplementary material.
Medical Writing/Editorial Assistance.
Medical writing support was provided by Jay Parekh, PharmD, of JB Ashtin, and funded by AbbVie.
Funding
AbbVie funded this study and participated in the study design, research, analysis, data collection, interpretation of data, reviewing, and approval of the publication. Funding for the Neurology and Therapy Rapid Service Fee is provided by AbbVie.
Author information
Authors and Affiliations
Contributions
Eric Freire-Alvarez: Research execution, review and critique of the manuscript throughout the editorial process, and approval of the final manuscript draft submitted for publication. Paola Vanni: Research execution, review and critique of the manuscript throughout the editorial process, and approval of the final manuscript draft submitted for publication. Egon Kurča: Research execution, review and critique of the manuscript throughout the editorial process, and approval of the final manuscript draft submitted for publication. Lydia Lopez-Manzanares: Research execution, review and critique of the manuscript throughout the editorial process, and approval of the final manuscript draft submitted for publication. Norbert Kovács: Research execution, review and critique of the manuscript throughout the editorial process, and approval of the final manuscript draft submitted for publication. Cleanthe Spanaki: Research execution, review and critique of the manuscript throughout the editorial process, and approval of the final manuscript draft submitted for publication. Tianming Gao: Design, review, and critique of the statistical analysis; review and critique of the manuscript throughout the editorial process; and approval of the final manuscript draft submitted for publication. Lars Bergmann: Research conception, design, review, and critique of the statistical analysis; review and critique of the manuscript throughout the editorial process; and approval of the final manuscript draft submitted for publication. Olga Sánchez-Soliño: Research conception, design, review, and critique of the statistical analysis; review and critique of the manuscript throughout the editorial process; and approval of the final manuscript draft submitted for publication. All authors agree to be accountable for all aspects of the work, ensuring the accuracy and integrity of the publication. All authors had access to relevant data and participated in the drafting, review, and approval of this publication. No honoraria or payments were made for authorship.
Corresponding author
Ethics declarations
Conflict of Interest
Eric Freire-Alvarez has received advisory, consulting, and lecture fees from AbbVie, Bial, Neuraxpharm, Teva, and Zambon, and was an investigator on the DYSCOVER study. He is an investigator on studies funded by AbbVie, Anavex, Bial, Cerevel, Impax, Irlab, Neuroderm, Roche, and Zambon. Paola Vanni has received speaker fees from AbbVie, Bial, Italfarmaco, and Piam; research grant support from AbbVie and Piam; and technical and logistical support from Medtronic, Pfizer, Sanofi, Teva, UCB, and Zambon. She was an investigator on the DYSCOVER study. Egon Kurča was an investigator on the DYSCOVER study. Lydia Lopez-Manzanares has been compensated for advisory services and has received consulting, research grant support, or speaker honoraria from AbbVie, Abbott, Bial, Italfarmaco, Krka, Lundbeck, Roche, Stada, and Zambon. She was an investigator on the DYSCOVER study. Norbert Kovács has received honorarium from AbbVie, Abbott, Boston Scientific, GlaxoSmithKline, Krka, MEdis, Medtronic, Richter Gedeon, and UCB for lecturing at symposia. He has been a consultant for AbbVie, Abbott, KrKa, and Teva, and has received research funding from Abbott; the Hungarian National Research, Development and Innovation Office; University of Pécs; and Medtronic. He was an investigator on the DYSCOVER study. Cleanthe Spanaki has received honoraria for lecturing, advisory fees, educational grants, and travel grants from AbbVie, ITF Hellas, Merck Serono, and Teva, and was an investigator on the DYSCOVER study. Tianming Gao, Lars Bergmann, and Olga Sánchez-Soliño are employees of AbbVie and may hold AbbVie stock and/or stock options.
Ethical Approval
Patients provided written informed consent before enrollment. Institutional review boards and ethics committees approved the DYSCOVER study protocol at all study sites in participating countries. This clinical study was conducted in accordance with the Good Clinical Practice guideline as defined by the International Council for Harmonisation guidelines, Declaration of Helsinki of 1964 and its later amendments, institutional review boards, and all applicable regional regulations. Where applicable, permissions were obtained to use the scales and assessment measures employed in this clinical trial (UDysRS, KPPS, PDQ-8, UPDRS II, CGI-S, CGI-C, and PD diary).
Additional information
Prior Presentation: Data included in this manuscript have been previously presented at the International Congress of Parkinson’s Disease and Movement Disorders (MDS), virtual meeting, September 17–22, 2021, and Congress of the European Academy of Neurology (EAN), Vienna, Austria, June 25–28, 2022.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Freire-Alvarez, E., Vanni, P., Kurča, E. et al. Dyskinesia and Pain in Advanced Parkinson’s Disease: Post Hoc Analysis from the Phase 3b, Open-Label, Randomized DYSCOVER Study. Neurol Ther 13, 437–447 (2024). https://doi.org/10.1007/s40120-024-00583-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-024-00583-z