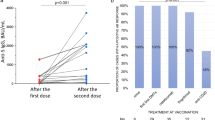
Abstract
Introduction
The risk of SARS-CoV-2 infection or severe coronavirus disease 2019 (COVID-19) has been shown to increase in patients with multiple sclerosis (MS). Vaccination is recommended in this patient population, and the effect of disease-modifying treatments (DMTs) on response to vaccination should be considered.
Methods
This prospective, observational, cross-sectional study investigated humoral response after COVID-19 vaccination as well as possible predictors for response in patients with MS and other neuroinflammatory diseases who received DMTs in routine clinical practice in Spain. Responses were compared versus those seen in healthy controls.
Results
After vaccination against COVID-19, most patients with MS developed an immune response comparable to that of healthy individuals. However, approximately half of patients receiving a sphingosine-1-phosphate modulator (SP1-M, fingolimod or siponimod) or a B-cell-depleting agent (aCD20, ocrelizumab or rituximab) did not develop protective antibodies, although patients receiving other DMTs had humoral immune responses comparable to healthy controls. Lymphocyte count was not associated with reduced humoral response in patients receiving an SP1-M or aCD20, whereas, in patients receiving an aCD20 or SP1-M, older age was associated with lower anti-SARS-CoV-2 spike protein immunoglobulin G antibody levels.
Conclusions
Treatment with aCD20 or SP1-M therapies appears to be associated with a lower humoral response to vaccines against SARS-CoV-2. Vaccination prior to initiation of these DMTs should be recommended whenever possible.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
With the approval of four coronavirus disease 2019 (COVID-19) vaccines in Spain, the impact of vaccination in patients with multiple sclerosis (MS) or other neuroinflammatory diseases who receive disease-modifying treatments (DMTs) in routine clinical practice needs to be elucidated |
This prospective, observational, cross-sectional study investigated humoral response after COVID-19 vaccination as well as possible predictors of response in patients with MS and other neuroinflammatory diseases |
What was learned from the study? |
After vaccination against COVID-19, patients with MS developed an immune response comparable to that of healthy individuals |
However, approximately half of patients receiving a sphingosine-1-phosphate modulator (SP1-M, fingolimod or siponimod) or a B-cell–depleting agent (aCD20, ocrelizumab or rituximab) did not develop protective antibodies |
These observational data suggest that vaccination prior to initiation of these DMTs should be recommended whenever possible |
Introduction
The ability of vaccines to induce a coordinated induction of both humoral and cell-mediated immunity is essential to fight severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) more effectively [1]. Since 2022 in Spain, four vaccines had been approved for the prevention of coronavirus disease 2019 (COVID-19) (BNT162b2 [Comirnaty, BioNTech/Pfizer], mRNA-1273 [Spikevax, Moderna], ChAdOx1-S [Vaxzevria, AstraZeneca] and Ad26COVS1 [Jcovden, Janssen]) [2,3,4,5].
Viral infections lead to disease exacerbations in patients with multiple sclerosis (MS) [6], and the risk of SARS-CoV-2 infection or severe COVID-19 increases in patients with MS and associated disability as well as in patients with progressive disease or those who are receiving some immunosuppressants such as disease-modifying treatments (DMTs) [7,8,9,10,11]. Furthermore, a pooled data analysis of 5634 patients with MS has shown that developing COVID-19 increased the risk of death by 24% in this population [12]. Therefore, vaccination against COVID-19 is widely recommended for patients with MS by medical associations [13] and experts [14].
It is still necessary to better understand vaccine responses in patients treated with DMTs, as the various immunomodulatory and immunosuppressive effects of DMTs add complexity to vaccinations. Mechanistically, agents that impact the adaptive immune system may decrease vaccines’ efficacy by impairing long-term memory development [13]. Studies suggest an insufficient humoral response after SARS-CoV-2 vaccination in patients with MS receiving some DMTs [1, 15,16,17,18,19]. Therefore, reliable quantification of the antibody response to SARS-CoV-2 is necessary to identify possible vaccine failure and estimate the duration of protection in these patients [20].
The aim of this study was to analyze humoral responses after anti-COVID-19 vaccination in patients with MS, neuromyelitis optica spectrum disorder (NMOSD), and clinically isolated syndrome (CIS) who received DMTs in routine clinical practice in Spain. The clinical experience obtained will help guide clinician decision-making and ultimately contribute to improving patient care.
Methods
Study Design
This prospective, observational, cross-sectional study was conducted in 2021 at a MS reference center in Murcia, Spain. Consecutive patients who met the inclusion criteria (see below) were followed as they attended scheduled consultations according to normal clinical practice.
Compliance with Ethics Guidelines
The study protocol was approved by the Institutional Review Board of the “Virgen de la Arrixaca” Clinical University Hospital (approval number: 2020-5-1-5-HCUVA), and the study was conducted under the principles in the Declaration of Helsinki. Patients were informed about the study’s goals, and written informed consent was obtained before enrollment, following Spanish law and Good Clinical Practices.
Study Population
Patients aged ≥ 18 years with MS, NMOSD, or CIS who were regularly seen in our Neuroimmunology Unit and received a complete anti-SARS-CoV-2 vaccination course, according to the Spanish Ministry of Health guidelines at the time of this study, were eligible for enrollment in our study. Patients were either untreated or receiving treatment with DMTs, including pleiotropic drugs (interferon β, glatiramer, dimethyl fumarate), B-cell-depleting (i.e., anti-CD20 antibody [aCD20]) agents (ocrelizumab, ofatumumab, rituximab), sphingosine-1-phosphate modulators (SP1-M; fingolimod, siponimod), pulsed-immune reconstitution therapies (PIRT; alemtuzumab, cladribine), or other DMTs (teriflunomide, natalizumab). For inclusion, effective contraceptive measures in women of childbearing age were required.
Patients were excluded if they had concomitant COVID-19 infection during the study period or in the previous 6 months (SARS-CoV-2 vaccination was not allowed in these instances according to the Spanish Ministry of Health guidelines at the time of this study), had received systemic corticosteroid therapy within 4 weeks of immunization, had any other concomitant uncontrolled/active disease, or were pregnant or breastfeeding.
Healthy subjects were also enrolled in the study as a control group and were selected from among the individuals accompanying the patients for consultation who had a temporal pattern of vaccination equal to that applied to the patients.
Study Objectives and Endpoints
The study’s main objective was to determine the post-vaccination humoral immunity of the study participants. A secondary objective was to explore how this humoral response was affected by other factors, such as the lymphocyte count, age, type of DMT received, and the time since the last dose of PIRT or aCD20 treatment.
The primary endpoint was the proportion of patients that developed a complete serological response with SARS-CoV-2 spike protein antibodies between a minimum of 2 weeks and a maximum of 12 weeks after complete vaccination or last dose (for individuals with only one dose due to previous infection or omission of the second dose). A complete serological response was defined as the presence of positive post-vaccination anti-SARS-CoV-2 spike protein (S-spike) immunoglobulin G (IgG) antibodies, i.e., all patients who, after receiving the vaccination regimen, had an antibody titer equal to or higher than the minimum IgG value (50 arbitrary units [AU]/ml) according to the values established by our local laboratory.
Secondary endpoints included differences between DMTs in complete serological response rates between subgroups of individuals based on total lymphocyte count, age, and time since the last dose of aCD20 or PIRT, and immunization to determine potential predictors of seroconversion.
Statistical Analysis
Descriptive statistics were used, with categorical variables presented as absolute frequency and percentage and continuous variables expressed as mean and standard deviation (SD) or median and interquartile range (IQR). The Kolmogorov-Smirnoff test was performed to assess the normality of continuous variables. For the bivariate analysis, Student's t-test was used to compare those variables that showed a normal distribution between groups. For variables that did not have a normal distribution, the Mann-Whitney U test was used. For categorical variables, the chi-squared test or Fisher's exact test was used when the expected frequency was less than five.
Results
Patients
Between January and June 2021, 250 participants were enrolled and analyzed (234 patients and 16 controls). Of the patients enrolled, 221 (93.2%) were diagnosed with MS, 10 (4.2%) with NMOSD, and 3 (1.2%) with CIS. Among the 221 patients diagnosed with MS, 184 had relapsing-remitting MS (RRMS), 12 primary progressive MS (PPMS), and 25 secondary progressive MS (SPMS). All patients were treated according to standard clinical practice. Most participants were women, 165 (70.5%) among the patients and 11 (68.8%) in the control group. The mean ± SD age was 47.7 ± 11.3 years for patients with MS and 54.7 ± 10.3 years for patients with other disorders, while the control group was younger (mean 42.5 ± 13.6 years) (Table 1).
Vaccines Administered
The most commonly used vaccine was BNT162b2, which was administered to 178 (71.0%) participants. ChAdOx1-S was administered to 40 (15.8%) participants; mRNA-1273 was administered to 19 (7.6%) participants and Ad26.COV2-S to 13 (7.1%) participants. Among the patients with MS who were immunized, 155 (70.1%), 18 (8.1%), 36 (16.3%), and 12 (5.4%) received the BNT162b2, mRNA-1273, ChAdOx1-S, and Ad26.COV2-S vaccines, respectively (Table 1). Of the 13 patients with other neuroinflammatory diseases who were immunized, 11 (84.6%) and 2 (15.4%) received the BNT162b2 and ChAdOx1-S vaccines, respectively. No patients in this subgroup received the mRNA-1273 or Ad26.COV2-S vaccine (Table 1).
A total of 240 patients were immunized with a vaccine that required a second dose according to their summary of product characteristics (i.e., BNT162b2, mRNA-1273, and ChAdOx1). According to the Spanish Ministry of Health guidelines, patients infected before active immunization should only receive a single dose of the BNT162b2, mRNA-1273, or ChAdOx1-S vaccines. It should be noted that among those 240 patients, 22 (9.2%) did not receive the second dose because of previous SARS-CoV-2 infection, and 7 (2.9%) did not complete the full vaccination for unknown reasons.
Treatments for Underlying Pathologies
Of the 234 patients included in the study, 214 received drug treatment for their disease. Of note, 17 (7.7%) patients with MS were not treated with DMTs (Table 2). The drug therapy received by the largest number of individuals was ocrelizumab (n = 37), followed by natalizumab (n = 34), teriflunomide (n = 28), and a S1P-M (fingolimod, siponimod; n = 23). Other less frequently received DNTs were glatiramer, alemtuzumab, dimethyl fumarate, interferon β, cladribine, rituximab, tocilizumab, ofatumumab, cyclophosphamide, corticosteroids, mycophenolate, or a Bruton’s tyrosine kinase inhibitor (BTKi).
Serological Response
Serum antibody determination was performed a median 46 (IQR 21–71) days after the full vaccination schedule. Concerning post-vaccination immunity, the mean virus-specific IgG titers detected against the S-spike were 1860 AU/ml, 3649 AU/ml, and 1912 AU/ml in patients with MS, patients with other disorders, and controls, respectively. The differences in antibody titers found in both patient groups versus controls were statistically significant (p < 0.001; Table 1).
Primary Endpoint
When analyzing the number of patients with IgG against SARS-CoV-2, 179 (81%) of those with MS and 12 (92.3%) of those with other disorders developed positivity (IgG against S-spike ≥ 50 AU/ml); in comparison, all the cohort of healthy subjects (n = 16) developed virus-specific IgG titers. There were no significant differences between the two groups of patients and the control group (Table 1).
A negative antibody titer (IgG against S-spike < 50 AU/ml) was observed in 56.8%, 3.6%, 56.5%, 60.0%, and 66.7% of patients receiving ocrelizumab, teriflunomide, an SP1-M, rituximab, and ofatumumab, respectively (Fig. 1).
Secondary Endpoints
Humoral Response According to Lymphocyte Count
When the humoral response was analyzed by lymphocyte count, there were no statistically significant differences between DMTs with regard to the number of patients achieving positive serology (IgG against S-spike ≥ 50 AU/ml; Fig. 2). In particular, among individuals with a lymphocyte count < 500/mm3 who received aCD20s, there was one patient each with negative and positive serology. Among those with a lymphocyte count between 500/mm3 and 1000/mm3, three (10.3%) patients had negative serology and two (10.5%) had positive serology. Finally, in those patients with a lymphocyte count > 1000/mm3, there were 25 (86.3%) and 16 (84.2%) with a negative and a positive serology, respectively (p > 0.999).
Immunoglobulin G (IgG) antibody titers according to lymphocyte count among different disease-modifying treatment categories. Positive serology = IgG against S-spike ≥ 50 AU/ml; negative serology = IgG against S-spike < 50 AU/ml. aCD20 B-cell-depleting agents (ocrelizumab, rituximab), FTY fingolimod, OCR ocrelizumab, RTX rituximab, SP1-M sphingosine-1-phosphate modulators (fingolimod, siponimod), SPN siponimod
Among patients with a lymphocyte count below 500/mm3 or between 500/mm3 and 1000/mm3 treated with an SP1-M, six (46.1%) had negative serology and four (40.0%) had positive serology. Finally, among those with a lymphocyte count > 1000/mm3, there were one (7.8%) and two (20.0%) with a negative and a positive serology, respectively (p = 0.722).
Humoral Response According to Age
With the available data, differences between the age of participants and the immunity developed could only be analyzed for two of the four categories of DMTs: aCD20s (ocrelizumab, rituximab) and SP1-Ms (fingolimod, siponimod; Fig. 3). In both these groups, a statistically significant difference (p < 0.001) between the age of the participants with positive and negative serology was observed, with the median age of patients with negative serology being higher than the median age of patients with positive serology.
Immunoglobulin G (IgG) antibody titers according to age among different disease-modifying treatment categories. Positive serology = IgG against S-spike ≥ 50 AU/ml; negative serology = IgG against S-spike < 50 AU/ml. aCD20 B-cell-depleting agents (ocrelizumab, rituximab), aCD20 B-cell depleting agents (ocrelizumab, rituximab), ALEMO alemtuzumab, CLD cladribine, DMF dimethyl fumarate; FTY fingolimod, GLAT glatiramer, INF interferon, NTZ natalizumab, OCR ocrelizumab, PIRT pulse-immune reconstitution therapies (alemtuzumab, cladribine), RTX rituximab, SP1-M sphingosine-1-phosphate modulators (fingolimod, siponimod), SPN siponimod, TRF teriflunomide
Humoral Response According to the Interval Between the Last Dose and Active Immunization
When we analyzed the relationship between the time elapsed between the last aCD20 pulse regimen (ocrelizumab, rituximab) and PIRTs (alemtuzumab, cladribine) and the time of active immunization (positive serology), data were scare and no statistically significant differences were seen between the groups (Supplementary Material; Table S1).
Humoral Response and COVID-19 Severity
When the relationship between anti-SARS-CoV-2 antibody titer and COVID-19 severity was investigated, no statistically significant differences were found (Supplementary Material; Table S2).
Discussion
We report the results of our observational monocentric study to investigate response to COVID-19 vaccination in patients with MS, NMOSD, or other neuroinflammatory diseases who were receiving DMTs. When median anti-S-spike IgG antibody titers were analyzed after vaccination, we observed that the levels obtained in patients with MS were similar to those obtained in healthy controls (Table 1). However, patients with NMOSD developed higher anti-S-spike IgG antibody levels, maybe because of the small sample size.
As for the vaccinations received, most patients received the BNT162b2 vaccine, which can be explained by the fact that our institution was following the guidelines of the COVID-19 vaccination strategy of the Interterritorial Council of the National Health System in force in Spain at the time of the study. It recommended the use of mRNA vaccines in persons with risk conditions. Of all the individuals immunized in the study, a large proportion adhered to the vaccination schedule established in Spain at the time of the study. Only a minority (2.9%) did not comply in the case of those vaccines that required a second dose.
By analyzing the serological response of patients according to the drug with which they were treated, our experience suggests that humoral response to SARS-CoV-2 vaccination is variable, particularly in patients treated with SP1-M and aCD20 agents, in line with previous prospective [1, 15,16,17,18] and retrospective [21] studies. As the study planned to determine serology from 2 to 12 weeks after immunization, there was some variability in this respect, with the highest number being at 1.5 months and, in some cases, > 2 months after immunization (data not shown). This can possibly be explained by the fact that all assessments were done in a real-life setting.
Ocrelizumab depletes circulating B cells within 2 weeks of treatment but spares CD20-negative plasma cells, stem cells, and pro-B cells, impairing the antibody response to non-live vaccines [22] including for SARS-CoV-2 vaccines [13, 15], a finding that was demonstrated in our study. In a single-center study performed by Brill and colleagues [15], patients with MS who were treated with ocrelizumab generated comparable SARS-CoV-2–specific T-cell responses to healthy controls but had lower antibody responses following vaccination compared with untreated patients or healthy controls.
As also described in the study by Pitzalis and colleagues [18], in our study, older age was associated with lower antibody levels in patients treated with an aCD20 or SP1-M. However, in a prospective observational, single-center cohort study of patients with MS treated with either ocrelizumab or natalizumab, there was no correlation between age or sex and antibody response [16].
Consistent with the findings of our study, no relation between serological response and white blood cell count was identified by Guerrieri and colleagues [21] or Katz and colleagues [16]. Hitchon and colleagues also prospectively analyzed the influence of different vaccines depending on the availability and regulatory indication at each moment of the COVID-19 pandemic. The sample size in this case is smaller (n = 72) than the one included in our study. In addition, this other study, although not without differentiation between the drugs, shows that the complete two-dose regimen of the vaccines achieves a good response, although this is lower in patients over 65 years of age after the second dose [23]. Another prospective study evaluated the serological response of 94 patients with MS getting DMTs that received mRNA-1273 or ChAdOx1-S vaccines for SARS-CoV-2. Although they developed a serological response with no differences between the vaccines used, stratified analysis according to treatment showed that fewer subjects developed antibodies against spike antigen in those receiving ocrelizumab (p ≤ 0.001) [24].
We consider that the value of the study is based on the fact that it provides data from routine clinical practice in a single center comparing the four vaccines that were in use at the time, without any intervention other than that determined by the health authority regarding vaccination. Not all patients have completed the planned immunization schedule (because of fear, reluctance, or incompatibility with their treatment), and peaks of reactivation of respiratory infections may occur in the future. The study findings are consistent with the results of previous studies and confirm that the humoral response differs depending on the immunosuppressive treatment used, helping to form a standard framework of doctrine in this regard. Although the COVID-19 pandemic has ended, the infection is still present in our environment and in many multiple sclerosis units; the lessons learned from the pandemic have led to the implementation of rapid antigen testing for influenza and COVID-19 before administration of immunosuppressive treatment. In fact, COVID-19 vaccination has been included in the vaccination schedules of many patients who are immunosuppressed. It is possible that, in the future, there will be new clusters of COVID-19 infection that may individually affect our patients; therefore, the results of our study will continue to be of interest. Our research updates knowledge in this field, and this learning should continue to be considered in clinical practice.
The main strength of this study is that the seroconversion effect of four different anti-COVID-19 vaccines was analyzed with a wide variety of DMTs (up to 11) with different mechanisms of action, including a drug for the treatment of NMOSD (tocilizumab), which was used off label. However, we also have to acknowledge some potential limitations of this study, such as the small sample size (meaning it was not possible to do a propensity score) and the fact we only assessed the impact of two doses of vaccine (bearing in mind that this was the vaccination schedule recommended at the time the study data were collected). Furthermore, the results of this study are based on observational data with limited follow-up. The fact that antibody determinations were performed over a wide time interval after full immunization (from 2 to 12 weeks) is another limitation of this study. As COVID-19 vaccine-induced immunity wanes over time, it is essential to study the persistence of IgG values. However, although antibodies following vaccination are thought to be a sign of protective immunity, little is known about the antibody levels needed to prevent or reduce infection severity. A New England Journal of Medicine editorial on rituximab noted that the US Food and Drug Administration advised against checking post-vaccination antibody levels as “positive titers may not reflect protection and negative titers may not indicate susceptibility” [25]. Another limitation of this study is that we exclusively publish data on IgG responses as a correlate of humoral immunity, although the adaptive immune response to SARS-CoV-2 appears also to be dependent on cellular responses [16, 25, 26]. It is currently unclear if a T-cell response is sufficient to prevent infection or decrease the severity of COVID-19 in patients who did not develop antibodies in response to vaccines. In our study, since when investigating the relationship between anti-SARS-CoV-2 antibodies and COVID-19 severity there was no significant difference, this may highlight the role of cell-mediated immunity. Finally, it would be reasonable to expect that, compared with our MS population with a mean age of 47.7 years, older patients with MS with other comorbidities would have a similar or lower immunogenic response to vaccination.
Conclusions
A humoral protective immune response was achieved with mRNA and viral vector SARS-CoV-2 vaccines in patients with MS, showing a protective humoral response similar to healthy controls. The differences in antibody titers found in patients versus healthy individuals were statistically significant. An attenuated humoral immune response was observed in patients receiving an SP1-M and aCD20, with nearly half not developing protective antibodies, while other DMTs showed similar humoral responses to healthy controls. Lymphopenia was not related to lower titers of humoral response in patients treated with an aCD20 or SP1-M. Older age was associated with lower antibody levels in patients treated with aCD20 and SP1-M therapies. It would be desirable to have more extensive studies and international registries to determine the best vaccine and DMTs options in this patient population.
Data Availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Tortorella C, Aiello A, Gasperini C, et al. Humoral- and T-cell-specific immune responses to SARS-CoV-2 mRNA vaccination in patients with MS using different disease-modifying therapies. Neurology. 2022;98(5):e541–54. https://doi.org/10.1212/WNL.0000000000013108.
The Spanish Agency of Medicines and Medical Devices (AEMPS). Comirnaty (BNT162b2, BioNTech/Pfizer): summary of product characteristics. 2020. https://cima.aemps.es/cima/pdfs/es/ft/1201528001/FT_1201528001.pdf. Accessed 26 Apr 2023.
The Spanish Agency of Medicines and Medical Devices (AEMPS). Spikevax (mRNA-1273, Moderna): summary of product characteristics. 2021. https://cima.aemps.es/cima/pdfs/es/ft/1201507001/FT_1201507001.pdf. Accessed 26 Apr 2023.
The Spanish Agency of Medicines and Medical Devices (AEMPS). Vaxzevria (ChAdOx1-S, AstraZeneca): summary of product characteristics. 2021. https://cima.aemps.es/cima/pdfs/es/ft/1211529001/FT_1211529001.pdf. Accessed 26 Apr 2023.
The Spanish Agency of Medicines and Medical Devices (AEMPS). Jcovden (Ad26COVS1, Janssen): summary of product characteristics. 2021. https://cima.aemps.es/cima/pdfs/es/ft/1201525001/FT_1201525001.pdf. Accessed 26 Apr 2023.
Steelman AJ. Infection as an environmental trigger of multiple sclerosis disease exacerbation. Front Immunol. 2015;6:520. https://doi.org/10.3389/fimmu.2015.00520.
Friedli C, Diem L, Hammer H, et al. Negative SARS-CoV2-antibodies after positive COVID-19-PCR nasopharyngeal swab in patients treated with anti-CD20 therapies. Ther Adv Neurol Disord. 2021;14:17562864211016640. https://doi.org/10.1177/17562864211016641.
Louapre C, Collongues N, Stankoff B, et al. Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol. 2020;77(9):1079–88. https://doi.org/10.1001/jamaneurol.2020.2581.
Reder AT, Centonze D, Naylor ML, et al. COVID-19 in patients with multiple sclerosis: associations with disease-modifying therapies. CNS Drugs. 2021;35(3):317–30. https://doi.org/10.1007/s40263-021-00804-1.
Salter A, Fox RJ, Newsome SD, et al. Outcomes and risk factors associated with SARS-CoV-2 infection in a North American registry of patients with multiple sclerosis. JAMA Neurol. 2021;78(6):699–708. https://doi.org/10.1001/jamaneurol.2021.0688.
Sormani MP, De Rossi N, Schiavetti I, et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol. 2021;89(4):780–9. https://doi.org/10.1002/ana.26028.
Prosperini L, Tortorella C, Haggiag S, Ruggieri S, Galgani S, Gasperini C. Increased risk of death from COVID-19 in multiple sclerosis: a pooled analysis of observational studies. J Neurol. 2022;269(3):1114–20. https://doi.org/10.1007/s00415-021-10803-3.
Achiron A, Mandel M, Dreyer-Alster S, et al. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther Adv Neurol Disord. 2021;14:17562864211012836. https://doi.org/10.1177/17562864211012835.
Reyes S, Cunningham AL, Kalincik T, et al. Update on the management of multiple sclerosis during the COVID-19 pandemic and post pandemic: an international consensus statement. J Neuroimmunol. 2021;357: 577627. https://doi.org/10.1016/j.jneuroim.2021.577627.
Brill L, Rechtman A, Zveik O, et al. Humoral and T-cell response to SARS-CoV-2 vaccination in patients with multiple sclerosis treated with ocrelizumab. JAMA Neurol. 2021;78(12):1510–4. https://doi.org/10.1001/jamaneurol.2021.3599.
Katz JD, Bouley AJ, Jungquist RM, Douglas EA, O’Shea IL, Lathi ES. Humoral and T-cell responses to SARS-CoV-2 vaccination in multiple sclerosis patients treated with ocrelizumab. Mult Scler Relat Disord. 2022;57: 103382. https://doi.org/10.1016/j.msard.2021.103382.
König M, Lorentzen ÅR, Torgauten HM, et al. Humoral immunity to SARS-CoV-2 mRNA vaccination in multiple sclerosis: the relevance of time since last rituximab infusion and first experience from sporadic revaccinations. J Neurol Neurosurg Psychiatry. 2023;94(1):19–22. https://doi.org/10.1136/jnnp-2021-327612.
Pitzalis M, Idda ML, Lodde V, et al. Effect of different disease-modifying therapies on humoral response to BNT162b2 vaccine in Sardinian multiple sclerosis patients. Front Immunol. 2021;12: 781843. https://doi.org/10.3389/fimmu.2021.781843.
Etemadifar M, Nouri H, Pitzalis M, et al. Multiple sclerosis disease-modifying therapies and COVID-19 vaccines: a practical review and meta-analysis. J Neurol Neurosurg Psychiatry. 2022;93(9):986–94. https://doi.org/10.1136/jnnp-2022-329123.
Perkmann T, Perkmann-Nagele N, Koller T, et al. Anti-spike protein assays to determine SARS-CoV-2 antibody levels: a head-to-head comparison of five quantitative assays. Microbiol Spectr. 2021;9(1): e0024721. https://doi.org/10.1128/Spectrum.00247-21.
Guerrieri S, Lazzarin S, Zanetta C, Nozzolillo A, Filippi M, Moiola L. Serological response to SARS-CoV-2 vaccination in multiple sclerosis patients treated with fingolimod or ocrelizumab: an initial real-life experience. J Neurol. 2022;269(1):39–43. https://doi.org/10.1007/s00415-021-10663-x.
Bar-Or A, Calkwood JC, Chognot C, et al. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis: the VELOCE study. Neurology. 2020;95(14):e1999–2008. https://doi.org/10.1212/WNL.0000000000010380.
Hitchon CA, Mesa C, Bernstein CN, et al. Immunogenicity and safety of mixed COVID-19 vaccine regimens in patients with immune-mediated inflammatory diseases: a single-centre prospective cohort study. BMJ Open. 2023;13(5): e071397. https://doi.org/10.1136/bmjopen-2022-071397.
Rojas JI, Alonso R, Cabrera M, et al. Serological response to SARS-CoV-2 vaccines in patients with multiple sclerosis in Argentina. Medicina (B Aires). 2023;83(3):358–65.
Kaul D. How should we advise our immunocompromised patients after COVID-19 vaccination? N Engl J Med J Watch. 2021. https://doi.org/10.1056/NEJM-JW.NA53599.
Ni L, Ye F, Cheng ML, et al. Detection of SARS-CoV-2-specific humoral and cellular immunity in COVID-19 convalescent individuals. Immunity. 2020;52(6):971-7 e3. https://doi.org/10.1016/j.immuni.2020.04.023.
Medical Writing, Editorial, and Other Assistance
The authors acknowledge Helena Martí Soler and María Romero, who provided statistical assistance and medical writing support, respectively, on behalf of Springer Healthcare. This medical writing assistance was funded by Merck, SLU, Madrid, Spain, an affiliate of Merck KGaA (CrossRef Funder ID: https://doi.org/10.13039/100009945).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Funding
No funding was received for the study design, collection of data, or statistical analysis of the results. Medical writing assistance was funded by Merck, S.LU, Madrid, Spain, an affiliate of Merck KGaA (CrossRef Funder ID: https://doi.org/10.13039/100009945). The journal´s Rapid Service Fee was funded by Springer Healthcare Ibérica.
Author information
Authors and Affiliations
Contributions
Conception/Design: Jorge Millán-Pascual, Gabriel Valero-López, Francisca Iniesta-Martinez, Judith Jimenez-Veiga, Ana Morales-Ortiz, José E. Meca-Lallana; Data collection: Jorge Millán-Pascual, Gabriel Valero-López, Francisca Iniesta-Martinez, Maria Fuensanta Hellin-Gil, Judith Jimenez-Veiga, Isabel Alejandra López-Tovar, Ana Morales-Ortiz, José. E. Meca-Lallana; Data analysis and interpretation: Jorge Millán-Pascual, José E. Meca-Lallana; Drafting manuscript: Jorge Millán-Pascual, José E. Meca-Lallana; Revising manuscript: Jorge Millán-Pascual, José E. Meca-Lallana; Final approval of manuscript: Jorge Millán-Pascual, Gabriel Valero-López, Francisca Iniesta-Martinez, Maria Fuensanta Hellin-Gil, Judith Jimenez-Veiga, Isabel Alejandra López-Tovar, Ana Morales-Ortiz, José. E. Meca-Lallana.
Corresponding author
Ethics declarations
Conflict of Interest
Jorge Millán-Pascual and Gabriel Valero-López have received honoraria for lecturing or assistance for conference attendance from Biogen Idec, Janssen-Cilag, Merck Serono, Roche, and Sanofi Aventis; have received consulting or speaking fees from Biogen Idec, Merck Serono, and Roche; and have received funding for research projects or in the form of conference fees, mentoring, and assistance for conference attendance from Sanofi Aventis. José E. Meca-Lallana has received honoraria as a consultant, as a chairman or lecturer in meetings and has participated in clinical trials and other research projects promoted by Alexion, Biogen, Bristol Myers Squibb, Janssen, Merck, Novartis, Roche, and Sanofi.. Francisca Iniesta-Martinez, Maria Fuensanta Hellin-Gil, Judith Jimenez-Veiga, Isabel Alejandra López-Tovar and Ana Morales-Ortiz have nothing to disclose.
Ethical Approval
The study protocol was approval by the Ethics Committee of the Virgen de la Arrixaca Clinical University Hospital (approval number: 2020-5-1–-5-HCUVA), and the study was conducted under the principles in the Declaration of Helsinki. Patients were informed about the study’s goals and written informed consent was obtained before enrolment, following Spanish law and Good Clinical Practices. We thank the participants of the study.
Additional information
Prior Presentation: SEN 2020/2021 (Spanish Society of Neurology). Online. 22/11/2021-2/12/2021. Oral communications (ID 16045). ECTRIM 2021 (European Committee for Treatment and Research in Multiple Sclerosis). Vienna, Austria and Online. 13–15 November 2021. P942 "Serological response to SARS-CoV-2 vaccination in multiple sclerosis patient in a real-life experience” published in Multiple Sclerosis Journal. Volume 27, Issue 2_suppl, October 2021, Pages 752-804. Doi: https://doi.org/10.1177/13524585211047080.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Millán-Pascual, J., Valero-López, G., Iniesta-Martinez, F. et al. Humoral Response to SARS-COV-2 Vaccination in Patients with Multiple Sclerosis and Neuromyelitis Optica Spectrum Disorder: A Real-World Study. Neurol Ther 13, 153–164 (2024). https://doi.org/10.1007/s40120-023-00572-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-023-00572-8