Abstract
Introduction
Similar white matter hyperintensities (WMH) burden may have varied cognitive outcomes in patients with cerebral small vessel disease (CSVD). This study aimed to evaluate whether blood–brain barrier (BBB) permeability is associated with cognitive impairment (CI) heterogeneity in patients with WMH.
Methods
We recruited 51 participants with WMH. We evaluated WMH burden using the Fazekas scale and WMH volume on structural magnetic resonance imaging (MRI), and assessed BBB permeability using dynamic contrast-enhanced (DCE)-MRI. We used permeability–surface area product (PS) from the Patlak model to represent BBB permeability. All patients underwent Mini-Mental State Examination (MMSE), Boston Naming Test (BNT) and animal verbal fluency test (VFT) for cognitive assessment. We divided patients into CI and non-CI groups based on their MMSE scores (< 27 or ≥ 27) and used multiple linear regression models to investigate the associations between MRI parameters and cognitive function.
Results
Patients in the two groups did not differ in Fazekas scores and WMH volume. However, patients in the CI group showed significantly higher PS in the WMH regions than those in non-CI group (1.89 × 10−3 versus 1.00 × 10−3, p = 0.032 in periventricular WMH [PVWMH]; 1.27 × 10−3 versus 0.74 × 10−3, p = 0.043 in deep WMH [DWMH]), indicating the breakdown of BBB in the CI group. In all patients with WMH, increased BBB permeability in PVWMH and DWMH was significantly associated with lower cognitive and language function after adjustment for age, education level (EL) and intracranial volume (ICV). In the CI group, this correlation remained significant. WMH volume was not associated with cognitive performance in either all patients or those with CI.
Conclusion
BBB impairment might be a more sensitive indicator for cognitive and language dysfunction than WMH volume in patients with WMH and possibly explains the heterogeneity of cognitive performance in patients with similar WMH burden.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
In patients with WMH of CSVD, WMH burden is related to cognition. However, a similar WMH burden may have varied cognitive outcomes. |
This study aimed to evaluate whether the permeability of the blood–brain barrier (BBB) is associated with the heterogeneity of cognitive impairment (CI) in patients with WMH. |
What was learned from the study? |
BBB impairment, especially in the periventricular WMH region, might be a more sensitive indicator for cognitive and language dysfunction than WMH volume in patients with WMH and possibly explains the heterogeneity of cognitive performance in patients with similar WMH burden. |
Introduction
Cerebral small vessel disease (CSVD) is a type of age-related cerebrovascular disease that is commonly observed clinically [1]. White matter hyperintensities (WMH) have received considerable attention as imaging markers of CSVD [2]. Previous studies on WMH have shown a progressive increase in prevalence with increasing age in adults, reaching 60–100% in individuals aged over 65 years [3]. In addition, the risk of stroke, dementia and death increased by 2.6–4.4-, 1.3–2.8- and 1.6–2.7-fold, respectively, in the WMH population, compared to the general population [3, 4].
Previous studies have found that a higher WMH burden is associated with a higher risk of cognitive impairment (CI) [4] and with the exacerbation of CI [5, 6]. In clinical practice, we observed that some patients with a very high WMH volume or visual score did not exhibit significant CI, whereas others with a low WMH volume or visual score exhibited significant CI. However, the reasons for the heterogeneity in cognitive performances in patients with WMH remain unclear.
Among all cognitive domains, language problems in patients with CSVD are usually subtle and might be easily neglected, and therefore under-researched. However, language is essential for daily life. Recent studies showed that patients with CSVD showed poor performance in verbal fluency along with executive deficiencies compared with normal healthy volunteers and patients with Alzheimer’s disease [7, 8]. Language tests such as the verbal fluency test (VFT) also reflect the executive functioning which has been regarded as the hallmark of vascular dementia [9]. Failure in executive-language functioning might be distinctive and of great importance in CI caused by CSVD [10].
Some studies showed that verbal abilities might be associated with more severe WMH but the results were not consistent in other studies [10, 11]. One of the pitfalls of WMH volumetric measurement or visual rating is that it could not reflect the underlying pathological changes in CSVD. Blood–brain barrier (BBB) dysfunction may be an early pathological change leading to WMH [12]. Recently, studies have used gadolinium-based contrast agents in dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) to detect subtle changes of BBB in patients with CSVD and dementia [13]. Therefore, we hypothesised that increased BBB permeability might be a more sensitive indicator of cognitive and language impairment than WMH in patients with CSVD and could possibly explain the heterogeneity of cognitive performances underlying similar WMH.
In this study, we used DCE-MRI to evaluate BBB permeability. We used the Boston Naming Test (BNT) [14] and VFT [15] to assess language abilities, and Mini-Mental State Examination (MMSE) [16] for global cognitive function. This study aimed to investigate the associations between BBB permeability in different WMH areas, WMH volume and cognitive performance in patients with WMH.
Methods
Patient Selection
Patients with WMH enrolled in the Department of Neurology of Beijing Tiantan Hospital between November 2019 and January 2022 were recruited as the study participants [17]. The selection criteria were as follows: aged 18–90 years; WMH with Fazekas grade ≥ 2 regardless of vascular risk factors, or WMH with Fazekas grade = 1 and two or more vascular risk factors; no new subcortical infarction within 14 days; independence in daily life (modified Rankin scale score ≤ 2).
The exclusion criteria were as follows: diagnosis of degenerative diseases of the central nervous system, such as Alzheimer’s disease, Parkinson’s disease and other non-vascular causes of brain injury-induced dementia; patients who were unable to cooperate in completing relevant cognitive function tests and head MRI examinations; patients with epilepsy, multiple sclerosis, head trauma, or previous structural brain abnormalities or non-vascular brain injury; those with white matter lesions caused by other non-vascular diseases, such as leukodystrophy and immune reactions to neurological infection; cortical infarction on MRI, acute cerebral infarction > 20 mm in diameter, acute cerebral haemorrhage, or subarachnoid haemorrhage within 14 days before recruitment; drug and alcohol abuse and diagnosis of psychiatric disorders, such as schizophrenia; clinically significant medical or neurological disorders, malignant diseases, pregnancy, chronic diseases with a poor 5-year prognosis, except for CI; and concurrent participation in other drug or instrumental clinical randomised controlled trials. Diagnosis of CSVD was performed by at least two trained neurologists after reviewing the clinical presentation, MRI features and other diagnostic test results.
Participant Consent
The study was approved by the Institutional Review Board of Beijing Tiantan Hospital (KY 2019-140-01) and followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies [17]. All participants voluntarily agreed to participate in the study, consented to the cognitive function assessment and MRI, agreed to the BBB assessment protocol and gave informed consent for the use of data in the study. The study was performed in accordance with the 1964 Declaration of Helsinki and its later amendments.
Cognitive Function Assessment
All patients completed the standardised measures of cognitive function tests, and all tests were administered and scored by trained psychologists in accordance with standard testing procedures using published criteria and sequences for each test. MMSE [16] was used to assess global cognitive function, because it has a large norm sample in China and has a recognised screening threshold and reliability [16]. We classified patients into CI (MMSE score < 27) and non-CI (MMSE score ≥ 27) groups. The cutoff value of MMSE was based on previous studies on vascular dementia in Asian and Chinese populations [18,19,20]. For very few patients whose scores were at the cutoff value, a senior cognitive specialist made the final decision of diagnosis. Considering the impact of education level (EL) on cognition, we recorded patients’ education years and classified EL into three categories (EL1 = illiterate, EL2 = 1–6 years, EL3 ≥ 7 years) [16]. To assess language performance separately, we used BNT [14] and VFT [15], according to the Vascular Impairment of Cognition Classification Consensus Study (VICCCS) [21]. Meanwhile, previous studies have shown that VFT can be used to assess executive function [15]. The duration of the cognitive test was approximately 30 min.
Brain Structural MRI
All enrolled participants underwent a routine brain MRI with a 3-T scanner (Siemens MAGNETOM Prisma, Erlangen, Germany) equipped with Siemens 64ch-head-coils. The sequences included a T1-weighted sequence [repetition time (TR)/inversion time (TI)/echo time (TE) = 2300/900/2.26 ms; field of view (FOV) = 256 × 256 × 192 mm3; voxel size = 1.0 × 1.0 × 1.0 mm3; and flip angle = 8°] for anatomic reference and a T2-weighted FLAIR sequence (TR/TI/TE = 5000/1800/388 ms; FOV = 256 × 256 × 192 mm3; voxel size = 1.0 × 1.0 × 1.0 mm3; and flip angle = 40°) for assessment of WMH. In addition, a DWI sequence (TR/TE = 4690/55 ms; FOV = 230 × 230 mm2; voxel size = 1.4 × 1.4 × 3.0 mm3, diffusion sensitising gradient directions b = 1000 s/mm2) was performed for the detection of new lacunar infarcts and an SWI sequence (TR/TE = 27/20 ms; FOV = 205 × 240 × 160 mm3; voxel size = 0.7 × 0.7 × 2.0 mm3; and flip angle = 15°) was used to detect microbleeds.
DCE Imaging Protocol
All patients had a DCE-MRI scan to assess BBB permeability. This consisted of a T1-weighted, whole-brain volume acquisition before and repeated sequentially up to 21 min after a slow intravenous injection of gadoteric acid (Gd-DOTA) (0.2 ml/kg body weight; Hengrui Pharmaceuticals Co., Ltd.). The injection lasted for about 130 s. The sequence acquisition was a spoiled gradient echo with TR/TE = 3.44/1.68 ms, flip angle = 15°, FOV = 240 × 240 mm2 and slice thickness = 2 mm [22].
Imaging Analysis
Structural Imaging Analysis
On the basis of the STRIVE criteria and the semi-quantitative Fazekas scale [23], two neurologists blindly scored WMH using 3D T1, T2 and FLAIR sequences. Senior neuroradiologists made the final decision regarding any inconsistent results.
On the basis of the Fazekas scale, WMH were classified as deep (0 = none, 1 = dotted, 2 = early confluence or 3 = confluent) or periventricular (0 = none, 1 = cap or pen thin layer, 2 = smooth halo or 3 = irregular WMH extending to the deep white matter) [2]. We calculated the total WMH score as the sum of the periventricular and deep WMH scores.
Intracranial volume (ICV) and grey matter volume (GMV) were extracted on the T1-weighted sequence using custom-built code in MATLAB (Mathworks; Natick, MA, USA), followed by manual correction [24].
Permeability and Volume Analysis
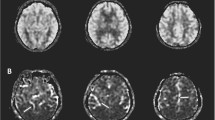
We performed DCE-MRI data analyses using custom-built code in MATLAB (Mathworks; Natick, MA, USA). All images were registered, and the venous input function (VIF) was obtained from the superior sagittal sinus. Voxels were used to acquire peak signal enhancement and smooth changes during the DCE-MRI time course. We used the Patlak model to calculate the BBB leakage parameters from tissue signal enhancement because it ignores back flux from extracellular extravascular space into the blood and is considered optimal for low and subtle leakage conditions [22, 25]. We generated the permeability–surface area product (PS) from the Patlak model to represent the leakage of the BBB, which is equivalent to the rate of leakage per unit tissue volume per unit capillary blood plasma Gd-DOTA concentration [25, 26]. We manually drew regions of interest (ROIs) for the WMH on the anterior and posterior corners of the bilateral periventricular and deep WMH, on the registered-FLAIR images, carefully excluding the vessels and cerebrospinal fluid (Fig. 1). In very few cases, when the periventricular and deep WMH fused, the periventricular white matter region was within 5 mm of the periventricular according to the distribution of the innervating vessels. The ROIs were overlapped on the voxel-wise PS (unit min−1) map, and the PS value in each ROI was calculated. Meanwhile, the volume of the WMH lesion was estimated on the basis of mask and voxel size (Fig. 2).
Selection of regions of interest (ROIs). ROIs for WMH were manually selected according to T2 and T2-FLAIR hyperintensity, T1-weighted sequence isointensity or slight hypointensity (different from the signal of cerebrospinal fluid, without cavitation). A Example of ROIs on FLAIR images for periventricular WMH. B Example of ROIs on FLAIR images for deep WMH. ROI region of interest, WMH white matter hyperintensities, T1 T1-weighted sequence, T2 T2-weighted sequence, T2-FLAIR T2-fluid attenuated inversion recovery
DCE imaging analysis process. SSS was used as the vascular input function, and the original DCE data were used to generate the Ktrans map by the Patlak model. Then, co-registration between the three-dimensional T1-weighted image and the Ktrans image was automatically performed. ROIs of WMHs were manually selected. DCE dynamic contrast-enhanced, ROIs regions of interest, SSS superior sagittal sinus, WMHs white matter hyperintensities
Statistical Analyses
All statistical analyses were performed using SPSS Statistics (version 25.0, IBM Corp., USA), and R v.4.1.2 (R Foundation for Statistical Computing, Vienna, Austria) for Windows. Statistical significance was set at p < 0.05. Measurement data conforming to a normal distribution are represented as mean and standard deviation and an independent sample t test was used to analyse differences between groups. Data with non-normal distribution are expressed as medians and interquartile ranges, and differences between groups were determined using the independent sample Wilcoxon rank-sum test. Counting data are represented as frequencies and component ratios, and differences between groups were assessed using the chi-squared or Fisher’s exact probability tests. We used multiple linear regression models for continuous outcome variables, including WMH volume and PS in different WMH areas. We natural-log transformed the PS of WMH in all regression models because they were not normally distributed. To correct for the effect of ICV on WMH volumes, WMH volumes were expressed as a percentage of ICV [24]. All regression models were adjusted for age, EL and GMV/ICV ratio [16, 27].
Results
Overall, 51 patients (23–82 years, F/M = 14/37) with WMH were included in the study, consisting of 31 in the CI group and 20 in the non-CI group. Patients in the CI group were significantly older than those in the non-CI group [median (interquartile range) 64.5 (57.5–67) versus 57 (52.5–63) years; p = 0.007). Patients in non-CI group had longer years of education than those in the CI group [12.2 ± 1.09 versus 9.26 ± 0.8 years; p = 0.031]. Otherwise, there were no significant differences in other baseline characteristics and vascular risk factors (Table 1).
Head MRI data from 51 participants were used for BBB permeability and volume analysis of WMH. Overall, 46 participants completed the BNT scale, and 44 participants completed the VFT scale. All 51 participants had completed MMSE scores, and thus the correlation between BBB permeability and volume and cognitive scores was analysed in 51 patients.
Comparison of Cognitive Function Tests Between the CI and Non-CI Groups
Comparisons of Cognitive Function Between Both Groups
Patients in the CI group showed significantly worse performance in MMSE (median (interquartile range) 24 (21–25) versus 28 (28–29) in the CI versus non-CI group, p < 0.001), VFT (13.00 ± 5.63 versus 19.37 ± 4.06, p < 0.001) and BNT (18.15 ± 5.28 versus 23.79 ± 3.47, p < 0.001) (Table 2).
Comparison of WMH Burden and BBB Permeability Between Both Groups
Patients did not differ in total Fazekas scores [28] or WMH volume between the two groups.
Patients in the CI group showed significantly higher PS value in both periventricular WMH (median [interquartile range] 1.89 [0.95–7.87] × 10−3 versus 1.00 [0.68–1.78] × 10−3 in the CI versus non-CI group, p = 0.032) and deep WMH (1.27 [0.69–13.91] × 10−3 versus 0.74 [0.38–1.84] × 10−3 in the CI versus non-CI group, p = 0.043) than those without CI, suggesting a higher BBB permeability in both areas (Table 2).
GMV and ICV in the CI group were significantly smaller than those in the non-CI group (GMV: 605.10 ± 12.66 versus 661.40 ± 19.93, p = 0.015; ICV: 1475.67 ± 22.63 versus 1572.26 ± 33.70, p = 0.017), but there were no significant differences between GMV/ICV and WMH/ICV ratios between two groups (Table 2).
Association Between BBB Permeability, WMH Volume and Cognitive Function in All Patients with WMH
Linear regression models revealed that increased BBB permeability in periventricular WMH and deep WMH areas was significantly associated with lower MMSE (β = − 0.579, p < 0.001 in periventricular WMH, β = − 0.442, p < 0.001 in deep WMH) score, lower BNT score (β = − 0.511, p < 0.001 in periventricular WMH, β = − 0.410, p = 0.004 in deep WMH) and lower VFT score (β = − 0.354, p = 0.01 in periventricular WMH, β = − 0.276, p = 0.046 in deep WMH). No significant associations were found between WMH volume and any cognitive test. All models were adjusted for age, education level and GMV/ICV (Table 3).
We plotted the receiver operating characteristic (ROC) curve and calculated the area under the ROC curve (AUC). The results showed that PS in periventricular WMH (AUC = 0.678) was the most sensitive to MMSE, followed by PS in deep WMH (AUC = 0.669) and finally WMH volume (AUC = 0.648) (Fig. 3).
Receiver operating characteristic curves based on PS in periventricular WMH, PS in deep WMH and total WMH volume with overall cognitive MMSE. aData are natural log-transformed in the models. bAll models are adjusted for age, education level and GMV/ICV. cTotal WMH volume is expressed as a percentage of ICV. Red, PS in periventricular WMH; Orange, PS in deep WMH; Yellow, total WMH volume; Blue, MMSE MMSE Mini-Mental State Examination, PS permeability–surface area product (unit min−1), WMH white matter hyperintensities, AUC area under the receiver operating characteristic curve
Association Between BBB Permeability, WMH Volume and Cognitive Function in Patients with Cognitive Impairment
We performed linear regression analyses separately in the CI group. The results showed that higher BBB permeability in periventricular WMH and deep WMH areas was still significantly associated with lower MMSE score (β = − 0.536, p < 0.001 in periventricular WMH, β = − 0.389, p = 0.014 in deep WMH). Increased BBB permeability in periventricular WMH was also significantly associated with lower BNT score (β = − 0.513, p = 0.006). There were no longer significant associations between BBB permeability in WMH areas and VFT scores. No significant associations were found between WMH volume and any cognitive test. All models were adjusted for age (Table 4).
Discussion
The main findings of this study are as follows. First, while most previous studies on DCE and cognition have focused on people with mild cognitive impairment (MCI) and Alzheimer’s disease (AD) [29] this study used DCE to explore the cause of the cognitive differences in the WMH population with CSVD and lay the imaging foundation for further research on the pathophysiological process of this difference. Second, we used a combination of global cognitive scales and cognitive domain scales, focusing on language and executive function. Third, we found a significant difference in BBB permeability between patients with WMH with and without CI. This difference appeared before any divergence of Fazekas scores and WMH volume in these patients, suggesting that BBB permeability is a more sensitive index than WMH volume and Fazekas score to evaluate CI. Further, this difference in BBB permeability may be the cause of cognitive heterogeneity in patients with a similar WMH burden. Fourth, in further related studies, we also confirmed that BBB PS was better correlated with overall cognition than WMH volume in patients with WMH, especially those with periventricular WMH PS. This suggests that the BBB permeability in the WMH region is more sensitive to CI than the WMH volume, and damage in key sites in the periventricular region may be more important than diffuse damage in the BBB. Fifth, correlations were observed between each cognitive domain and BBB permeability in WMH, and specific cognitive domains, including executive function, and language, correlated with periventricular WMH. Impairments in language and specific cognitive domains in patients with WMH may be related to the impairment of the BBB in key sites such as the periventricular region. The location with higher BBB permeability and its effect on each cognitive domain will receive our attention in future studies.
WMH are associated with the occurrence and development of CI [5]. In patients with WMH, the baseline severity of WMH is considered an independent predictor of clinical vascular CI, and WMH can precede clinical symptoms of CI [3]. However, WMH exhibits considerable heterogeneity for the following reasons: (1) Development of WMH varies (can grow or shrink) [30]. (2) WMH may have different histopathological manifestations (slightly diffuse matrix/varying degrees of myelin and axonal loss) [31]. (3) There are differences in clinical symptoms associated with WMH (periventricular WMH are associated with hypertension, and deep WMH are associated with poor sleep quality) [32]. Moreover, studies on the heterogeneity of WMH in patients with CI are lacking.
Conventional vascular risk factors may not be strongly associated with CI in patients with WMH. This study assigned patients with WMH to the CI and non-CI groups. We found significant age differences between the two groups, whereas differences in conventional vascular risk factors were insignificant in the rest. Accordingly, the relationship between WMH severity and CI may be independent of previously reported vascular risk factors [32].
We found a “mismatch” between WMH severity and CI. The Fazekas scores, WMH volume and each cognitive domain score were compared between groups; significant differences in global cognition and each cognitive domain remained after adjusting for age, whereas no significant differences in the Fazekas scores and WMH volume were observed between the two groups. Although previous studies have suggested that higher Fazekas scores and larger volume of WMH are associated with lower overall brain function or deficits in specific regions of cognitive ability, these associations were weak in our study and insufficient to reflect the heterogeneity of CI due to WMH [33]. We believe that using a visual scoring scale or WMH volume does not clearly reflect the dynamic changes in WMH, and the inability to assess WMH more accurately.
With advances in imaging technology, a theory of impaired white matter integrity based on WMH has been developed to explain the heterogeneity of WMH that leads to CI. As BBB dysfunction and fluid leakage into the perivascular area are recognised as early pathological changes in WMH, BBB function can be used to characterise white matter integrity. DCE-MRI is a reliable technique for assessing white matter integrity through the BBB function [34]. Using multimodal imaging, we confirmed that BBB permeability was significantly altered in the CI group before the appearance of differences in Fazekas visual scores and WMH volume, resulting in an earlier and more accurate assessment of CI, further suggesting that the presence of CI in patients is associated with altered BBB permeability.
We observed that the presence of CI in patients with WMH may have resulted from BBB damage in key regions. The breakdown of the lateral periventricular white matter BBB may be more closely associated with cognitive decline than the deep white matter. Previous studies used diffusion tensor imaging to map neuronal circuits of cognition located in the periventricular white matter, which supports our results that cognition may be more susceptible to periventricular white matter damage [35, 36]. This may explain why patients with similar visual WMH scores had different cognitive outcomes.
Analysis of each cognitive domain changes in BBB at different sites of the white matter further extends our acknowledgement that the key domain of cognitive function may rely on the preservation of BBB permeability in specific brain locations. The effects of WMH volume on CI, especially language and executive function, were weaker than the location effects of WMH [37]. Consistently, our study found that executive function and language were associated with PS in periventricular WMH. On the basis of this, altered BBB permeability in key regions may be a sensitive indicator of cognitive performance. This also implies that damage in key sites of the BBB may have a greater effect on cognition than increased diffuse permeability.
This study had some limitations. First, the overall sample size was small, which may introduce statistical bias; future studies require a larger sample size to validate and make the results more informative. Second, about the DCE technique: consensus recommendations on the acquisition protocol for BBB permeability in MRI were not proposed until recently [22]. Automatic segmentation and manual delineation of the ROIs were used to obtain PS values before the start of the experiment, which needs to be optimised. Automatic segmentation did not adequately represent our study ROIs; thus, we assessed the inter-rater agreement for manual ROI analysis. In future clinical use, automatic segmentation of specific ROIs would be more advantageous than manual segmentation in preventing unnecessary measurement variability. Third, the healthy population may show changes in BBB permeability at different locations. From this perspective, a control group should be considered in future DCE studies, and shortening the duration of DCE image acquisition may contribute to better cooperation and enrolment [38, 39]. Fourth, although cross-sectional studies limit causal inferences that can be drawn, our results encourage mechanistic inference and validation in longitudinal or basic studies to investigate the role of the BBB in the development of clinical symptoms in patients with WMH. Finally, measurements of biomarkers associated with BBB damage were not included in this study. In future studies, blood biomarkers associated with various components of the BBB and advanced imaging techniques that can detect neurometabolites, metabolic states or molecular profiles can be employed [40, 41] to improve our understanding of the pathophysiology of WMH and their relationship with vascular CI, and the related pathophysiological mechanisms, to discover targets for further interventional treatments.
Conclusion
There was a significant difference in BBB permeability between patients with WMH with and without significant CI when the Fazekas visual score and WMH volume did not indicate a significant difference, which may account for the cognitive heterogeneity exhibited by WMH. BBB permeability, especially periventricular permeability, was a more sensitive measure of overall cognitive changes than WMH volume in patients with WMH. Additionally, language and executive functions were significantly correlated with BBB permeability in periventricular WMH regions, suggesting that BBB damage in key sites may have a more significant impact on cognition than diffuse damage. Future studies that used more sophisticated methods to evaluate the brain structure changes are needed to investigate the key regions of BBB impairment in patients with CSVD and language impairment. Longitudinal studies are also required to observe the associations between changes in cognition and BBB permeability.
References
Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701.
Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–38.
van Leijsen EMC, van Uden IWM, Ghafoorian M, et al. Nonlinear temporal dynamics of cerebral small vessel disease: the RUN DMC study. Neurology. 2017;89:1569–77.
Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341: c3666.
Benedictus MR, van Harten AC, Leeuwis AE, et al. White matter hyperintensities relate to clinical progression in subjective cognitive decline. Stroke. 2015;46:2661–4.
Jokinen H, Kalska H, Ylikoski R, et al. Longitudinal cognitive decline in subcortical ischemic vascular disease—the LADIS study. Cerebrovasc Dis. 2009;27:384–91.
Lafosse JM, Reed BR, Mungas D, Sterling SB, Wahbeh H, Jagust WJ. Fluency and memory differences between ischemic vascular dementia and Alzheimer’s disease. Neuropsychology. 1997;11:514–22.
Herbert V, Brookes RL, Markus HS, Morris RG. Verbal fluency in cerebral small vessel disease and Alzheimer’s disease. J Int Neuropsychol Soc. 2014;20:413–21.
Vasquez BP, Zakzanis KK. The neuropsychological profile of vascular cognitive impairment not demented: a meta-analysis. J Neuropsychol. 2015;9:109–36.
Camerino I, Sierpowska J, Reid A, et al. White matter hyperintensities at critical crossroads for executive function and verbal abilities in small vessel disease. Hum Brain Mapp. 2021;42:993–1002.
Salvadori E, Brambilla M, Maestri G, et al. The clinical profile of cerebral small vessel disease: toward an evidence-based identification of cognitive markers. Alzheimers Dement. 2023;19:244–60.
Ter Telgte A, van Leijsen EMC, Wiegertjes K, Klijn CJM, Tuladhar AM, de Leeuw FE. Cerebral small vessel disease: from a focal to a global perspective. Nat Rev Neurol. 2018;14:387–98.
Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol. 2013;12:483–97.
Cheung RW, Cheung MC, Chan AS. Confrontation naming in Chinese patients with left, right or bilateral brain damage. J Int Neuropsychol Soc. 2004;10:46–53.
Brucki SM, Rocha MS. Category fluency test: effects of age, gender and education on total scores, clustering and switching in Brazilian Portuguese-speaking subjects. Braz J Med Biol Res. 2004;37:1771–7.
Li H, Jia J, Yang Z. Mini-mental state examination in elderly Chinese: a population-based normative study. J Alzheimers Dis. 2016;53:487–96.
von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4:e296.
Khaw J, Subramaniam P, Abd Aziz NA, Ali Raymond A, Wan Zaidi WA, Ghazali SE. Current update on the clinical utility of MMSE and MoCA for stroke patients in Asia: a systematic review. Int J Environ Res Public Health. 2021;18:8962.
Zhu Y, Zhao S, Fan Z, et al. Evaluation of the mini-mental state examination and the Montreal cognitive assessment for predicting post-stroke cognitive impairment during the acute phase in Chinese minor stroke patients. Front Aging Neurosci. 2020;12:236.
Shen YJ, Wang WA, Huang FD, et al. The use of MMSE and MoCA in patients with acute ischemic stroke in clinical. Int J Neurosci. 2016;126:442–7.
Skrobot OA, O’Brien J, Black S, et al. The vascular impairment of cognition classification consensus study. Alzheimers Dement. 2017;13:624–33.
Thrippleton MJ, Backes WH, Sourbron S, et al. Quantifying blood-brain barrier leakage in small vessel disease: review and consensus recommendations. Alzheimers Dement. 2019;15:840–58.
Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol. 1987;149:351–6.
Clancy U, Makin SDJ, McHutchison CA, et al. Impact of small vessel disease progression on long-term cognitive and functional changes after stroke. Neurology. 2022;98:e1459–69.
Patlak CS, Blasberg RG. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations. J Cereb Blood Flow Metab. 1985;5:584–90.
Heye AK, Thrippleton MJ, Armitage PA, et al. Tracer kinetic modelling for DCE-MRI quantification of subtle blood-brain barrier permeability. Neuroimage. 2016;125:446–55.
van Loenhoud AC, Groot C, Bocancea DI, et al. Association of education and intracranial volume with cognitive trajectories and mortality rates across the Alzheimer disease continuum. Neurology. 2022;98:e1679–91.
Fazekas F, Kleinert R, Offenbacher H, et al. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993;43:1683–9.
Raja R, Rosenberg GA, Caprihan A. MRI measurements of blood-brain barrier function in dementia: a review of recent studies. Neuropharmacology. 2018;134:259–71.
Wardlaw JM, Chappell FM, Valdes Hernandez MDC, et al. White matter hyperintensity reduction and outcomes after minor stroke. Neurology. 2017;89:1003–10.
Prins ND, Scheltens P. White matter hyperintensities, cognitive impairment and dementia: an update. Nat Rev Neurol. 2015;11:157–65.
Jung KH, Stephens KA, Yochim KM, et al. Heterogeneity of cerebral white matter lesions and clinical correlates in older adults. Stroke. 2021;52:620–30.
Hu HY, Ou YN, Shen XN, et al. White matter hyperintensities and risks of cognitive impairment and dementia: a systematic review and meta-analysis of 36 prospective studies. Neurosci Biobehav Rev. 2021;120:16–27.
Li Y, Li M, Zhang X, et al. Higher blood-brain barrier permeability is associated with higher white matter hyperintensities burden. J Neurol. 2017;264:1474–81.
O’Sullivan M. Imaging small vessel disease: lesion topography, networks, and cognitive deficits investigated with MRI. Stroke. 2010;41:S154–8.
Otsuka Y, Yamauchi H, Sawamoto N, Iseki K, Tomimoto H, Fukuyama H. Diffuse tract damage in the hemispheric deep white matter may correlate with global cognitive impairment and callosal atrophy in patients with extensive leukoaraiosis. AJNR Am J Neuroradiol. 2012;33:726–32.
Smith EE, Salat DH, Jeng J, et al. Correlations between MRI white matter lesion location and executive function and episodic memory. Neurology. 2011;76:1492–9.
Ha IH, Lim C, Kim Y, Moon Y, Han SH, Moon WJ. Regional differences in blood-brain barrier permeability in cognitively normal elderly subjects: a dynamic contrast-enhanced MRI-based study. Korean J Radiol. 2021;22:1152–62.
Farrall AJ, Wardlaw JM. Blood-brain barrier: ageing and microvascular disease–systematic review and meta-analysis. Neurobiol Aging. 2009;30:337–52.
Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV. Blood-brain barrier: from physiology to disease and back. Physiol Rev. 2019;99:21–78.
Gasparovic C, Prestopnik J, Thompson J, et al. 1H-MR spectroscopy metabolite levels correlate with executive function in vascular cognitive impairment. J Neurol Neurosurg Psychiatry. 2013;84:715–21.
Acknowledgements
We are grateful to all participants and researchers in this study for their support and contribution to this work.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Xing Wang, Yulu Shi, Tingting Wang, Zhengyang Li, Ying Gao and Yiyi Chen. The first draft of the manuscript was written by Xing Wang and all authors commented on all versions of the manuscript. All authors read and approved the final manuscript. Xing Wang and Yulu Shi were the co-first authors.
Funding
Sponsorship for this study and Rapid Service Fee were funded by the National Natural Science Foundation of China (No. 81825007). Sponsorship for this study was funded by the National Natural Science Foundation of China (No. 82001241), Beijing Outstanding Young Scientist Program (No. BJJWZYJH01201910025030), Capital's Funds for Health Improvement and Research (2022-2-2045), National Key R&D Program of China (2022YFF1501500, 2022YFF1501501, 2022YFF1501502, 2022YFF1501503, 2022YFF1501504, 2022YFF1501505), Youth Beijing Scholar Program (No. 010), Beijing Laboratory of Oral Health (PXM2021_014226_000041), Beijing Talent Project - Class A: Innovation and Development (No. 2018A12) , National Ten-Thousand Talent Plan - Leadership of Scientific and Technological Innovation and National Key R&D Program of China (No. 2017YFC1307900, 2017YFC1307905).
Data Availability
Data are available from the corresponding author on reasonable request.
Ethical Approval
The study was approved by the Institutional Review Board of Beijing Tiantan Hospital (KY 2019-140-01) and followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. All participants voluntarily agreed to participate in the study, consented to the cognitive function assessment and MRI, agreed to the BBB assessment protocol, and gave informed consent for the use of data in the study. The study was performed in accordance with the 1964 Declaration of Helsinki and its later amendments.
Conflict of interest
Xing Wang, Yulu Shi, Yiyi Chen, Ying Gao, Tingting Wang, Zhengyang Li and Yilong Wang declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Wang, X., Shi, Y., Chen, Y. et al. Blood–Brain Barrier Breakdown is a Sensitive Biomarker of Cognitive and Language Impairment in Patients with White Matter Hyperintensities. Neurol Ther 12, 1745–1758 (2023). https://doi.org/10.1007/s40120-023-00527-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-023-00527-z