Abstract
Introduction
Multiple sclerosis (MS) clinical trials have included low numbers of patients from racial and ethnic minority populations; therefore, it is uncertain whether differences exist in response to disease-modifying therapies. We evaluated the real-world safety and effectiveness of dimethyl fumarate (DMF) treatment over 5 years in four patient cohorts: Black, non-Black, Hispanic, and non-Hispanic people with relapsing–remitting MS.
Methods
ESTEEM is an ongoing, 5-year, multinational, prospective study evaluating the long-term safety and effectiveness of DMF in people with MS. The analysis included patients newly prescribed DMF in routine practice at 393 sites globally.
Results
Overall, 5251 patients were analyzed (220 Black, 5031 non-Black; 105 Hispanic, 5146 non-Hispanic). Median (min−max) months of follow-up was 32 (0–72) for Black, 29 (1–77) for Hispanic, and 41 (0–85) for both the non-Black and non-Hispanic populations. In total, 39 (18%) Black and 29 (28%) Hispanic patients reported adverse events leading to treatment discontinuation versus 1126 (22%) non-Black and 1136 (22%) non-Hispanic patients; gastrointestinal disorders were the most common in all subgroups. Median lymphocyte counts decreased by 37% in Black, 40% in non-Black, 10% in Hispanic, and 39% in non-Hispanic patients in the first year, then remained stable and above the lower limit of normal in most patients. Annualized relapse rates (ARRs) (95% confidence intervals) up to 5 years were 0.054 (0.038–0.078) for Black, 0.077 (0.072–0.081) for non-Black, 0.069 (0.043–0.112) for Hispanic, and 0.076 (0.072–0.081) for non-Hispanic populations, representing reductions of 91–92% compared with ARR 12 months before study entry (all p < 0.0001).
Conclusion
The safety profile of DMF in these subgroups was consistent with the overall ESTEEM population. Relapse rates remained low in Black and Hispanic patients, and consistent with non-Black and non-Hispanic patients. These data demonstrate a comparable real-world treatment benefit of DMF in Black and Hispanic patients.
Trial Registration
ClinicalTrials.gov identifier NCT02047097.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Black and Hispanic people with multiple sclerosis (PwMS) are reported to exhibit greater disease severity than non-Black and non-Hispanic PwMS. |
This analysis from ESTEEM, a 5-year multinational, prospective, observational study, evaluated the real-world safety and effectiveness of dimethyl fumarate (DMF) treatment over 5 years in Black, non-Black, Hispanic, and non-Hispanic people with relapsing–remitting multiple sclerosis. |
What was learned from the study? |
Relapse rates remained low in Black and Hispanic patients, consistent with non-Black and non-Hispanic patients. |
The safety profile of DMF in Black and Hispanic patients was consistent with that in the non-Black and non-Hispanic ESTEEM population. |
This study represents one of the largest Black and Hispanic cohorts to date and demonstrates a real-world long-term benefit of DMF for Black and Hispanic patients that is consistent with findings in the overall ESTEEM population. |
Introduction
It has been reported that clinical course and disability outcomes associated with multiple sclerosis (MS) may vary by race and ethnicity [1,2,3]. However, MS has not been well studied in populations such as racial and ethnic minorities because they are generally underrepresented in clinical trials [3, 4]. Black or African Americans comprise 13.4% of the US population, but as little as 5% participate in clinical trials. Similarly, although Hispanic Americans comprise 18.1% of the US population, only 1% are clinical trial participants [4]. Thus, data describing the effectiveness of disease-modifying therapies in these patients in real-world settings are limited [5, 6]. Moreover, social determinants of health—the conditions into which people are born, live, work, and age—could affect MS outcomes. The magnitude of racial and ethnic influences of social determinants of health are likely to differ across countries and regions [7].
Dimethyl fumarate (DMF; also known as gastro-resistant DMF) is approved for the treatment of relapsing forms of MS. DMF has demonstrated efficacy, effectiveness, and a stable benefit–risk profile in both clinical and real-world studies [6, 8,9,10,11,12]. As of 31 December 2022, ~ 587,200 patients had been treated with DMF, representing > 1,333,500 patient-years of exposure. Of these, 6450 patients (14,508 patient-years) were from clinical trials. DMF was efficacious in small groups of Black and Hispanic patients in the phase 3 DEFINE/CONFIRM trials [10] as well as a retrospective chart review [13]. A previous analysis of these subgroups in ESTEEM demonstrated effectiveness during 3 years of DMF treatment [5, 6]; however, data describing the long-term effects of DMF in these subgroups in real-world settings are limited.
ESTEEM (ClinicalTrials.gov identifier NCT02047097) is an ongoing, phase 4, 5-year, multinational, prospective, observational study characterizing the long-term safety and effectiveness of DMF in real-world clinical practice. In this analysis, we evaluated the real-world safety and effectiveness of DMF treatment over 5 years in Black, non-Black, Hispanic, and non-Hispanic people with relapsing–remitting MS.
Methods
Study Design and Patients
This interim analysis of ESTEEM (NCT02047097; data cutoff August 2021) included patients newly prescribed DMF in routine clinical practice at 393 sites globally [5, 6]. Patients enrolling in ESTEEM were required to have a diagnosis of a relapsing form of MS and be newly prescribed DMF under routine clinical care. Patients aged < 18 years were excluded from ESTEEM. In total, > 5000 patients have been enrolled in ESTEEM and will be monitored for 5 years via routine clinical visits. The study case report forms utilized the term “Black or African American” to specify race, and the term “Hispanic or Latino” to specify ethnicity; for ease of reference, we will utilize the terms Black and Hispanic throughout the paper. We acknowledge that this is an oversimplification and that there may have been non-Black patients of African descent who identified as Black or African American. We also acknowledge that there are differences between Hispanic and Latino. We have simplified the terminology here for the sake of readability and clarity.
The primary objective of ESTEEM was to determine the incidence, type, and pattern of serious adverse events (SAEs), and also adverse events (AEs) leading to treatment discontinuation. A secondary objective was to evaluate the effectiveness of DMF, determined through the assessment of the annualized relapse rate (ARR), the proportion of patients who relapsed, and the distribution of the number of relapses. The incidence of treatment discontinuation for any reason was also assessed. In this observational study, patients experienced routine clinical care as per the site standard of care procedures used when initiating a new DMT. In line with the US prescribing information for DMF, serum aminotransferase, alkaline phosphatase, and total bilirubin levels are suggested to be obtained prior to initiating DMF; however, hepatic evaluations were not required for participation in the study.
In this analysis, the safety and effectiveness of DMF were evaluated in a post hoc subgroup analysis in Black, non-Black, Hispanic, and non-Hispanic patients. Race and ethnicity were self-reported: those who selected “Black or African American” comprised the Black population, and any who did not were defined as non-Black; those who selected “Hispanic or Latino” were defined as the Hispanic population and those who did not comprised the non-Hispanic population. Of the population who identified as Hispanic (n = 105), five (4.8%) also identified as Black.
The study was conducted in accordance with relevant US federal regulations, the Declaration of Helsinki, and the International Council for Harmonisation Guideline for Good Clinical Practice. The study was approved by local ethics committees at each of the 393 study sites, which were overseen by the ESTEEM Study Contract Research Organization ethics committee. It was the responsibility of participating physicians to ensure that all aspects of institutional review were conducted in accordance with current governmental regulations. All ethics committee approvals for study protocol and amendments were documented, and progress reports had to be submitted to the local ethics committee at required intervals and not less than annually. Written assent and consent forms were obtained from each patient or his or her parent or legal guardian. ESTEEM was registered at ClinicalTrials.gov (NCT02047097).
Statistical Analysis
Descriptive statistics for categorical variables were presented as proportions and presented as means or medians for continuous variables, where specified. The proportion of patients who did not experience a relapse was estimated using the Kaplan–Meier method. ARR was defined as the total number of relapses experienced per patient divided by the number of years followed in the study. Mean differences in ARRs in the 1 year prior to DMF initiation and through years 1, 2, 3, 4, and 5 following DMF initiation were calculated via the generalized estimating equation Poisson model, adjusting for the correlation of patient-specific repeated measures and follow-up time. Median absolute lymphocyte count (ALC) was calculated and presented with distribution-free 95% confidence intervals (CIs). The significance level for all results was set to 0.05.
Results
Study Population
Overall, 5251 patients received at least one dose of DMF and were included in the analysis, with follow-up over 60 months. Of those, 220 (4%) were Black, 5031 (96%) were non-Black, 105 (2%) were Hispanic, and 5146 (98%) were non-Hispanic. Mean (SD) age at enrollment was 42.5 (11.1) years in the Black population and 40.1 (11.6) years in the Hispanic population.
Baseline characteristics (Table 1) were generally similar across populations, although the majority of Black (97%) and Hispanic (98%) patients were from the USA (which includes Puerto Rico). Black patients were older than other patient groups, with 60% (131/220) ≥ 40 years old, compared with 49% of non-Black, 47% of Hispanic, and 50% of non-Hispanic patients. The median (min−max) duration of DMF treatment in ESTEEM was 32 (0–72) months in the Black population, 29 (1–77) months in the Hispanic population, and 41 (0–85) months in both the non-Black and non-Hispanic populations.
Safety and Discontinuations
AEs leading to treatment discontinuation occurred in 39 (18%) Black patients and 29 (28%) Hispanic patients (Table 2) compared with 1126 (22%) non-Black patients and 1136 (22%) non-Hispanic patients. Gastrointestinal (GI) disorders were the most common AEs leading to discontinuation in all subgroups: 17 (8%) Black patients; 429 (9%) non-Black patients; 11 (10%) Hispanic patients; and 435 (8%) non-Hispanic patients. SAEs were reported in 348/5146 (6.8%) non-Hispanic patients, 8/105 (7.6%) Hispanic patients, 344/5031 (6.8%) non-Black patients, and 12/220 (5.5%) Black patients.
Available ALCs were assessed during the study (longitudinal data are limited by small patient numbers with these data in this observational study). In the first year, estimated median lymphocyte counts declined by 37% (n = 29) in Black patients, 40% (n = 1811) in non-Black patients, 10% (n = 17) in Hispanic patients, and 39% (n = 1823) in non-Hispanic patients. ALCs then stabilized, remaining above the lower limit of normal (LLN; 0.91 × 109/L) for most patients (Fig. 1). For all subgroup cohorts, the 60-month data were not depicted due to low patient numbers at these later time points.
Median absolute lymphocyte counts over time. n represents number of patients with absolute lymphocyte count assessment at the specified time point. For both subgroup cohorts, the 60-month data aligned with the 48-month data, but are not depicted due to low patient numbers at these later time points. Distribution-free 95% confidence limits are presented. LLN lower limit of normal
ARR and Relapses
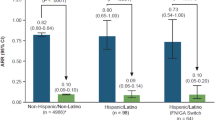
ARRs up to 5 years were 0.054 (95% CI 0.038–0.078) for Black patients, 0.077 (95% CI 0.072–0.081) for non-Black patients, 0.069 (95% CI 0.043–0.112) for Hispanic patients, and 0.076 (95% CI 0.072–0.081) for non-Hispanic patients, representing reductions ranging from 91 to 92% compared with ARR 12 months prior to study entry (p < 0.0001 for all subgroups; Fig. 2). Kaplan–Meier-estimated proportion of patients relapse-free at 54 months was 70.6% in non-Black and non-Hispanic patients, 74.7% in Black patients, and 77.3% in Hispanic patients (Table 3, Fig. 3). Kaplan–Meier estimates are reported out to month 54 instead of month 60 because some of the subgroups had fewer than five patients at risk at the month 60 time point.
ARR in the 12 months prior to and up to 5 years after DMF initiation. Each group has the same N for Fig. 2 across time points because the analysis includes all patients with data through 5 years and compares their 5-year ARR to the prior 1-year ARR. ARR annualized relapse rate, CI confidence interval, DMF dimethyl fumarate
Kaplan–Meier-estimated proportion of patients without MS relapse at 54 months after DMF initiation. DMF dimethyl fumarate, MS multiple sclerosis. Kaplan–Meier estimates are reported out to month 54 instead of month 60 because some of the subgroups had fewer than 5 patients at risk at the month 60 time point
Discussion
We report an updated analysis of the safety and effectiveness of DMF in Black versus non-Black patients and Hispanic versus non-Hispanic patients, including those who have been treated with DMF in a real-world clinical setting for up to 5 years. The safety profile of DMF in these subgroups was consistent with the overall ESTEEM population. Lymphocyte dynamics were generally consistent across the patient subgroups, although the validity of the ALC data was limited by small patient numbers. GI disorders were the most common AE leading to treatment discontinuation in all subgroups. Compared with the 12 months before DMF initiation, ARR was significantly lower up to 5 years after DMF initiation across all of the subgroups, including the analysis in Black and Hispanic patients. These data are consistent with the previous interim analysis of ESTEEM and demonstrate the real-world treatment benefit of DMF in Black and Hispanic patients.
Future studies should aim to increase the numbers and relative proportions of racial and ethnic minorities in clinical trials. Although Black and Hispanic patients represent 4% and 2% of the total ESTEEM population, and 13.4% and 18.1% of the US population, respectively, they represent the largest Black and Hispanic cohorts to date in a prospective MS therapeutic study [14]. This is indicative of the small numbers of people with MS (pwMS) from Black and Hispanic populations enrolled in clinical studies. There is a need for clinicians to encourage clinical trial participation in these populations, to help improve our understanding of potential ethnic or racial variations in MS pathophysiology, as well as treatment safety and efficacy in minority racial populations.
A limitation of this analysis is that most of the patients in both the Black and Hispanic groups were from the USA and therefore may not be representative of a global MS population. Although these are the largest cohorts of Black and Hispanic patients in a study of this kind to date, the numbers of patients in these groups are still small (220 in the Black population and 105 in the Hispanic population vs. 5251 in the overall population). Also, the attrition rate in prospective, observational studies such as this one limits conclusions on efficacy. Another potential limitation was convenience sampling, which could introduce bias. Also, median lymphocyte counts were reported, which has not been the typical metric for lymphocyte dynamics in other studies of DMF safety and effectiveness but which has been the approach for ESTEEM given the variability of reporting in this observational study. A further consideration is that factors such as social determinants of health (socioeconomic status and access to care) in different groups may have a significant impact on MS disease outcomes [7]. Interestingly, enrolled Black patients were, on average, older than in other patient subgroups, which may suggest that Black pwMS are diagnosed later or are being managed differently. It is possible that response to therapies may vary due to clinically important differences in the course of MS in various ethnic and racial populations [3, 5, 6, 13, 15,16,17]. Previous literature has suggested that pwMS of Black or African American descent have more frequent relapses, worse post-relapse recovery, faster progression of secondary-progressive MS, and more rapid disability progression than non-Black or African American populations [1, 3, 13, 18, 19]. Studies have shown that Hispanic American pwMS tend to be younger at onset and are more likely to experience optic neuritis and transverse myelitis, but otherwise have similar disease progression as White American populations [2, 20,21,22]. Previous studies examining disease-modifying therapy response in Black and Hispanic pwMS are limited by small sample size [13, 16, 17]. One study in the literature reports a poorer response to interferon beta therapy in Black African Americans than in a White American population [16]. This less optimal response in Black African Americans may be due to the more severe course of disease activity and/or reduced efficacy response to interferon beta therapy in Black African American pwMS. Of note, in this study, responses to DMF in both Black and Hispanic cohorts were consistent with the overall population.
Conclusions
This study represents one of the largest Black and Hispanic MS cohorts to date and reports outcomes over the longest follow-up compared with previous studies. It confirms the real-world long-term benefit of DMF in both Black and Hispanic populations, with safety and effectiveness findings that are consistent with the overall ESTEEM population.
References
Kister I, Chamot E, Bacon JH, Niewczyk PM, De Guzman RA, Apatoff B, et al. Rapid disease course in African Americans with multiple sclerosis. Neurology. 2010;75(3):217–23. https://doi.org/10.1212/WNL.0b013e3181e8e72a.
Langer-Gould A, Brara SM, Beaber BE, Zhang JL. Incidence of multiple sclerosis in multiple racial and ethnic groups. Neurology. 2013;80(19):1734–9. https://doi.org/10.1212/WNL.0b013e3182918cc2.
Khan O, Williams MJ, Amezcua L, Javed A, Larsen KE, Smrtka JM. Multiple sclerosis in US minority populations: clinical practice insights. Neurol Clin Pract. 2015;5(2):132–42. https://doi.org/10.1212/CPJ.0000000000000112.
Clinical Research Pathways. Diversity Statistics (infographic). https://clinicalresearchpathways.org/diversity/diversity-statistics-infographic. Accessed 14 July 2022.
Williams MJ, Amezcua L, Okai A, Okuda DT, Cohan S, Su R, et al. Real-world safety and effectiveness of dimethyl fumarate in Black or African American patients with multiple sclerosis: 3-year results from ESTEEM. Neurol Ther. 2020;9(2):483–93. https://doi.org/10.1007/s40120-020-00193-5.
Chinea A, Amezcua L, Vargas W, Okai A, Williams MJ, Su R, et al. Real-world safety and effectiveness of dimethyl fumarate in Hispanic or Latino patients with multiple sclerosis: 3-year results from ESTEEM. Neurol Ther. 2020;9(2):495–504. https://doi.org/10.1007/s40120-020-00192-6.
Dobson R, Rice DR, D’Hooghe M, Horne R, Learmonth Y, Mateen FJ, et al. Social determinants of health in multiple sclerosis. Nat Rev Neurol. 2022;18(12):723–34. https://doi.org/10.1038/s41582-022-00735-5.
Fox RJ, Miller DH, Phillips JT, Hutchinson M, Havrdova E, Kita M, CONFIRM Study Investigators, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med. 2012;367(12):1087–97. https://doi.org/10.1056/NEJMoa1206328.
Gold R, Kappos L, Arnold DL, Bar-Or A, Giovannoni G, Selmaj K, DEFINE Study Investigators, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med. 2012;367(12):1098–107. https://doi.org/10.1056/NEJMoa1114287.
Fox RJ, Gold R, Phillips JT, Okwuokenye M, Zhang A, Marantz JL. Efficacy and tolerability of delayed-release dimethyl fumarate in Black, Hispanic, and Asian patients with relapsing-remitting multiple sclerosis: post hoc integrated analysis of DEFINE and CONFIRM. Neurol Ther. 2017;6(2):175–87. https://doi.org/10.1007/s40120-017-0077-5.
Gold R, Arnold DL, Bar-Or A, Fox RJ, Kappos L, Chen C, et al. Safety and efficacy of delayed-release dimethyl fumarate in patients with relapsing-remitting multiple sclerosis: 9 years’ follow-up of DEFINE, CONFIRM, and ENDORSE. Ther Adv Neurol Disord. 2020;13:1756286420915005. https://doi.org/10.1177/1756286420915005.
Gold R, Arnold DL, Bar-Or A, Fox RJ, Kappos L, Mokliatchouk O, et al. Long-term safety and efficacy of dimethyl fumarate for up to 13 years in patients with relapsing-remitting multiple sclerosis: final ENDORSE study results. Mult Scler. 2022;28(5):801–16. https://doi.org/10.1177/13524585211037909.
Zhovtis Ryerson L, Green R, Confident G, Pandey K, Richter B, Bacon T, et al. Efficacy and tolerability of dimethyl fumarate in White-, African- and Hispanic- Americans with multiple sclerosis. Ther Adv Neurol Disord. 2016;9(6):454–61. https://doi.org/10.1177/1756285616661929.
Onuorah HM, Charron O, Meltzer E, Montague A, Crispino A, Largent A, et al. Enrollment of non-white participants and reporting of race and ethnicity in phase III trials of multiple sclerosis DMTs: a systematic review. Neurology. 2022;98(9):e880–92. https://doi.org/10.1212/WNL.0000000000013230.
Chinea Martinez AR, Correale J, Coyle PK, Meng X, Tenenbaum N. Efficacy and safety of fingolimod in Hispanic patients with multiple sclerosis: pooled clinical trial analyses. Adv Ther. 2014;31(10):1072–81. https://doi.org/10.1007/s12325-014-0154-4.
Cree BA, Al-Sabbagh A, Bennett R, Goodin D. Response to interferon beta-1a treatment in African American multiple sclerosis patients. Arch Neurol. 2005;62(11):1681–3. https://doi.org/10.1001/archneur.62.11.1681.
Klineova S, Nicholas J, Walker A. Response to disease modifying therapies in African Americans with multiple sclerosis. Ethn Dis. 2012;22(2):221–5.
Cree BA, Khan O, Bourdette D, Goodin DS, Cohen JA, Marrie RA, et al. Clinical characteristics of African Americans vs Caucasian Americans with multiple sclerosis. Neurology. 2004;63(11):2039–45. https://doi.org/10.1212/01.wnl.0000145762.60562.5d.
Naismith RT, Trinkaus K, Cross AH. Phenotype and prognosis in African-Americans with multiple sclerosis: a retrospective chart review. Mult Scler. 2006;12(6):775–81. https://doi.org/10.1177/1352458506070923.
Hadjixenofontos A, Beecham AH, Manrique CP, Pericak-Vance MA, Tornes L, Ortega M, et al. Clinical expression of multiple sclerosis in Hispanic Whites of primarily Caribbean ancestry. Neuroepidemiology. 2015;44(4):262–8. https://doi.org/10.1159/000431375.
Amezcua L, Lund BT, Weiner LP, Islam T. Multiple sclerosis in Hispanics: a study of clinical disease expression. Mult Scler. 2011;17(8):1010–6. https://doi.org/10.1177/1352458511403025.
Buchanan RJ, Zuniga MA, Carrillo-Zuniga G, Chakravorty BJ, Tyry T, Moreau RL, et al. Comparisons of Latinos, African Americans, and Caucasians with multiple sclerosis. Ethn Dis. 2010;20(4):451–7.
Acknowledgements
We thank the participants of the study.
Funding
This study was sponsored by Biogen (Cambridge, MA, USA). Biogen also provided funding for the journal’s Rapid Service Fee.
Medical Writing and/or Editorial Assistance
Biogen provided funding for medical writing and editorial support in the development of this manuscript; Annabel Campbell, PhD (Excel Medical Affairs), wrote the first draft of the manuscript based on input from authors, and Miranda Dixon (Excel Medical Affairs) copyedited and styled the manuscript per journal requirements.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Mitzi Williams: Concept and design of study, data collection, data interpretation, drafting and critically revising the manuscript. Lilyana Amezcua: Concept and design of study, data collection, data interpretation, drafting and critically revising the manuscript. Angel Chinea: Concept and design of study, data collection, data interpretation, drafting and critically revising the manuscript. Stanley Cohan: Concept and design of study, data collection, data interpretation, drafting and critically revising the manuscript. Annette Okai: Concept and design of study, data collection, data interpretation, drafting and critically revising the manuscript. Darin T. Okuda: Concept and design of study, data collection, data interpretation, drafting and critically revising the manuscript. Wendy Vargas: Concept and design of study, data collection, data interpretation, drafting and critically revising the manuscript. Nick Belviso: Concept and design of study, data collection, statistical analyses, data interpretation, drafting and critically revising the manuscript. Ivan Božin: Concept and design of study, data interpretation, drafting and critically revising the manuscript. Xiaotong Jiang: Concept and design of study, data interpretation, drafting and critically revising the manuscript. James B. Lewin: Concept and design of study, data interpretation, drafting and critically revising the manuscript. Jennifer Lyons: Concept and design of study, data interpretation, drafting and critically revising the manuscript. Changyu Shen: Concept and design of study, data interpretation, drafting and critically revising the manuscript. Sarah M. England: Concept and design of study, data interpretation, drafting and critically revising the manuscript. Nydjie Grimes: Concept and design of study, data collection, data interpretation, drafting and critically revising the manuscript.
Prior Presentation
These data have previously been presented in abstract and poster form at the Consortium of Multiple Sclerosis Centers 2022 Annual Meeting (June 1–4, 2022); National Harbor, MD.
Disclosures
Mitzi J. Williams received advisory/consulting fees from Biogen, Celgene, EMD Serono, Genentech, Sanofi Genzyme, and Teva Neuroscience; speaker fees from Biogen, Genentech, Sanofi Genzyme, and Teva. Lilyana Amezcua received advisory/consulting fees from EMD Serono, Genzyme, and Novartis; research support from Biogen and MedDay. Angel Chinea received advisory/consulting fees from Biogen, Genentech, Novartis, Sanofi Genzyme, and Teva Neuroscience; research support from Biogen, Novartis, and Sanofi Genzyme; speaker fees from Biogen, Genentech, Novartis, Sanofi Genzyme, and Teva. Stanley Cohan advisory/consulting fees from Biogen, Celgene, Novartis, Pear Therapeutics, Roche Genentech, Sage Therapeutics, and Sanofi Genzyme; research support from AbbVie, Adamas, Alithios, Biogen, EMD Serono, MedDay, Novartis, Roche Genentech, and Sanofi Genzyme; speaker fees from Biogen, Roche Genentech, and Sanofi Genzyme. Annette Okai received advisory/consulting fees from Biogen, Celgene, EMD Serono, Genentech, Novartis, and Sanofi Genzyme; research support from Biogen, Novartis, Sanofi Genzyme, and TG Therapeutics; speaker fees from Biogen, Genentech, Novartis, Sanofi Genzyme, and Teva. Darin T. Okuda received personal compensation for consulting and advisory services from Alexion, Biogen, Celgene/Bristol Myers Squibb, EMD Serono, Genentech, Genzyme, Janssen, Novartis, Osmotica, RVL, TG Therapeutics, and Viela Bio; research support from Biogen and EMD Serono/Merck; has issued national and international patents along with pending patents related to other developed technologies; received royalties for intellectual property licensed by The Board of Regents of The University of Texas System. Wendy Vargas received advisory/consulting fees from Alexion, Biogen, Genentech, and Octapharma; research support from Teva. Nick Belviso, Ivan Božin, Xiaotong Jiang, James B. Lewin, Jennifer Lyons, Changyu Shen and Sarah M. England are employees of and hold stock/stock options in Biogen. Nydjie Grimes was an employee of Biogen at the time of analysis.
Compliance with Ethics Guidelines
The study was conducted in accordance with relevant US federal regulations, the Declaration of Helsinki, and the International Council on Harmonisation Guideline for Good Clinical Practice. Approvals were granted by relevant institutional ethics committees for study protocol and amendments, and written assent and consent forms were obtained from each patient and his or her parent or legal guardian. ESTEEM was registered at ClinicalTrials.gov (NCT02047097).
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Williams, M.J., Amezcua, L., Chinea, A. et al. Real-World Safety and Effectiveness After 5 Years of Dimethyl Fumarate Treatment in Black and Hispanic Patients with Multiple Sclerosis in ESTEEM. Neurol Ther 12, 1669–1682 (2023). https://doi.org/10.1007/s40120-023-00517-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-023-00517-1