Abstract
Introduction
Although acute and preventive treatments for migraine are commonly given in combination, data on the real-world effectiveness of ubrogepant as an acute treatment when used with an anti-calcitonin gene-related peptide (CGRP) monoclonal antibody (with or without onabotulinumtoxinA) are limited. This analysis sought to evaluate the real-world effectiveness, treatment satisfaction, and optimization of ubrogepant for the acute treatment of migraine when used in combination with an anti-CGRP monoclonal antibody, with or without concomitant onabotulinumtoxinA.
Methods
This prospective, multiple-attack, open-label, observational study (COURAGE) assessed meaningful pain relief (MPR), return to normal function (RNF), treatment satisfaction, and acute treatment optimization of ubrogepant (50 or 100 mg) when combined with an anti-CGRP monoclonal antibody, onabotulinumtoxinA, or both in adult users of Migraine Buddy, a migraine tracking application.
Results
In the ubrogepant and anti-CGRP monoclonal antibody arm (n = 245), following the first ubrogepant-treated attack, 61.6% (151/245) and 80.4% (197/245) of ubrogepant-treated participants achieved MPR at 2 and 4 h post-dose, respectively, and 34.7% (85/245) and 55.5% (136/245) achieved RNF at 2 and 4 h post-dose, respectively. Across up to 10 ubrogepant-treated attacks (N = 1153), MPR was achieved in 51.3% (592/1153) and 73.5% (847/1153) at 2 and 4 h post-dose, respectively. RNF was achieved by 32.2% (371/1153) and 53.2% (613/1153) at 2 and 4 h post-dose. After 30 days, 72.7% (168/231) of participants reported satisfaction (using a 7-point scale) with ubrogepant when used in combination with an anti-CGRP monoclonal antibody, and 79.7% (184/231) of participants achieved acute treatment optimization (defined as moderate-maximum treatment efficacy using the Migraine Treatment Optimization Questionnaire-4).
Conclusion
Real-world ubrogepant use with an anti-CGRP monoclonal antibody was associated with MPR, RNF, satisfaction, and acute treatment optimization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Early clinical experience with the use of a calcitonin gene-related peptide (CGRP) receptor antagonist with an anti-CGRP monoclonal antibody (mAb) suggests that the combination is both safe and effective; however more data demonstrating the real-world effectiveness of the combination are needed. |
The objective of this analysis was to assess the real-world effectiveness of ubrogepant for the acute treatment of migraine when used in combination with an anti-CGRP mAb, with or without concomitant onabotulinumtoxinA. |
What was learned from the study? |
The real-world use of ubrogepant with an anti-CGRP mAb, with or without onabotulinumtoxinA, was associated with meaningful pain relief, return to normal function, treatment satisfaction, and acute treatment optimization. |
This study also showed the feasibility of an app-based study design to collect real-world data on migraine treatment effectiveness. |
Introduction
Ubrogepant, an oral calcitonin gene-related peptide (CGRP) receptor antagonist, is approved by the US Food and Drug Administration for the acute treatment of migraine with or without aura [1]. Pivotal and long-term safety studies have demonstrated the safety, tolerability, and efficacy of ubrogepant for the acute treatment of migraine [2,3,4]. Ubrogepant treatment has also been associated with reduced functional disability and increased patient satisfaction [5, 6]. In clinical studies and in practice, ubrogepant is often used to treat breakthrough migraine in patients also receiving preventive treatment [7, 8]. Current preventive treatments for migraine include antiepileptics, beta-blockers, antidepressants, onabotulinumtoxinA, and medications that target CGRP, both monoclonal antibodies (mAbs) and gepants [7, 9]. Although ubrogepant and anti-CGRP mAbs are commonly prescribed together in clinical practice, limited data exist on their combined use. Given that the CGRP pathway is a target for both acute treatments and preventive treatments, and that these treatments are sometimes used together in the same patient, there is a need for data on the effects of the co-administration of ubrogepant and anti-CGRP mAbs in clinical practice.
The interactions of ubrogepant with two CGRP-targeted mAbs, erenumab and galcanezumab, were explored in a multicenter, open-label, drug–drug interaction study of adults with migraine. The pharmacokinetic profile of ubrogepant before and after administration of either one of the mAbs was evaluated. The study also included a 30-day follow-up for safety and tolerability. A total of 40 participants were included in this study, and findings showed no significant differences in the maximum plasma concentration (Cmax), concentration–time curve from 0 to t post-dose (AUC0–t), or from time 0 to infinity (AUC0–inf) of ubrogepant before compared with after erenumab or galcanezumab administration. Furthermore, no serious treatment-emergent adverse events (TEAEs) or TEAEs leading to discontinuation were observed in this study [10].
Although early clinical experience with the use of a CGRP receptor antagonist with an anti-CGRP mAb suggested that the combination was both safe and effective, more data demonstrating the effectiveness of the combination are needed [11, 12]. The objective of the COURAGE study was to prospectively evaluate the real-world effectiveness of ubrogepant when used in combination with an anti-CGRP mAb or onabotulinumtoxinA, or both anti-CGRP mAb and onabotulinumtoxinA.
The COURAGE study was conducted using Migraine Buddy version 30 and above (Healint, Singapore). Migraine Buddy is a downloadable application (app) that allows users to track aspects of their migraine or headache condition, and also has the ability to deliver baseline questionnaires, customized surveys, and daily customizable diaries. Migraine Buddy was used to identify potentially eligible participants on the basis of their medications, to screen, consent, and enroll them, to capture daily data on treated attacks, and to collect data at the end of a 30-day observation period.
This analysis assessed meaningful pain relief (MPR) and return to normal function (RNF) for multiple ubrogepant-treated attacks at 2 and 4 h post-dose. MPR evaluates treatment benefit from the patient perspective [13] and has been commonly used in previous migraine studies [14, 15]. RNF is an important goal of acute treatments for migraine [7]. Additionally, at the end of 30 days, satisfaction and acute treatment optimization were evaluated. Herein, we report results for ubrogepant when taken in addition to an anti-CGRP mAb, with and without concomitant onabotulinumtoxinA.
Methods
Study Design
COURAGE was a prospective, observational study designed to evaluate the real-world effectiveness, as measured by MPR and RNF, satisfaction, and acute treatment optimization, of ubrogepant for the acute treatment of migraine when taken with an anti-CGRP mAb, onabotulinumtoxinA, or both. Potentially eligible participants were identified from the Migraine Buddy app user population as those who indicated taking ubrogepant and an anti-CGRP mAb or onabotulinumtoxinA, or both. Migraine Buddy is an app used by individuals with self-identified migraine to record and track their migraine attacks, characteristics of attacks, and treatment or management strategies for their attacks. Potentially eligible participants were screened to determine eligibility, consented electronically to participate in the study, and then enrolled in the study. Participants were informed their personal study-related data would be used in accordance with local privacy and data protection laws. The study was conducted in accordance with the Declaration of Helsinki. The study protocol was approved by Advarra Institutional Review Board in the USA (Pro00045512).
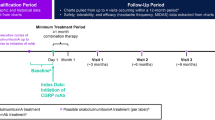
The study included a screening questionnaire, a baseline questionnaire, a 30-day data collection period, and a final questionnaire (Fig. 1). During the screening, information was collected on current use and 30-day availability of medications, informed consent, and consent of data sharing. A baseline questionnaire was completed to report previous and current use of ubrogepant and other acute treatments. During the 30-day observation period, participants completed daily questionnaires at least 4 h after treating their migraine attack to report on the effectiveness of ubrogepant-treated migraine attacks, as measured by MPR and RNF. Any attack was eligible for a day’s diary reporting if treatment was taken at least 4 h before completing the evening diary. If treatment was taken less than 4 h before the evening diary was administered, participants were prompted the following morning to complete a retrospective diary entry characterizing the attack. At the end of the 30-day treatment period, a final questionnaire assessed satisfaction with ubrogepant and acute treatment optimization. There were three observed study arms: ubrogepant and anti-CGRP mAb, ubrogepant and onabotulinumtoxinA, and ubrogepant with both anti-CGRP mAb and onabotulinumtoxinA. Here, we present data collected from the ubrogepant and anti-CGRP mAb arm and the ubrogepant with both anti-CGRP mAb and onabotulinumtoxinA arm. The effectiveness, satisfaction, and optimization in the ubrogepant and onabotulinumtoxinA arm were evaluated in a separate analysis [16].
Participants
Eligible participants were US adult Migraine Buddy app users who reported at least three migraine attacks in the last 30 days and had treated at least three prior attacks with ubrogepant 50 or 100 mg and used onabotulinumtoxinA, an anti-CGRP mAb, or both, for migraine prevention. Participants had to plan to continue use of their preventive treatments.
Endpoints
All questionnaires were completed after dosing in an electronic diary format via the Migraine Buddy app.
Meaningful Pain Relief (MPR)
Participants rated their headache pain level when ubrogepant was taken as none, mild, moderate, or severe. At least 4 h after taking ubrogepant, participants indicated if they had achieved MPR and how long it took. A patient-centered definition of MPR occurred when the level of headache pain was reduced to a degree that was meaningful to the participant. Achievement of pain relief, evaluated separately for 2 h post-dose and 4 h post-dose, was defined as having pain prior to taking ubrogepant and reporting meaningful relief of pain at 2 or 4 h post-dose, or remaining free of pain in those who reported no pain pre-dose. The endpoint variable of MPR was binary, achieving pain relief or not, and has been widely used as an endpoint in studies of acute treatments for migraine and other pain disorders [14, 15, 17].
Return to Normal Function (RNF)
Participants rated the time to RNF, defined as the timepoint when they were able to function normally. Participants rated the ability to perform daily activities at the point when ubrogepant was taken using the Functional Disability Scale (FDS). Responses ranged from no disability (able to function normally) to severely impaired (cannot do all or most things, bed rest may be necessary). Achieving normal function, evaluated separately for 2 h post-dose and 4 h post-dose, was defined as indicating having functional disability prior to taking ubrogepant and then indicating normal function at 2 or 4 h post-dose, or remaining free of disability in those who reported normal function predose. The endpoint variable was binary: achieving normal function or not.
Satisfaction and Acute Treatment Optimization
Satisfaction with study medication was reported at the final questionnaire via the Migraine Buddy app. Participants reported on their overall satisfaction with ubrogepant, as well as satisfaction with ubrogepant in combination with their current preventive treatment, at the final questionnaire using a 7-point rating scale ranging from extremely satisfied to extremely dissatisfied. Treatment satisfaction was defined as those who reported being “satisfied,” “very satisfied,” or “extremely satisfied,” making the final endpoint variable binary: satisfied or not satisfied.
Participants completed the Migraine Treatment Optimization Questionnaire (mTOQ-4) at the final questionnaire via the Migraine Buddy app. The mTOQ has been validated and used in forms of various lengths [18,19,20]. The mTOQ-4 uses frequency-based response options with sum scores ranging from 0 to 8. A higher sum score indicates greater treatment optimization. Acute treatment optimization was defined as reporting an mTOQ-4 score of at least 4. The final endpoint variable was binary: having achieved acute treatment optimization or not.
Statistical Analysis
The target sample size for this analysis was 200 participants in the ubrogepant and anti-CGRP mAb arm; however, no formal sample size calculation was performed. Continuous variables were summarized by using descriptive statistics. Categorical variables were reported as frequency counts and the percentage of participants in corresponding categories. For the analysis of populations, missing data were not imputed, and the observed data were employed. Percentage calculations were based on the number of participants with nonmissing data in each of the treatment groups. All analyses were conducted using a combination of SAS (SAS Software, Version 9.4, SAS Institute Inc, Cary, NC) and R statistical software (R, version 3.4.3, R Development Core Team).
The odds of achieving MPR and RNF were modeled under two frameworks: the first treated attack only and data for up to 10 repeated attacks over 30 days. First attack, satisfaction, and mTOQ endpoints were modeled via generalized linear models parameterized with binomial distribution and logit link. The outcome event was endpoint achievement, and the outcome reference was the failure to achieve the endpoint. The repeated attack diary endpoints were modeled via generalized linear models parameterized with a binomial distribution and logit link, adjusted for correlated repeated attacks via generalized estimating equations. This analysis evaluated up to the first 10 treated attacks per participant because few participants treated more than 10 attacks, and the small sample sizes beyond the first 10 attacks caused convergence issues and prevented the statistical model from successfully fitting to the data. This analysis reports results from the unadjusted models. The point and interval estimates reported corresponded to the odds of achieving the endpoint in each model.
Results
Ubrogepant and Anti-CGRP mAb Arm
Participants
Of the 271 participants enrolled in the ubrogepant and anti-CGRP mAb arm, 26 participants were excluded from this analysis because there were no diary data on treated attacks. This analysis included 245 participants who reported at least one ubrogepant-treated attack and reported current use of an anti-CGRP mAb without onabotulinumtoxinA. The diary study was open from September 2020 through April 2021. Each participant contributed up to 30 days of data. The majority of participants were female (89.0%) and the mean (standard deviation [SD]) age was 41.2 (10.8) years (Table 1). Most participants had a prescription for ubrogepant 100 mg (58.4%) compared with 41.6% for ubrogepant 50 mg. For preventive treatment of migraine, 44.5% (109/245) of participants reported use of erenumab, 35.1% (86/245) reported galcanezumab, 18.0% (44/245) reported fremanezumab, and 2.9% (7/245) reported eptinezumab. For each participant, the median (interquartile range [IQR]) number of recorded attacks was 8.0 (5.0; 13.0), and the median (IQR) number of ubrogepant-treated attacks was 4.0 (3.0; 7.0). Participants reported a total of 1217 migraine attacks treated with ubrogepant. Of these, 959 attacks were treated with one ubrogepant dose and 258 attacks were treated with two ubrogepant doses.
Effectiveness
For the first treated attack (first dose only), MPR was achieved by 61.6% (151/245; 95% CI 55–68%) and 80.4% (197/245; 95% CI 75–85%) of participants at 2 and 4 h post-dose, respectively (Fig. 2). Only two participants were treated with a reported predose pain level of none, and both participants reported maintaining pain-free status at 2 h. RNF was achieved by 34.7% (85/245; 95% CI 29–41%) and 55.5% (136/245; 95% CI 49–62%) of participants at 2 and 4 h post-dose, respectively. A total of 20 participants were treated with a reported predose function level of no disability; 15 participants maintained normal function and 5 developed functional impairment. The analysis across up to 10 ubrogepant-treated attacks included 1153 attacks in total (Supplemental Table 1). Across up to 10 ubrogepant-treated attacks, MPR was achieved in 51.3% (592/1153; 95% CI 48–54%) and 73.5% (847/1153; 95% CI 71–76%) at 2 and 4 h post-dose, respectively (Fig. 3). Across up to 10 attacks, MPR remained relatively stable (Supplemental Fig. 1). RNF was achieved by 32.2% (371/1153; 95% CI 29–35%) and 53.2% (613/1153; 95% CI 50–56%) at 2 and 4 h post-dose. Similar to MPR, RNF remained relatively stable across up to 10 attacks (Supplemental Fig. 2).
Ubrogepant and anti-CGRP mAb groupa: achievement of meaningful pain relief and return to normal function for first treated attack. CGRP calcitonin gene-related peptide, mAb monoclonal antibody, MPR meaningful pain relief, RNF return to normal function. aIncluded participants who used an anti-CGRP mAb without onabotulinumtoxinA for current preventive treatment
Ubrogepant and anti-CGRP mAb groupa: achievement of meaningful pain relief and return to normal function in up to 10 treated attacks. CGRP calcitonin gene-related peptide, mAb monoclonal antibody, MPR meaningful pain relief, RNF return to normal function. aIncluded participants who used an anti-CGRP mAb without onabotulinumtoxinA for current preventive treatment
Satisfaction and Acute Treatment Optimization
Following the 30-day study period, 72.7% (168/231) of participants reported being satisfied, very satisfied, or extremely satisfied with ubrogepant; 58.9% (136/231) of participants reported being satisfied, very satisfied, or extremely satisfied with ubrogepant in combination with their current preventive (Fig. 4).
Ubrogepant and anti-CGRP mAb groupa: satisfaction with ubrogepant for acute treatment of migraine and ubrogepant in combination with current preventive treatment. CGRP calcitonin gene-related peptide, mAb monoclonal antibody. aIncluded participants who used an anti-CGRP mAb without onabotulinumtoxinA for current preventive treatment
After 30 days of real-world use of ubrogepant with an anti-CGRP mAb, acute treatment optimization (mTOQ-4 score ≥ 4) was achieved by 79.7% (184/231) of participants. The mean (SD) mTOQ-4 score was 5.6 (2.4), indicating moderate acute treatment optimization. The median (IQR) mTOQ-4 score was 6.0 (4.0; 8.0).
Ubrogepant, Anti-CGRP mAb, and OnabotulinumtoxinA Arm
Participants
A total of 79 participants using ubrogepant with an anti-CGRP mAb and onabotulinumtoxinA were enrolled, but 10 participants were excluded because they did not record any diaries or because none of their diaries contained ubrogepant-treated attacks, leaving 69 participants included in this analysis. Most of the participants in this treatment arm were female (89.9%; 62/69), and the mean (SD) age was 43.8 (10.1) years (Table 1). More participants (53.6%; 37/69) received ubrogepant 100 mg than ubrogepant 50 mg (46.4%; 32/69). The median (IQR) number of recorded attacks for each participant was 10.0 (6.0; 14.0), and the median (IQR) number of treated attacks was 5.0 (3.0; 7.0). Participants reported a total of 354 migraine attacks treated with ubrogepant, with 78.8% (279/354) of attacks treated with one ubrogepant dose and 21.2% (75/354) of attacks treated with two ubrogepant doses.
Effectiveness
For the first treated attack, MPR was achieved in 63.8% (44/69; 95% CI 52–74%) and 84.1% (58/69; 95% CI 73–91%) of participants at 2 and 4 h post-dose, respectively (Fig. 5). One participant was treated with a reported predose pain level of none, and the participant reported maintaining no pain at 2 h. RNF was achieved in 39.1% (27/69; 95% CI 28–51%) and 55.1% (38/69; 95% CI 43–66%) of participants at 2 and 4 h post-dose. A total of four participants were treated with a reported predose function level of no disability, and all four participants maintained normal function. In total, 347 attacks were included in the analysis across up to 10 ubrogepant-treated attacks (Supplemental Table 2). Across up to 10 ubrogepant-treated attacks, MPR was achieved in 49.6% (172/347; 95% CI 44–55%) and 66.6% (231/347; 95% CI 62–72%) of ubrogepant-treated attacks at 2 and 4 h post-dose, respectively, and RNF was achieved by 30.8% (107/347; 95% CI 26–36%) and 48.4% (168/347; 95% CI 43–54%) at 2 and 4 h post-dose, respectively (Fig. 6). Across up to 10 treated attacks, MPR and RNF remained relatively stable (Supplemental Figs. 3 and 4).
Satisfaction and Acute Treatment Optimization
Satisfaction with ubrogepant was reported by 68.8% (44/64 [only 64 of the 69 participants completed the final questionnaire, which included satisfaction questions and the mTOQ-4]) of participants and satisfaction with ubrogepant in combination with their current preventive was reported by 59.4% (38/64; Fig. 7). Acute treatment optimization (mTOQ-4 score ≥ 4) was achieved by 78.1% (50/64) of participants. The mean (SD) mTOQ-4 score was 5.4 (2.5), indicating moderate acute treatment optimization, and the median (IQR) mTOQ-4 was 6.0 (4.0; 7.2).
Discussion
Using a novel app-based design, the COURAGE study collected entirely remote questionnaires and provided a unique opportunity to continue to understand treatment value and practice patterns. In this real-world study of combination use, ubrogepant was associated with MPR and RNF when used in combination with either an anti-CGRP mAb or an anti-CGRP mAb and onabotulinumtoxinA. Additionally, treatment satisfaction and acute treatment optimization were reported after 30 days of combination use. For the first treated attack, at 2 h post-dose, approximately 60% and more than 30% of participants reported MPR and RNF, respectively. At 4 h post-dose, more than 80% and 50% of participants reported MPR and RNF, respectively. The percentages of participants achieving MPR and RNF remained relatively stable over up to 10 ubrogepant-treated attacks, indicating a consistent response with ubrogepant when used in combination with an anti-CGRP mAb. More than 75% of participants reported acute treatment optimization. Moreover, results from a separate analysis of the ubrogepant and onabotulinumtoxinA arm demonstrated relatively similar effectiveness and acute treatment optimization [16]. Overall, the use of ubrogepant, a small-molecule CGRP receptor antagonist, with either an anti-CGRP mAb alone or with an anti-CGRP mAb and onabotulinumtoxinA was effective in the real world.
These results are generally consistent with another smaller study that demonstrated that the use of a CGRP receptor antagonist with an anti-CGRP mAb was safe and efficacious [21, 22]. Moreover, these findings support results from a real-world study of 251 participants who were prescribed ubrogepant, which showed that 19.0% and 47.6% of participants achieved headache freedom and headache relief for at least 75% of all treated attacks, respectively [23]. Additionally, in that real-world study, 31.1% of participants reported being “very satisfied” with ubrogepant. However, it should be noted that findings from the present study demonstrated slightly higher satisfaction with ubrogepant among participants who reported current use of an anti-CGRP mAb compared with those who reported current use of an anti-CGRP mAb and onabotulinumtoxinA, which may suggest that participants in the latter group had greater disease severity given the number of branded preventive treatments needed to manage their migraine. Furthermore, the present findings complement results from the pivotal randomized, double-blind, placebo-controlled, single-attack, phase 3 clinical trials (ACHIEVE I and ACHIEVE II) of ubrogepant [2, 3]. In those clinical trials, compared with placebo-treated participants, significantly higher proportions of ubrogepant-treated participants reported being able to function normally at 2 h post-dose (ACHIEVE I: 40.6% with ubrogepant 50 mg and 42.9% with ubrogepant 100 mg vs 29.8% with placebo; ACHIEVE II: 40.5% with ubrogepant 50 mg vs 34.2% with placebo) and 4 h post-dose (ACHIEVE I: 60.6% with ubrogepant 50 mg and 60.7% with ubrogepant 100 mg vs 45.2% with placebo; ACHIEVE II: 60.8% with ubrogepant 50 mg vs 47.6% with placebo). Significantly higher proportions of ubrogepant-treated compared with placebo-treated participants also reported being satisfied or extremely satisfied with study medication at 2 h post-dose (ACHIEVE I: 36.3% with ubrogepant 50 mg and 35.8% with ubrogepant 100 mg vs 24.1% with placebo; ACHIEVE II: 37.8% with ubrogepant 50 mg vs 24.8% with placebo).
Individuals with migraine should all be offered acute treatment, with the goals of rapidly relieving pain, restoring normal function, and providing consistent results from attack to attack [7]. Some individuals with migraine also need a preventive treatment to reduce the frequency, severity, and duration of attacks and avoid escalating acute treatment use. In clinical practice, health care providers have successfully used onabotulinumtoxinA and anti-CGRP mAbs in people with migraine [24]; however, there are a number of practical and hypothetical reasons that suggest that the combination of ubrogepant and an anti-CGRP mAb would be an effective paradigm to maximize outcomes. First, anti-CGRP mAbs have demonstrated efficacy in the preventive treatment of migraine; however, they are administered monthly or quarterly, and people have reported wearing-off of effectiveness towards the end of a treatment cycle [25]. In a real-world study of erenumab, self-reported wearing-off occurred in 34.7% of participants who received erenumab for 6 months or discontinued treatment. Of those who experienced wearing-off, 80.0% reported that wearing-off occurred 1 week prior to the next scheduled injection [25]. In addition, a pharmacokinetic study of galcanezumab demonstrated that CGRP plasma levels initially dropped following administration and returned to higher levels prior to the next monthly dose [26]. As CGRP plasma levels rise, the use of ubrogepant may be of benefit in the treatment of breakthrough attacks to bridge the prevention cycle. Additionally, higher doses of the anti-CGRP mAbs are commonly used, suggesting that there may not be complete coverage of the CGRP pathway when used for prevention; thus, ubrogepant may be an effective acute treatment if there is an additional surge of CGRP at any point. Furthermore, the CGRP pathway may play a pivotal role in the pathophysiology of pain [27]. As people with migraine may experience other chronic pain disorders, medications that target the CGRP pathway may potentially alleviate symptoms of these comorbid conditions [27, 28].
Strengths and Limitations
Real-world studies provide important information on treatment effectiveness and safety in a more representative patient population compared with those in clinical trials; real-world studies also prioritize the patient experience and focus on the clinical outcomes that are the most meaningful to patients [29]. By administering daily questionnaires, this study collected self-reported data as close as possible to the time of treatment, which reduced recall bias compared with weekly and quarterly questionnaires. The study design leveraged automated reminders to administer the relevant questionnaires, which significantly reduced the workload on data collection and subsequent processing, highlighting the value of this design in similar platforms for large-scale, real-world evidence studies. A potential limitation of this study was that data were self-reported by Migraine Buddy app users and not confirmed by a health care provider; however, the diagnosis of migraine could be assumed, as participants completed a migraine screening assessment. Additionally, all participants reported using migraine-specific products, which are not prescribed for any other diseases and require a prescription from a health care provider. Another limitation was the absence of a contemporaneous placebo control group, a feature of pivotal efficacy studies that is rarely included in studies designed to generate real-world evidence. Even though pain assessments may be subjective and inherent variability among pain scales exists, the use of MPR as a key endpoint is aligned with the use of MPR in prior studies of migraine and has been widely used in studies of other pain disorders [14, 15, 17, 30]. Our approach allowed us to determine if MPR occurred within 4 h to estimate the time at which it occurred using a single recall-based question. This efficient approach to data collection generated logically consistent results across multiple attacks. As the interval increases from the achievement of MPR to the collection of time to MPR, the estimate is subject to recall bias. Although pain freedom after 2 h is a recommended primary endpoint in clinical trials, the use of MPR within 4 h was a clinically relevant endpoint for this real-world study. MPR relies on patient engagement and provides an understanding of the treatment benefit from the perspective of the patient [13]. Furthermore, the evaluation of RNF in this study provided real-world insight into the restoration of normal function, which is a primary goal of acute treatment for migraine [31]. To fully assess the safety and effectiveness of this combination, controlled trials and real-world studies with longer-term safety data collection are needed. The successful implementation of this study, however, demonstrated the feasibility of an app-based study design to collect real-world data on migraine treatment effectiveness during the COVID-19 pandemic.
Conclusion
In this real-world study, ubrogepant was effective when used either in combination with an anti-CGRP mAb or an anti-CGRP mAb and onabotulinumtoxinA, as indicated by meaningful pain relief and restoration of function. Ubrogepant was also associated with treatment satisfaction and acute treatment optimization when used either in combination with an anti-CGRP mAb or an anti-CGRP mAb and onabotulinumtoxinA.
Data Availability
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual and trial-level data (analysis data sets), as well as other information (e.g., protocols, clinical study reports, or analysis plans), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time after approval in the US and Europe and after acceptance of this manuscript for publication. The data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://vivli.org/ourmember/abbvie/ then select “Home.”
References
Ubrelvy [package insert]: AbbVie; 2023.
Dodick DW, Lipton RB, Ailani J, et al. Ubrogepant for the treatment of migraine. N Engl J Med. 2019;381:2230–41.
Lipton RB, Dodick DW, Ailani J, et al. Effect of ubrogepant versus placebo on pain and the most bothersome associated symptom in the acute treatment of migraine: the ACHIEVE II randomized clinical trial. JAMA. 2019;322:1887–98.
Ailani J, Lipton RB, Hutchinson S, et al. Long-term safety evaluation of ubrogepant for the acute treatment of migraine: phase 3, randomized, 52-week extension trial. Headache. 2020;60:141–52.
Dodick DW, Lipton RB, Ailani J, et al. Ubrogepant, an acute treatment for migraine, improved patient-reported functional disability and satisfaction in 2 single-attack phase 3 randomized trials, ACHIEVE I and II. Headache. 2020;60:686–700.
Lipton RB, Singh RBH, Revicki DA, et al. Functionality, satisfaction, and global impression of change with ubrogepant for the acute treatment of migraine in triptan insufficient responders: a post hoc analysis of the ACHIEVE I and ACHIEVE II randomized trials. J Headache Pain. 2022;23:50.
Ailani J, Burch RC, Robbins MS. The American Headache Society consensus statement: update on integrating new migraine treatments into clinical practice. Headache. 2021;61:1021–39.
Curto M, Capi M, Cipolla F, Cisale GY, Martelletti P, Lionetto L. Ubrogepant for the treatment of migraine. Expert Opin Pharmacother. 2020;21:755–9.
Qulipta [package insert]: AbbVie; 2023.
Jakate A, Blumenfeld AM, Boinpally R, et al. Pharmacokinetics and safety of ubrogepant when coadministered with calcitonin gene-related peptide-targeted monoclonal antibody migraine preventives in participants with migraine: a randomized phase 1b drug-drug interaction study. Headache. 2021;61:642–52.
Freitag FG, Tolebeyan A, Sivakumar D. CGRP monoclonal antibodies along with CGRP receptor antagonists are safe and effective together and compared to standard of care [P-155]. Headache. 2021;61(S1):113.
Berman G, Croop R, Kudrow D, et al. Safety of rimegepant, an oral CGRP receptor antagonist, plus CGRP monoclonal antibodies for migraine. Headache. 2020;60:1734–42.
Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole MR. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149–58.
Matsumori Y, Komori M, Tanji Y, Ozeki A, Sakai F. Rapid onset and sustained efficacy of lasmiditan among Japanese patients with migraine: prespecified analyses of a randomized controlled trial. Neurol Ther. 2022;11:1721–34.
Sunshine A, Mulhern SA, Olson N, Elkind A, Almas M, Sikes C. Comparative sensitivity of stopwatch methodology and conventional pain assessment measures for detecting early response to triptans in migraine: results of a randomized, open-label pilot study. Clin Ther. 2006;28:1107–15.
Manack Adams A, Hutchinson S, Engstrom E, et al. Real-world effectiveness, satisfaction, and optimization of ubrogepant for the acute treatment of migraine in combination with onabotulinumtoxinA: results from the COURAGE Study. J Headache Pain. 2023;24:102.
Black P, Max MB, Desjardins P, Norwood T, Ardia A, Pallotta T. A randomized, double-blind, placebo-controlled comparison of the analgesic efficacy, onset of action, and tolerability of ibuprofen arginate and ibuprofen in postoperative dental pain. Clin Ther. 2002;24:1072–89.
Lipton RB, Kolodner K, Bigal ME, et al. Validity and reliability of the migraine-treatment optimization questionnaire. Cephalalgia. 2009;29:751–9.
Lipton RB, Fanning KM, Serrano D, Reed ML, Cady R, Buse DC. Ineffective acute treatment of episodic migraine is associated with new-onset chronic migraine. Neurology. 2015;84:688–95.
Serrano D, Buse DC, Manack Adams A, Reed ML, Lipton RB. Acute treatment optimization in episodic and chronic migraine: results of the American Migraine Prevalence and Prevention (AMPP) Study. Headache. 2015;55:502–18.
Berman G, Kudrow D, Halverson P, et al. Oral rimegepant 75 mg is well-tolerated when used concomitantly with injectable anti-CGRP monoclonal antibodies: results from a multicenter, long-term, open-label safety study [abstract]. Neurology. 2020;94(suppl 15):4553.
Mullin K, Kudrow D, Croop R, et al. Potential for treatment benefit of small molecule CGRP receptor antagonist plus monoclonal antibody in migraine therapy. Neurology. 2020;94:e2121–5.
Chiang CC, Arca KN, Dunn RB, et al. Real-world efficacy, tolerability, and safety of ubrogepant. Headache. 2021;61:620–7.
Argyriou AA, Dermitzakis EV, Xiromerisiou G, Vikelis M. OnabotulinumtoxinA add-on to monoclonal anti-CGRP antibodies in treatment-refractory chronic migraine. Toxins (Basel). 2022;14:847.
Robblee J, Devick KL, Mendez N, Potter J, Slonaker J, Starling AJ. Real-world patient experience with erenumab for the preventive treatment of migraine. Headache. 2020;60:2014–25.
Kielbasa W, Helton DL. A new era for migraine: pharmacokinetic and pharmacodynamic insights into monoclonal antibodies with a focus on galcanezumab, an anti-CGRP antibody. Cephalalgia. 2019;39:1284–97.
Schou WS, Ashina S, Amin FM, Goadsby PJ, Ashina M. Calcitonin gene-related peptide and pain: a systematic review. J Headache Pain. 2017;18:34.
Wang SJ, Chen PK, Fuh JL. Comorbidities of migraine. Front Neurol. 2010;1:16.
Blonde L, Khunti K, Harris SB, Meizinger C, Skolnik NS. Interpretation and impact of real-world clinical data for the practicing clinician. Adv Ther. 2018;35:1763–74.
Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9–19.
Ailani J, Lipton RB, Goadsby PJ, et al. Atogepant for the preventive treatment of migraine. N Engl J Med. 2021;385:695–706.
Acknowledgements
We thank the participants of this study.
Medical Writing/Editorial Assistance
Writing and editorial assistance was provided to the authors by Anny Wu, PharmD, of Peloton Advantage, LLC, an OPEN Health company, and was funded by AbbVie.
Funding
Allergan (prior to its acquisition by AbbVie) funded this study and the Rapid Service Fee and participated in the study design, research, analysis, data collection, interpretation of data, reviewing, and approval of the publication. All authors had access to relevant data and participated in the drafting, review, and approval of this publication. No honoraria or payments were made for authorship.
Author information
Authors and Affiliations
Contributions
Study design: Weijie Poh. Enrolled patients: Weijie Poh. Collection and assembly of data: Weijie Poh. Data analysis: Daniel Serrano, Ella Engstrom, Nicolai D. Ayasse. Data interpretation: Richard B. Lipton, Janette Contreras-De Lama, Daniel Serrano, Ella Engstrom, Nicolai D. Ayasse, Weijie Poh, François Cadiou, Aubrey Manack Adams. Manuscript preparation: Richard B. Lipton, Janette Contreras-De Lama, Daniel Serrano, Ella Engstrom, Nicolai D. Ayasse, Weijie Poh, François Cadiou, Aubrey Manack Adams. Manuscript review and revisions: Richard B. Lipton, Janette Contreras-De Lama, Daniel Serrano, Ella Engstrom, Nicolai D. Ayasse, Weijie Poh, François Cadiou, Aubrey Manack Adams. Final approval of the manuscript: Richard B. Lipton, Janette Contreras-De Lama, Daniel Serrano, Ella Engstrom, Nicolai D. Ayasse, Weijie Poh, François Cadiou, Aubrey Manack Adams.
Corresponding author
Ethics declarations
Conflict of Interest
Richard B. Lipton, MD, has received research support from the National Institutes of Health, the FDA, and the National Headache Foundation. He serves as consultant, advisory board member, or has received honoraria or research support from AbbVie/Allergan, Amgen, Biohaven, Dr. Reddy’s Laboratories (Promius), electroCore, Eli Lilly, GlaxoSmithKline, Lundbeck, Merck, Novartis, Teva, Vector, and Vedanta Research. He receives royalties from Wolff’s Headache, 8th edition (Oxford University Press, 2009), and Informa. He holds stock in Biohaven and Manistee. Janette Contreras-De Lama, PhD, and Aubrey Manack Adams, PhD, are employees of AbbVie and may hold AbbVie stock. Daniel Serrano, PhD, and Ella Engstrom, BS, were employees of OPEN Health Group at the time of the study. Nicolai D. Ayasse, PhD, was an employee of OPEN Health Group at the time of the study and is currently an employee of Critical Path Institute. Weijie Poh, PhD, was an employee of Healint Pte Ltd at the time of the study and is currently an employee of Digital Life Line. François Cadiou, MBA, is an employee of Healint Pte Ltd.
Ethical Approval
Potentially eligible participants were screened to determine eligibility, consented electronically to participate in the study, and then enrolled in the study. Participants were informed their personal study-related data would be used in accordance with local privacy and data protection laws. The study was conducted in accordance with the Declaration of Helsinki. The study protocol was approved by Advarra Institutional Review Board in the USA (Pro00045512).
Additional information
Affiliations for Daniel Serrano, Ella Engstrom, Nicolai D. Ayasse, and Weijie Poh are those at the time of study conduct.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Lipton, R.B., Contreras-De Lama, J., Serrano, D. et al. Real-World Use of Ubrogepant as Acute Treatment for Migraine with an Anti-Calcitonin Gene-Related Peptide Monoclonal Antibody: Results from COURAGE. Neurol Ther 13, 69–83 (2024). https://doi.org/10.1007/s40120-023-00556-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-023-00556-8