Abstract
Cladribine tablets (CladT) is a highly active oral disease-modifying therapy (DMT) for the management of relapsing multiple sclerosis (RMS). CladT acts as an immune reconstitution therapy, in that two short courses of treatment 1 year apart have been shown to suppress disease activity for a prolonged period in most patients, without need for continued DMT. Each course of CladT induces a profound reduction in B lymphocytes that recovers over months, and serious lymphopenia (Grade 3–4) is uncommon. Smaller reductions in levels of T lymphocytes occur slightly later: on average, these remain within the normal range and repopulate progressively. A larger effect occurs on CD8 vs. CD4 cells. Reactivation of latent or opportunistic infections (e.g. varicella zoster, tuberculosis) is mostly associated with very low lymphocyte counts (< 200/mm3). Screening and managing pre-existing infections, vaccinating non-exposed patients and delaying the 2nd year of treatment with CladT to allow lymphocytes to recover to > 800/mm3 (if necessary) are important for avoiding infections and higher-grade lymphopenia. There was no demonstrable or apparent effect of CladT on the efficacy of vaccinations, including against Covid-19. Adverse events consistent with drug-induced liver injury (DILI) represent a rare but potentially serious complication of CladT therapy in spontaneous adverse event reporting; patients should be screened for liver dysfunction before starting treatment. Ongoing hepatic monitoring is not required, but CladT must be withdrawn if signs and symptoms of DILI develop. There was a numerical imbalance for malignancies when comparing cladribine to placebo in the clinical programme, particularly in short-term data, but recent evidence shows that the risk of malignancy with CladT is similar to the background rate in the general population and to that with other DMTs. Overall, CladT is well tolerated with a favorable safety profile appropriate for the management of RMS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Cladribine tablet (CladT) is a high-efficacy disease-modifying therapy (DMT) for relapsing multiple sclerosis (RMS) that acts as an immune reconstitution therapy |
Treatment with CladT is associated with a low risk of severe lymphopenia and of opportunistic infections when recommended prophylaxis for common infections is given before treatment |
CladT does not appear to impair the efficacy of vaccinations, including for Covid-19 |
Rare cases of serious liver dysfunction have occurred during treatment with CladT and labelling now contains monitoring and management recommendations on this |
CladT does not appear to increase the risk of malignancy |
Overall, CladT is well tolerated with a safety profile appropriate for the management of RMS |
Immune Reconstitution Therapy in the Care of Relapsing MS
Most highly active disease-modifying therapies (DMTs) used in the management of relapsing multiple sclerosis (RMS) are administered regularly according to a fixed dosing schedule and act via continuous suppression of the immune system [1, 2]. This approach has led to concerns regarding the potential for increased risk of serious infections and/or malignancy in patients with compromised immune systems [3]. Pharmacological immune reconstitution therapy (IRT; currently exemplified by cladribine tablets [CladT] and alemtuzumab) takes a different approach [1, 3, 4]. Two short courses of IRT given 1 year apart induce a temporary reduction in the number of circulating immune cells (B and T cells) that reverses over a period of months, with no requirement for further continuous treatments in the subsequent 2 years in patients who respond to the treatment.
Clinical evidence, summarised briefly below, confirms that IRT is effective in reducing MS disease activity. In the 2-year randomised phase of the CLARITY trial, which recruited a population with at least one MS relapse during the previous year, 79.7% of patients who received the recommended total dose of 3.5 mg/kg of CladT remained relapse-free vs. 60.9% for placebo (odds ratio [OR] 2.5 in favour of CladT, p < 0.001) [5]. An extension to this trial showed that similar proportions of patients who received the initial two courses of CladT followed by 2 years of placebo, or those who received four annual courses of treatment, remained relapse-free over 4 years (75.6% vs. 81.2%) [6]. Further follow-up of the trial extension population showed that almost half (up to 44%) of patients achieved the outcome of “no evidence of disease activity” (NEDA-3) for up to 6 years following the initiation of treatment [7]. Durable efficacy (reduced relapse rates and MS disease activity) was also seen with alemtuzumab, following its pivotal CARE-MS-I and CARE-MS-II randomised trials [8, 9]. Similarly, about one-third (30%) of patients treated with alemtuzumab reported a similar benefit during 4 years of follow-up [10]. Long-term treatment with both of these IRTs was associated with reduced progression of disability [11, 12]. Real-world evidence has shown that treatment with IRT can lead to years of life free of disease activity in a substantial proportion of patients in the absence of continuous treatment with a DMT [13,14,15,16,17,18,19].
Durable efficacy that far outlasts the period of suppression of levels of immune cells, as described above, is therefore the hallmark of IRT. However, the benefits of any treatment must be determined by its overall balance of efficacy and safety. The use of alemtuzumab is currently restricted in France (the location of the authors of this article) because of safety concerns [20,21,22]. Accordingly, this article focuses mainly on current data on the safety of CladT in the management of RMS.
Posology of Cladribine Tablets
CladT acts in a manner consistent with an IRT, as described above. An understanding of the mode of administration of this treatment is essential for consideration of its efficacy and safety, and this is described briefly here. Treatment is given as two annual courses of treatment 1 year apart (Fig. 1) [23]. A treatment course consists of two periods of treatment, given during the 1st week of the first and 2nd month of each year. In each treatment period, one or two 10 mg tablets of CladT daily for 4 or 5 consecutive days, depending on body weight, are administered (i.e. a cumulative dose of 1.75 mg/kg per annual treatment course, resulting in an overall cumulative dose of 3.5 mg/kg over the full 2 years). There is no requirement for further treatment in years 3 and 4 in the absence of disease reactivation.
Posology of cladribine tablets for the management of multiple sclerosis. Cladribine tablets are given as two treatment courses 1 year apart. Red and blue arrows show treatment days at the beginning of month 1 and month 2 of each treatment course (5 treatment days are shown for each course, but in practice this could be 4 or 5 days, depending on body weight). Compiled from information presented in Ref. [23]
Overview of Adverse Events
The incidence of all-cause adverse events (AE) in the randomised Phase 3 CLARITY trial was 80.7% for CladT 3.5 mg/kg and 73.3% for placebo [5]. The most common all-cause AEs in the CladT/placebo groups were headache (24.2%/17.2%), lymphopenia (21.6%/1.8%), nasopharyngitis (14.4%/12.9%), upper respiratory tract infection (12.6%/9.7%) and nausea (10.0%/9.0%). All-cause serious AEs occurred in 8.4% of the CladT group and 6.4% of the placebo group. AE led to discontinuation of therapy in 3.5% of the CladT group and 2.1% of the placebo group in the randomised phase of CLARITY.
The most comprehensive overview of serious adverse events (SAE) with CladT is available from an integrated review of the CLARITY trial, its extension, the ORACLE-MS trial and the PREMIERE registry [24]. This analysis included 923 patients exposed to CladT and 641 patients exposed to placebo for up to 8 years (the CladT group was larger because some patients were switched from placebo to CladT in the CLARITY Extension study and are thus counted in the CladT group). Figure 2 shows the incidence of SAE overall and SAEs of particular interest (described in more detail below) for the CladT and placebo groups. Table 1 shows data on AEs of special interest from an integrated analysis of clinical trials and the PREMIERE registry [25] compared with real-world incidences of these AE from a post-approval cohort [25]. The incidences of these AE were generally lower in the post-approval cohort, and no adverse safety signal for CladT appeared over time. A separate analysis of AEs occurring during the first 12 weeks of administration of CladT found a higher incidence of AE for CladT vs. placebo (61% vs. 55%, respectively) as well as a higher incidence of AE considered to be drug-related (35% vs. 23%, respectively) but similar proportions of AE leading to discontinuation (1.6% vs. 1.4%, respectively) [26].
Incidence of serious adverse events and SAE of interest in an integrated analysis of randomised evaluations of cladribine tablets for the management of multiple sclerosis. Drawn from data presented in Ref. [24]
A recent (2022) network meta-analysis [27] found generally similar rates of AE and SAE between CladT 3.5 mg/kg and other high-efficacy DMTs, except for a higher rate of AE for CladT vs. ozanimod (odds ratio [OR] 1.8 [95% CI 1.1–2,8] or placebo (OR 1.5 [1.1–2.1]); SAEs were more frequent for CladT vs. ocrelizumab (OR 2.9 [1.0–4.7]), with no other significant differences between CladT and other DMTs. There were no differences between CladT and other high-efficacy DMTs for AE leading to treatment discontinuation in this analysis. An earlier network meta-analysis (2018) did not find differences in tolerability or safety between CladT and other high-efficacy DMTs [28].
Most (84.1%) of 270 patients reported at least one treatment-emergent AE and 5.4% reported at least one SAE in the 2-year MAGNIFY-MS Phase 4 trial [29]. Real-world data from Europe suggested that the safety of CladT was similar for patients above and below 50 years of age (suggesting that immunosenescence was not an important factor here); female gender and disability were the main predictors of AEs on CladT treatment [30]. Additional data from 6 months of CladT treatment from the CLARIFY-MS Phase IV study cohort showed that 18.7% and 9.1% reported mild and moderate AE respectively and 0.6% severe AE: the incidence of these AEs did not vary clearly or consistently according to receipt or not of prior DMT therapy [31]. The mean score for side effects from the Treatment Satisfaction Questionnaire for Medication was high (91.9 [SD 17.68]/100, where 100 = the highest level of satisfaction).
Adverse Events of Special Interest
Effects on Immune Cells
Immune Cell Counts
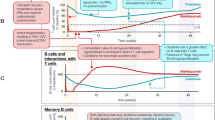
The randomised CLARITY study and its extension conducted in 685 patients with RMS receiving the recommended cumulative total dose of CladT 3.5 mg/kg over two annual courses are the largest individual studies evaluating the effects of CladT on levels of immune cells [32]. Figure 3 shows the time course of key subsets of lymphocytes during the randomised and extension phases of the study. Median absolute lymphocyte count (ALC) fell rapidly after the first course of CladT, reaching a nadir of 1 × 109 mm−3 at 2 months. Median ALC recovered steadily (to a level of 1.2 × 109/l) until the time of the second course of treatment in year 2, after which a new nadir of 0.8 × 109/l occurred again about 2 months later. The median ALC returned within the normal range (> 1 × 109/l) at 36 weeks after the second treatment course. The reduction in B lymphocytes (CD19 +) was rapid and profound, with median levels of these cells reduced from 0.2 × 109/l to 0.02 × 109/l for the year 1 course and 0.02 × 109/l following the year 2 course. Lymphopenia recovery was again rapid, with recovery to a nominal normal value occurring at about 30 weeks following each treatment course. Effects on T helper lymphocytes (CD4 +) or cytotoxic T cells (CD8 +) were less marked (median T cell count was never reduced below the normal range), with slower reduction and recovery phases (nadirs in cell counts occurred at 3–4 months following each treatment course, with a slow recovery thereafter). The lowest nadirs in year 2 of treatment indicated reductions in cell counts of about 55% for CD4 + and about 40% for CD8 + , based on changes in median counts.
It should be noted when considering effects of CladT on lymphocyte counts in the CLARITY study that the year 2 course of CladT was administered irrespective of ALC, and most Grade 3 or 4 lymphopenia occurred in the 2nd year of treatment [5, 32]. Drug labelling was amended subsequently to require ALC to be > 800/mm3 before administration of the 2nd year treatment course (with the possibility of delaying treatment up to 6 months to allow lymphocytes to recover) to limit the risk of potential severe lymphopenia during year 2 (see below). The incidence of severe or serious lymphopenia was much lower in an observational post-approval cohort compared with the incidence from an integrated clinical trial analysis (Table 1), which is consistent with this change in practice [25].
Another analysis evaluated the effects of the first annual course of CLadT (i.e. a cumulative dose of 1.75 mg/kg) including study populations of CLARITY and its extension and also subjects from the randomised ORACLE-MS trial in patients with a clinically isolated syndrome, as defined at the time [33]. Although this study does not tell us about the recommended clinical dosage of CladT (the CLARITY study showed us that full efficacy was not achieved until after the year 2 dose [5]), it is of interest as it measured effects on subsets of immune cells. Effects on ALC, CD19 +, CD4 + and CD8 + cells were similar to those described above. There was a modest effect of CLadT on natural killer T cells (CD16 +/56 +; average 30–44%% reduction from baseline across the studies). An extended analysis of effects on immune cell subtypes from the ORACLE-MS population revealed reductions in naïve (CD4 +/CD45RA +) and memory (CD4 +/CD45RO +) T helper cells, in naïve (CD8 + /CD45RA +) and memory (CD8 +/CD45RO +) cytotoxic T cells, and central (CD4 + RO + CCR7 +) and effector (CD4 +/RO +/CCR7–) memory T cells of 30–65%. Changes in Th1-type helper T cells and T regulatory cells were similarly relatively modest after CladT. Importantly, there were no substantive effects on the innate immune system: monocytes were essentially unaffected and neutrophil counts remained in the normal range and had recovered by 5 weeks post-treatment.
There is no rebound in ALC or in any subset of lymphocyte counts above the pre-treatment level following immune reconstitution with CladT [32, 33]. Such rebounds have been observed in patients receiving alemtuzumab or autologous haematopoietic stem cell transplantation and are thought to contribute to the occurrence of secondary non-MS (especially thyroid) autoimmunity, which is a recognised side effect of these treatments [34,35,36]. The mechanism of action of CladT does not include sequestration of lymphocytes, so there is no clinical rebound of MS disease activity after treatment, in contrast to the potentially severe MS relapses that have been observed following withdrawal of S1P inhibitors or natalizumab [37,38,39,40].
Clinical Lymphopenia
During the randomised CLARITY study and its extension (96 weeks overall), administration of CladT 3.5 mg/kg resulted in reports of Common Terminology Criteria for Adverse Events (CTCAE) lymphopenia of Grade 1 (800–1000 mm−3) in 26.3% of patients, Grade 2 lymphopenia (500–800 mm−3) in 37.7%, Grade 3 lymphopenia (200–500 mm−3) in 24.9% and Grade 4 lymphopenia (< 200 mm−3) in 0.7% [5]. All 3 cases of Grade 4 lymphopenia occurred in patients redosed with CladT while still demonstrating Grade 3 lymphopenia. The ALC remained within the normal range for 10% [5]. Severe lymphopenia was of limited duration for most patients, consistent with the data on cell counts described above; data submitted for the approval of CladT in the USA showed that the median time to recover from Grade 3 lymphopenia was 28 weeks [41]. Post hoc integrated analyses of data from the CLARITY and ORACLE-MS studies showed that the aggregate incidence of lymphopenia as an SAE was 0.1 event/100 patient-years for CladT [24] and 0/100 patient-years for placebo (Fig. 2) and that the time course of effects of CladT on immune cells was similar for patients above or below age 50 years [42].
A recent real-world safety analysis of CladT included spontaneous AE reports and data from post-marketing studies, among other sources, and reflected clinical experience from 56,300 patients treated with CladT over a total duration of exposure to drug of 95,664 patient-years [25]. There were 112 reported cases of serious lymphopenia (incidence 0.12/100 patient-years [95% CI 0.10–0.14]); the potential clinical consequences of severe lymphopenia, particularly with reference to reactivation of herpes zoster, are described in a later section. In another real-world evaluation, longitudinal data on lymphocyte counts were available for 226 patients followed for 2 years [43]. Grade 3 and 4 lymphopenia developed in 18% and 1% of patients, respectively. The incidence of Grade 3–4 lymphopenia in this study was slightly lower for patients who had received platform DMTs, fingolimod or natalizumab before CladT compared with DMT-naïve patients (13% vs. 19%, respectively). However, half (50%) of patients previously treated with dimethyl fumarate (DMF) developed Grade 3–4 lymphopenia, although there was no difference in mean ALC for patients receiving different prior DMTs at baseline. A retrospective study showed that 3/12 people with MS demonstrated delayed lymphopenia > 1 year after initiation of DMF treatment, and 5/9 patients switched from DMF to other DMTs experienced recurrent lymphopenia afterwards [44]. Further study is required to understand this phenomenon and to evaluate to what extent it contributes to cases of lymphopenia attributed to CladT. Other real-world experience from a number of different countries demonstrated comparable or lower rates of lymphopenia than those seen following randomised treatment [45,46,47,48,49,50,51,52].
A 6-month analysis of Phase IV data concerning people with MS who received CladT demonstrated an incidence of lymphopenia of 8.3%, with an incidence approximately threefold higher in patients who had received prior DMT therapy (10.1%) vs. DMT-naïve patients (3.6%) [31]. Grade 3 lymphopenia occurred in in 24.4% and Grade 4 lymphopenia in 0.7% during 2 years of treatment in the MAGNIFY-MS study [29]. Pre-treatment ALC was a predictor of lymphopenia during treatment with CladT in another real-world analysis [53].
Infections
Overview of Infections
Two principal analyses of Phase III clinical trial data are available, based on the CLARITY study [5] and from an integrated analysis that included data from patients enrolled in CLARITY, its extension phase and the ORACLE-MS trial [24]. The incidence of any infections/infestations in the randomised phase of the CLARITY study was 47.7% for CladT 3.5 g/kg and 42.5% for placebo, of which 99.6% and 98.6%, respectively, were graded as being of mild or moderate severity by investigators [5]. The frequency of infections/infestations reported as SAE in the randomised phase of the CLARITY study was 2.3% for CladT 3.5 mg/kg and 1.6% for placebo [5]. In the integrated analysis [24], infections/infestations reported as SAE occurred in 2.5% of the CladT group (0.6 events/100 patient-years) and in 1.6% of the placebo group. (0.4 events/100 patient-years) (Fig. 2). The incidence of infections rated as severe was low in both groups in this analysis (0.76/100 patient-years for CladT and 0.81/100 patient-years for placebo).
Opportunistic infections were also infrequent in the integrated analysis (incidence 0.31/100 patient-years for CladT 3.5 mg/kg vs. 0.17/100 patient-years for placebo), with localised fungal infections making up most of the difference between treatments [24].
Infections occurring following treatment with CladT were driven mainly by an increased incidence of reactivation of latent herpes zoster. Eight patients receiving CladT 3.5 mg/kg reported herpes zoster in the main analysis of the CLARITY trial [5], while the integrated analysis reported a twofold higher incidence of herpetic infections (not defined further) for CladT (1.61/100 patient-years) vs. placebo (0.81/100 patient-years) [24]. The risk of herpes zoster was markedly higher during periods of Grade 3 or 4 lymphopenia vs. periods of higher ALC (incidences of 4.15/100 patient-years versus 0.64/100 patient-years, respectively) [24]. A significant correlation between low ALC and higher risk of zoster had been noted previously in the CLARITY Study [5].
An analysis of infections is available from the CLARION cohort, a long-term real-world safety evaluation measuring the incidence of AE of interest in patients with MS newly initiating CladT (N-742) or fingolimod (N = 583) [54]. Similar incidence rates ([95% CI]/1000 patient-years) were observed in the CladT and fingolimod groups for any severe infection (7.4 [2.8–19.6] vs. 6.6 [2.5–17.4), herpes zoster (5.5 [1.8–17.1] and opportunistic infections (0 vs. 1.6 [0.2–11.5]) [55]. Other real-world evaluations included single-centre evaluations in Norway [56], Qatar [57] and Canada [58], where 3/90, 1/49 and 3/111 patients, respectively, reported reactivation of herpes zoster after receiving CladT, and a study of a nationwide database in Sweden in which 8/208 patients developed infections or infestations on CladT [59]. Two of 138 patients in a real-world study in Portugal experienced herpes zoster as a SAE [60]. Effective prophylaxis for zoster was shown to prevent occurrence of this infection in one real-world study [51].
Post-approval surveillance data (Table 1) have shown an incidence of serious infections of 0.79/100 patient-years and of herpes zoster of 0.54/100 patient-years [25]. Similarly low rates of opportunistic infections/100 patient-years were reported for tuberculosis (TB; 0.02) and opportunistic infections (0.02) in this analysis. An updated safety analysis for CladT, drawing data from the trial and real-world settings, estimated crude incidences of infections as 0.008 for herpes zoster, 0.0004 for TB, 0.009 for severe infections and 0.001 for opportunistic infections [61].
Progressive Multifocal Leukoencephalopathy
No cases of confirmed progressive multifocal leukoencephalopathy (PML) have been reported to date in patients receiving CladT for the management of RMS, including during post-approval follow-up (Table 1) [25, 61].
Covid-19
Data from real-world cohorts, including the MAGNIFY-MS and CLARIFY-MS cohorts, suggest that treatment with CladT does not appear to increase either the risk of infection with Covid-19 or the risk of adverse clinical outcomes once Covid-19 infection is established; real-world data showed that most CladT-treated patients experienced a mild or moderate severity of Covid-19 infection [62,63,64,65,66,67]. By contrast, a higher risk of Covid-19 outcomes appears to be associated with anti-CD20 therapies, especially where corticosteroids were given within the previous month [68, 69]. The effects of CladT on the response to vaccination against the SARS-CoV2 virus is described below.
Effects on Efficacy of Vaccinations
Expert consensus statements have considered vaccination against SARS-CoV2 to be safe for people with MS and have provided strong support for the vaccination of CladT-treated patients with MS against Covid-19 [70, 71]. A study in 32 patients who received the Pfizer/BioNTech SARS-CoV2 vaccine 2–3 weeks after the second course of CladT demonstrated similar production of antibodies against the SARS-CoV2 spike protein compared with 30 healthy controls [72]. Another study showed that the humoral response to this vaccine was unimpaired compared with healthy control subjects in people with MS treated with CladT, interferons, dimethyl fumarate, natalizumab or teriflunomide, with a decreased humoral response in patients treated with ocrelizumab, fingolimod or alemtuzumab [73, 74]. Similar results were obtained elsewhere, with maintained antibody responses to CladT and other DMTs used in MS, except for anti-CD20 agents and fingolimod, where responses were blunted [75, 76].
The efficacy of other vaccines is also maintained in people with MS treated with CladT (reviewed elsewhere [77,78,79]). Treatment with CladT did not diminish pre-existing humoral immunological memory from previous vaccines commonly given in childhood (measles, mumps, rubella, zoster, hepatitis B, diphtheria and tetanus): loss of seroprotection from those diseases was seen in < 1% of patients treated with CladT [80].
Liver Injury
The Phase III studies with CladT did not reveal any signal for serious hepatic AE. However, reports of rare but potentially serious liver toxicity have arisen from post-approval AE reporting [81, 82]. Initially, a single case was noted, where a patient developed elevations of liver transaminases of up to 33 × the upper limit of normal approximately 1 month following the conclusion of the first treatment course of CladT [81]. Liver transaminases returned to within the normal range over approximately 2 months. Examination of the CladT clinical trials database revealed five further cases (0.3% of patients) of CladT-related liver SAEs that were serious or led to treatment discontinuation [41]. In all but one case these arose 2–4 weeks after the end of the first treatment course (all survived). There were no cases of autoimmune hepatitis. A total of 60 spontaneous AE reports of liver dysfunction that were in temporal association with CladT administration have been made to date [25]. Of the 60 cases, 44 were rated as serious, of which two were Grade 3 AEs and one was a Grade 4 AE (all others were Grade 1 or 2). One patient with a Grade 4 AE died because of liver toxicity associated with the use of isoniazid; this elderly patient had a history of alcohol-related liver disease and persistent TB.
Malignancies
Analysis of patient populations treated within controlled clinical trials of CladT and their extensions revealed rates of malignancy of 0.27 events/100 patient-years with CladT compared with 0.13 events/100 patient-years for placebo [41]. These data drove an apparently higher incidence of malignancy for CladT 3.5 mg/kg in the integrated analysis (Fig. 2) [24]. In contrast, a meta-analysis showed that the incidence of malignancy in placebo-treated patients in CladT trials was lower than observed in other placebo-treated clinical trial populations and that the incidence of malignancy was not higher with CladT vs. other DMTs: excess risk = 0.20 events (95% CI − 0.08 to 0.39)/100 patient-years (p = 0.46) [90]. In addition, the integrated analysis showed that the longer-term (5 years onwards) rate of malignancy on CladT was not higher than that on placebo (0.17 vs. 0.29 events/100 patient-years [24]. The integrated analysis of CladT trials also showed that the predicted rate of malignancy in the population that received CladT did not differ significantly from that from a matched population from the Global Cancer Observatory (GLOBOCAN) database (Fig. 4) [24]. These data confirmed the results of earlier integrated analyses [91, 92]. There has been no suggestion of clustering of specific cancer types or haematological cancers in CladT-treated populations to date [24, 42].
Comparison of rates of malignancy from the cladribine tablets clinical database for use in the management of multiple sclerosis with the expected background incidence from the Global Cancer Observatory (GLOBOCAN) database. Reproduced from Ref. [24] under the Creative Commons CC-BY-NC-ND license (https://creativecommons.org/licenses)
Post-approval AE reporting to date provided a rate for malignancy of 0.20 (95% CI 0.17–0.23), which is consistent with the rates observed in clinical trials (Table 1) [25]. In another study, spontaneous AE reports made to the US Food and Drug Administration between 2004 and 2020 found no difference in the age- and gender-adjusted risk of malignancy for any DMT (including CladT) compared with interferon β1a [92]. Finally, experimental evidence showed that cladribine inhibited proliferation of a cultured melanoma cell line irrespective of the oncogene/mutational status of the cells, which is not consistent with a risk of promotion of malignancy [93].
Pregnancy
Many diagnoses of MS occur during or before young adulthood; for example, one real-world study from Germany found that 65% of women receiving CladT for MS were aged < 40 years [95]. Pregnancy is a contraindication for CladT: women receiving the drug are required to avoid pregnancy until 6 months after the last dose and male patients should ensure pregnancy does not occur in their partner during this time period [23]. Should a pregnancy occur during treatment, further treatment with CladT should be withheld until after delivery.
Some pregnancies have occurred during or soon after treatment with CladT. An analysis of 70 pregnancies in patients exposed to oral or parenteral CladT for MS (n = 49) or placebo (n = 21) in CladT trials or from registry data found comparable rates of live births (39% vs. 43%), spontaneous abortions (22% vs. 24%, respectively) and therapeutic abortions (10% for each group) [96]. The rate of elective terminations was numerically higher for CladT (29%) vs. placebo (19%), likely due to this being offered to CladT recipients in view of the association of the pregnancy with treatment. Two cases of birth malformation (one for each treatment group) occurred more than 2 years after the last ingestion of cladribine. Restricting the analysis to pregnancies occurring strictly within the period of contraindication (during or < 6 months after treatment) limited the study population to 27 pregnancies; 10/16 pregnancies (63%) in women in the CladT group were terminated electively, rendering comparisons with the placebo-exposed group difficult (2/11 pregnancies [18%] ended with elective terminations in this group). The rate of spontaneous termination was 2/16 (13%) vs. 3/11 (27%) for the CladT- and placebo-exposed cohorts, respectively, and there were no congenital malformations in either group. Similar data were presented from an analysis of the trials database and the German Multiple Sclerosis and Pregnancy Registry [97]. An ongoing cohort study of 39 pregnancies in women who received cladribine < 6 months before, or after, their last period before pregnancy has described 27 live births so far that included a single major congenital malformation [98]. There are no reports to date of adverse pregnancy outcomes from pregnancies fathered by male partners who received CladT during the at-risk period [96, 97].
Conclusions
CladT was generally well tolerated in clinical trials, with the main AE reported consisting of lymphopenia and reactivation of latent infections, particularly varicella zoster. Grade 3 or 4 lymphopenia was relatively uncommon, and lymphocytes recovered over a period of months for most patients. The two issues are linked, as the risk of reactivation or latent infections or of opportunistic infections increased markedly where lymphopenia was more profound (ALC < 200 mm3). Pragmatic alterations to the administration regimen of CladT since the conduct of the pivotal Phase III trials (especially the requirement that ALC must be > 800/mm3 before administration of the second course and that the second cycle can be delayed for up to 6 months to allow ALC to recover sufficiently) have limited the potential for severe lymphopenia (and thus for opportunistic infections). There appears to be no increased risk of malignancy with CladT vs. untreated, non-RMS populations or vs. other DMTs used in the management of RMS. Finally, the emergence of drug-induced liver disease as a new and potentially serious (if uncommon) SAE associated with CladT emphasises the importance of withholding CLadT treatment from people with pre-existing liver disease and maintaining vigilance for the emergence of liver disease during treatment (further information on the aetiology of this AE is required). The CLARION registry will continue to collect real-world efficacy and safety data on the use of CladT in the management of RMS [54].
Supporting Information
Search Strategy for this Review
A PubMed search was conducted for cladribine AND "multiple sclerosis" AND safety (abstracts of 137 hits, restricted to articles in English, were examined manually for articles of interest). Recent presentations to conferences (for the most up-to-date information), references in identified publications and authors’ reference collections provided further material for review.
Change history
20 November 2023
A Correction to this paper has been published: https://doi.org/10.1007/s40120-023-00564-8
References
AlSharoqi IA, Aljumah M, Bohlega S, et al. Immune reconstitution therapy or continuous immunosuppression for the management of active relapsing-remitting multiple sclerosis patients? A narrative review. Neurol Ther. 2020;9:55–66.
Alroughani R, Inshasi JS, Deleu D, et al. An overview of high-efficacy drugs for multiple sclerosis: Gulf region expert opinion. Neurol Ther. 2019;8:13–23.
Sorensen PS, Sellebjerg F. Pulsed immune reconstitution therapy in multiple sclerosis. Ther Adv Neurol Disord. 2019;28(12):1756286419836913.
De Sèze J, Suchet L, Mekies C, et al. The place of immune reconstitution therapy in the management of relapsing multiple sclerosis in France: an expert consensus. Neurol Ther 2023;12:351–369.
Giovannoni G, Comi G, Cook S, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med. 2010;362:416–26.
Giovannoni G, Soelberg Sorensen P, Cook S, et al. Safety and efficacy of cladribine tablets in patients with relapsing-remitting multiple sclerosis: results from the randomized extension trial of the CLARITY study. Mult Scler. 2018;24:1594–604.
Giovannoni G, Singer BA, Issard D, Jack D, Vermersch P. Durability of no evidence of disease activity-3 (NEDA-3) in patients receiving cladribine tablets: the CLARITY extension study. Mult Scler. 2022;28:1219–28.
Cohen JA, Coles AJ, Arnold DL, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet. 2012;380:1819–28.
Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet. 2012;380:1829–39.
Brecl Jakob G, Barun B, Gomezelj S, et al. Effectiveness and safety of alemtuzumab in the treatment of active relapsing-remitting multiple sclerosis: a multicenter, observational study. Neurol Sci. 2021;42:4591–7.
Moser T, Ziemssen T, Sellner J. Real-world evidence for cladribine tablets in multiple sclerosis: further insights into efficacy and safety. Wien Med Wochenschr. 2022. https://doi.org/10.1007/s10354-022-00931-4.
Giovannoni G, Cohen JA, Coles AJ, et al. Alemtuzumab improves preexisting disability in active relapsing-remitting MS patients. Neurology. 2016;87:1985–92.
Bose G, Rush C, Atkins HL, Freedman MS. A real-world single-centre analysis of alemtuzumab and cladribine for multiple sclerosis. Mult Scler Relat Disord. 2021;52:102945.
Rauma I, Viitala M, Kuusisto H, et al. Finnish multiple sclerosis patients treated with cladribine tablets: a nationwide registry study. Mult Scler Relat Disord. 2022;61:103755.
Patti F, Visconti A, Capacchione A, Roy S, Trojano M, CLARINET-MS Study Group. Long-term effectiveness in patients previously treated with cladribine tablets: a real-world analysis of the Italian multiple sclerosis registry (CLARINET-MS). Ther Adv Neurol Disord. 2020;13:1756286420922685.
Frau J, Coghe G, Lorefice L, Fenu G, Musu L, Cocco E. Efficacy and safety of alemtuzumab in a real-life cohort of patients with multiple sclerosis. J Neurol. 2019;266:1405–11.
Coles AJ, Arnold DL, Bass AD, et al. Efficacy and safety of alemtuzumab over 6 years: final results of the 4-year CARE-MS extension trial. Ther Adv Neurol Disord. 2021;14:1756286420982134.
Steingo B, Al Malik Y, Bass AD, et al. Long-term efficacy and safety of alemtuzumab in patients with RRMS: 12-year follow-up of CAMMS223. J Neurol. 2020;267:3343–53.
Russo CV, Saccà F, Frau J, et al. A real-world study of alemtuzumab in a cohort of Italian patients. Eur J Neurol. 2022;29:257–66.
Ruck T, Barman S, Schulte-Mecklenbeck A, et al. Alemtuzumab-induced immune phenotype and repertoire changes: implications for secondary autoimmunity. Brain. 2022;145:1711–25.
Killestein J, van Oosten B. Emerging safety issues in alemtuzumab-treated MS patients. Mult Scler. 2019;25(9):1206–8.
European Medicines Agency. Lemtrada. Measures to minimise risk of serious side effects of multiple sclerosis medicine. 2019. https://www.ema.europa.eu/en/medicines/human/referrals/lemtrada. Accessed Nov 2022.
European Medicines Agency. Mavenclad, INN-cladribine. Available at https://www.ema.europa.eu/en/documents/product-information/mavenclad-epar-product-information_en.pdf. Accessed Nov 2022.
Leist T, Cook S, Comi G, et al. Long-term safety data from the cladribine tablets clinical development program in multiple sclerosis. Mult Scler Relat Disord. 2020;46:102572.
Giovannoni G, Leist T, Jack D, Galazka A. Updated post-approval safety of cladribine tablets in the treatment of multiple sclerosis, with particular reference to liver safety. Abstract 321 at the 2022 meeting of ECTRIMS, October 26–28 2022. https://doi.org/10.1177/13524585221123687. Accessed Nov 2022.
Oh J, Walker B, Giovannoni G, et al. Treatment-emergent adverse events occurring early in the treatment course of cladribine tablets in two phase 3 trials in multiple sclerosis. Mult Scler J Exp Transl Clin. 2021;7:20552173211024296.
Śladowska K, Kawalec P, Holko P, Osiecka O. Comparative safety of high-efficacy disease-modifying therapies in relapsing-remitting multiple sclerosis: a systematic review and network meta-analysis. Neurol Sci. 2022;43:5479–500.
Siddiqui MK, Khurana IS, Budhia S, Hettle R, Harty G, Wong SL. Systematic literature review and network meta-analysis of cladribine tablets versus alternative disease-modifying treatments for relapsing-remitting multiple sclerosis. Curr Med Res Opin. 2018;34:1361–71.
De Stefano N. Early onset of action and sustained efficacy of MRI outcomes during cladribine tablets treatment in highly active relapsing multiple sclerosis: results of the 2-year MAGNIFY-MS study. Abstract P717 at the 2022 meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), Amsterdam, Netherlands, 26–28 October 2022. https://ectrims2022.abstractserver.com/program/#/details/presentations/871 (accessed Jan 2023).
Disanto G, Moccia M, Sacco R, et al. Monitoring of safety and effectiveness of cladribine in multiple sclerosis patients over 50 years. Mult Scler Relat Disord. 2022;58:103490.
Brochet B, Hupperts R, Langdon D, et al. Treatment satisfaction, safety, and tolerability of cladribine tablets in patients with highly active relapsing multiple sclerosis: CLARIFY-MS study 6-month interim analysis. Mult Scler Relat Disord. 2022;57:103385.
Comi G, Cook S, Giovannoni G, et al. Effect of cladribine tablets on lymphocyte reduction and repopulation dynamics in patients with relapsing multiple sclerosis. Mult Scler Relat Disord. 2019;29:168–74.
Stuve O, Soelberg Soerensen P, Leist T, et al. Effects of cladribine tablets on lymphocyte subsets in patients with multiple sclerosis: an extended analysis of surface markers. Ther Adv Neurol Disord. 2019;12:1756286419854986.
Sellner J, Rommer PS. Immunological consequences of “immune reconstitution therapy” in multiple sclerosis: a systematic review. Autoimmun Rev. 2020;19:102492.
Coles AJ, Jones JL, Vermersch P, et al. Autoimmunity and long-term safety and efficacy of alemtuzumab for multiple sclerosis: benefit/risk following review of trial and post-marketing data. Mult Scler. 2022;28:842–6.
Willison AG, Ruck T, Lenz G, Hartung HP, Meuth SG. The current standing of autologous haematopoietic stem cell transplantation for the treatment of multiple sclerosis [published correction appears in J Neurol. 2022 May 24;:]. J Neurol. 2022;269:3937–58.
Lohmann L, Janoschka C, Schulte-Mecklenbeck A, et al. Immune cell profiling during switching from natalizumab to fingolimod reveals differential effects on systemic immune-regulatory networks and on trafficking of non-t cell populations into the cerebrospinal fluid-results from the ToFingo Successor Study. Front Immunol. 2018;9:1560.
Sica F, Centonze D, Buttari F. Fingolimod immune effects beyond its sequestration ability. Neurol Ther. 2019;8:231–40.
Prosperini L, Kinkel RP, Miravalle AA, Iaffaldano P, Fantaccini S. Post-natalizumab disease reactivation in multiple sclerosis: systematic review and meta-analysis. Ther Adv Neurol Disord. 2019;12:1756286419837809.
Barry B, Erwin AA, Stevens J, Tornatore C. Fingolimod rebound: a review of the clinical experience and management considerations. Neurol Ther. 2019;8:241–50.
Food and Drug Administration. Center for drug evaluation and research. Clinical review. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/022561Orig1s000MedR.pdf. Accessed Nov 2022.
Giovannoni G, Coyle PK, Vermersch P, et al. Integrated lymphopenia analysis in younger and older patients with multiple sclerosis treated with cladribine tablets. Front Immunol. 2021;12: 763433.
Pfeuffer S, Rolfes L, Hackert J, et al. Effectiveness and safety of cladribine in MS: real-world experience from two tertiary centres. Mult Scler. 2022;28:257–68.
Borrelli S, Mathias A, Goff GL, Pasquier RD, Théaudin M, Pot C. Delayed and recurrent dimethyl fumarate induced-lymphopenia in patients with multiple sclerosis. Mult Scler Relat Disord. 2022;63: 103887.
Thakre M, Inshasi J. Real world experience of oral immune reconstitution therapy (cladribine) in the treatment of multiple sclerosis in the United Arab Emirates. Abstract P0140 at the 2020 virtual joint meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) and the American Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS). Mult Scler J 2020;26(S3):185. https://doi.org/10.1177/1352458520974938 (accessed Jan 2023).
Kantorová E, Szilásiová J, Vítková M, et al. Real world experience with cladribine treatment in Slovakia. Abstract EP1085 at the 2022 meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), Amsterdam, Netherlands, 26–28 October 2022. Available at https://ectrims2022.abstractserver.com/program/#/details/presentations/1176 (accessed Jan 2023).
Sokmen O, Acar Ozen P, Tuncer A, Karabudak R. Efficacy and safety profiles of cladribine in highly active multiple sclerosis: a tertiary MS center experience in Turkey Abstract EP1088 at the 2022 meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), Amsterdam, Netherlands, 26–28 October 2022. https://ectrims2022.abstractserver.com/program/#/details/presentations/1543 (accessed Jan 2023).
Garcia Dominguez JM, Cuello JP, Alba Suárez E, et al. Experience with cladribine in a real world MS cohort. Abstract EP1129 at the 2022 meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), Amsterdam, Netherlands, 26–28 October 2022. https://ectrims2022.abstractserver.com/program/#/details/presentations/1109 (accessed Jan 2023).
Negroski D, Sellers D, Khiabani A, Khiabani D. Real-world experience with cladribine tablets in an aged ≥50 years cohort. Abstract P311 at the 2022 meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), Amsterdam, Netherlands, 26–28 October 2022. https://ectrims2022.abstractserver.com/program/#/details/presentations/1206 (accessed Jan 2023).
Margoni M, Annovazzi P, Prosperini L, et al. A multicentre, real-life study on the risk of lymphopenia and infections discloses a favourable safety profile of cladribine in MS patients. Abstract P0273 at the 2020 virtual joint meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) and the American Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS). Mult Scler J 2020;26(S3):252. https://doi.org/10.1177/1352458520974938 (accessed Jan 2023).
Dotor García-Soto J, López Ruiz R, Eichau S, et al. Real world experience with cladribine: patient profile, efficacy and safety. Abstract P0377 at the 2020 virtual joint meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) and the American Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS). Mult Scler J 2020;26(S3):300. https://doi.org/10.1177/1352458520974938 (accessed Jan 2023).
Suslak T, Macdougall N, Murray N. Cladribine is a safe and effective treatment for highly active relapsing-remitting multiple sclerosis. Abstract P0309 at the 2020 virtual joint meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) and the American Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS). Mult Scler J 2020;26(S3):269. https://doi.org/10.1177/1352458520974938 (accessed Jan 2023).
Petracca M, Ruggieri S, Barbuti E, et al. Predictors of cladribine effectiveness and safety in multiple sclerosis: a real-world, multicenter, 2-year follow-up study. Neurol Ther. 2022;11:1193–208.
Butzkueven H, Moore N, Aydemir A, et al. The CLARION study design and status update: a long-term, registry-based study evaluating adverse events of special interest in patients with relapsing multiple sclerosis newly started on cladribine tablets. Curr Med Res Opin. 2022;38:1167–76.
Hillert J, Butzkueven H, Soilu-Hänninen M, et al. Incidence of infections and severe lymphopenia in patients newly initiating cladribine tablets or fingolimod for treatment of multiple sclerosis: CLARION study. Abstract P767 at the at the 2021 virtual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS). Mult Scler J 2021;27(2_Suppl):639. https://doi.org/10.1177/13524585211044667 (accessed Jan 2023).
Celius EG, Berg-Hansen P. Cladribine as treatment of multiple sclerosis, real world experience. Abstract P998 at the 2019 meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), Stockholm, Sweden, 11–13 September 2019. https://doi.org/10.1177/1352458519868080 (accessed Jan 2023).
Garcia-Cañibano B, Zamrath Zahir F, Safan A, Ibrahim F, Deleu D. Two years efficacy and safety results from real world experience for cladribine tablets in management of relapsing multiple sclerosis in Qatar. Abstract P375 at the 2022 meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), Amsterdam, Netherlands, 26–28 October 2022. https://ectrims2022.abstractserver.com/program/#/details/presentations/471 (accessed Jan 2023).
Bain J, Oh J, Jones J, Selchen D, Overholt S, Guenette M. Early real-world safety, tolerability, and efficacy of cladribine tablets: a single center experience. Abstract P0319 at the 2020 virtual joint meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) and the American Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS). Mult Scler J 2020;26(S3):274. https://doi.org/10.1177/1352458520974938 (accessed Jan 2023).
Rosengren V, Forsberg LL, Ekström E, et al. Clinical effectiveness and safety of cladribine tablets for patients treated at least 12 months in the Swedish post-market surveillance study “immunomodulation and multiple sclerosis epidemiology 10” (IMSE 10). Abstract P728 at the 2022 meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), Amsterdam, Netherlands, 26–28 October 2022. https://ectrims2022.abstractserver.com/program/#/details/presentations/910 (accessed Jan 2023).
Pato-Pato A, Rodríguez-Regal A, Alvarez E, et al. Real life efficacy and tolerability of cladribine: multicentre study in galicia (CLADRIGAL). Abstract EP1134 at the 2022 meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), Amsterdam, Netherlands, 26–28 October 2022. https://ectrims2022.abstractserver.com/program/#/details/presentations/518 (accessed Jan 2023).
Giovannoni G, Berger J, Leist T, et al. Updated post-approval safety of cladribine tablets in the treatment of multiple sclerosis, with particular reference to respiratory viral infections. Abstract P0415 at the 2020 virtual joint meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) and the American Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS). Mult Scler J 2020;26(S3):318. https://doi.org/10.1177/1352458520974938 (accessed Jan 2023).
Yavorskaya V, Karan R, Borsi L, Alexandri N. Clinical outcomes in patients with COVID-19 During Two Phase IV studies of cladribine tablets for treatment of multiple sclerosis: an update. Neurology 2022;98 (18 Supplement):1358 (Astract). https://n.neurology.org/content/98/18_Supplement/1358. Accessed Nov 2022.
Preziosa P, Rocca MA, Nozzolillo A, Moiola L, Filippi M. COVID-19 in cladribine-treated relapsing-remitting multiple sclerosis patients: a monocentric experience. J Neurol. 2021;268:2697–9.
Karan R, Roy S, Alexandri NN. Clinical outcomes in patients with Covid-19 infection during phase Iv studies of cladribine tablets for treatment of multiple sclerosis. Abstract LB1151 at the 2020 virtual joint meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) and the American Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS). Mult Scler J 2020;26(S3):53. https://doi.org/10.1177/1352458520974938 (accessed Jan 2023)
Oreja-Guevara C, Meca-Lallana V, Brieva L, et al. Covid-19 in cladribine-treated patients with multiple sclerosis. Abstract LB1165 at the 2020 virtual joint meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) and the American Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS). Mult Scler J 2020;26(S3):59. https://doi.org/10.1177/1352458520974938 (accessed Jan 2023)
Giovannoni G, Berger J, Leist T, et al. Post-approval safety of cladribine tablets with particular reference to Covid-19 outcomes: an update. Abstract at the 2021 Consortium of Multiple Sclerosis Centers Annual Meeting. https://2021abstracts.cmscscholar.org/2021/10/25/updated-post-approval-safety-of-cladribine-tablets-in-the-treatment-of-multiple-sclerosis-with-particular-reference-to-respiratory-viral-infections-and-covid-19. Accessed Nov 2022.
Albanese A, Sormani MP, Gattorno G, Schiavetti I. COVID-19 severity among patients with multiple sclerosis treated with cladribine: a systematic review and meta-analysis. Mult Scler Relat Disord. 2022;68: 104156.
Sormani MP, De Rossi N, Schiavetti I, et al. Disease-modifying therapies and coronavirus disease severity in multiple sclerosis. Ann Neurol. 2021;89:780–9.
Simpson-Yap S, Pirmani A, Kalincik T, et al. Updated Results of the COVID-19 in MS Global Data Sharing Initiative: anti-CD20 and other risk factors associated with COVID-19 severity. Neurol Neuroimmunol Neuroinflamm. 2022;9:e200021.
Yamout BI, Zakaria M, Inshasi J, et al. MENACTRIMS practice guideline for COVID-19 vaccination in patients with multiple sclerosis. Mult Scler Relat Disord. 2021;56:103225.
Rieckmann P, Centonze D, Giovannoni G, et al. Expert opinion on COVID-19 vaccination and the use of cladribine tablets in clinical practice. Ther Adv Neurol Disord. 2021;14:17562864211058298.
Brill L, Rechtman A, Zveik O, et al. Effect of cladribine on COVID-19 serology responses following two doses of the BNT162b2 mRNA vaccine in patients with multiple sclerosis. Mult Scler Relat Disord. 2022;57:103343.
Achiron A, Mandel M, Dreyer-Alster S, et al. Humoral immune response in multiple sclerosis patients following PfizerBNT162b2 COVID19 vaccination: up to 6 months cross-sectional study. J Neuroimmunol. 2021;361:577746.
Achiron A, Mandel M, Dreyer-Alster S, et al. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther Adv Neurol Disord. 2021;14:17562864211012836.
Sormani MP, Inglese M, Schiavetti I, et al. Effect of SARS-CoV-2 mRNA vaccination in MS patients treated with disease modifying therapies. EBioMedicine. 2021;72:103581.
Etemadifar M, Nouri H, Pitzalis M, et al. Multiple sclerosis disease-modifying therapies and COVID-19 vaccines: a practical review and meta-analysis. J Neurol Neurosurg Psychiatry. 2022;93:986–94.
Moiola L, Riva A, Nicoletti F, et al. Vaccination opportunities in multiple sclerosis patients treated with cladribine tablets. Curr Neuropharmacol. 2022;20(10):1811–5.
Inshasi J, Alroughani R, Al-Asmi A, et al. Expert consensus and narrative review on the management of multiple sclerosis in the Arabian Gulf in the COVID-19 Era: focus on disease-modifying therapies and vaccination against COVID-19. Neurol Ther. 2021;10(2):539–55.
Gold R, Fätkenheuer G, Hartung HP, et al. Vaccination in multiple sclerosis patients treated with highly effective disease-modifying drugs: an overview with consideration of cladribine tablets. Ther Adv Neurol Disord. 2021;14:17562864211019598.
Moser T, O’Sullivan C, Puttinger C, et al. Pre-existing humoral immunological memory is retained in patients with multiple sclerosis receiving cladribine therapy. Biomedicines. 2021;9:1584.
Saraceno L, Pirro F, Stigliano R, Agostoni EC, Protti A. Acute idiosyncratic liver injury after Cladribine treatment for multiple sclerosis: first case report and review on associated hepatic disorders. Mult Scler. 2022;28:2142–5.
Brownlee WJ. Cladribine-induced liver injury: implications for practice. Mult Scler. 2022;28:2146.
European Medicines Agency. Tecfidera. Summary of product characteristics. https://www.ema.europa.eu/en/medicines/human/EPAR/tecfidera. Accessed Nov 2022.
European Medicines Agency. Tysabri. Summary of product characteristics. https://www.ema.europa.eu/en/medicines/human/EPAR/tysabri. Accessed Nov 2022.
European Medicines Agency. Aubagio. Summary of product characteristics. https://www.ema.europa.eu/en/medicines/human/EPAR/aubagio. Accessed Nov 2022.
European Medicines Agency. Gilenya. Summary of Product Characteristics. https://www.ema.europa.eu/en/medicines/human/EPAR/gilenya. Accessed Nov 2022.
European Medicines Agency. Glatiramer – Scientific conclusions and grounds for the variation to the terms of the Marketing Authorisation - PSUSA-00001529–202011. https://www.ema.europa.eu/documents/psusa/glatiramer-scientific-conclusions-grounds-variation-terms-marketing-authorisation-psusa-00001529_en.pdf. Accessed Nov 2022.
European Medicines Agency. Rebif. Summary of product characteristics. https://www.ema.europa.eu/en/medicines/human/EPAR/rebif. Accessed Nov 2022.
National Institute for Health and Care Excellence. Cladribine. Important safety information. https://bnf.nice.org.uk/drugs/cladribine/#important-safety-information. Accessed Nov 2022.
Pakpoor J, Disanto G, Altmann DR, Pavitt S, Turner BP, Marta M, Juliusson G, Baker D, Chataway J, Schmierer K. No evidence for higher risk of cancer in patients with multiple sclerosis taking cladribine. Neurol Neuroimmunol Neuroinflamm. 2015;2:e158.
Galazka A, Nolting A, Cook S, et al. An analysis of malignancy risk in the clinical development programme of cladribine tablets in patients with relapsing multiple sclerosis. J Neurol Neurosurg, Psychiatry. 2018;89:A17.
Stamatellos VP, Siafis S, Papazisis G. Disease-modifying agents for multiple sclerosis and the risk for reporting cancer: a disproportionality analysis using the US Food and Drug Administration Adverse Event Reporting System database. Br J Clin Pharmacol. 2021;87:4769–79.
Lebrun-Frenay C, Berestjuk I, Cohen M, Tartare-Deckert S. Effects on melanoma cell lines suggest no significant risk of melanoma under cladribine treatment. Neurol Ther. 2020;9:599–604.
Sørensen PS, Centonze D, Giovannoni G, et al. Expert opinion on the use of cladribine tablets in clinical practice. Ther Adv Neurol Disord. 2020;13:1756286420935019.
Platzbecker K, Wentzell N, Kollhorst B, Haug U. Fingolimod, teriflunomide and cladribine for the treatment of multiple sclerosis in women of childbearing age: description of drug utilization and exposed pregnancies in Germany. Mult Scler Relat Disord. 2022;67:104184.
Giovannoni G, Galazka A, Schick R, et al. Pregnancy outcomes during the clinical development program of cladribine in multiple sclerosis: an integrated analysis of safety. Drug Saf. 2020;43:635–43.
Hellwig K, Thiel S, Ciplea A, Galazka A, Nolting A, Huebschen M. Pregnancy of MS patients treated with cladribine tablets (1477). Neurology 2020;94 (15 Supplement):Abstract 1477.
Dost-Kovalsky K, Thiel S, Ciplea AI, Gold R, Hellwig K. Cladribine and pregnancy in women with multiple sclerosis: the first cohort study [published online ahead of print, 2022 Oct 22]. Mult Scler. 2022. https://doi.org/10.1177/13524585221131486.
Dobson R, Dassan P, Roberts M, Giovannoni G, Nelson-Piercy C, Brex PA. UK consensus on pregnancy in multiple sclerosis: “Association of British Neurologists” guidelines. Pract Neurol. 2019;19:106–14.
Simone IL, Tortorella C, Ghirelli A. Influence of pregnancy in multiple sclerosis and impact of disease-modifying therapies. Front Neurol. 2021;12: 697974.
Alroughani R, Inshasi J, Al-Asmi A, et al. Disease-modifying drugs and family planning in people with multiple sclerosis: a consensus narrative review from the gulf region. Neurol Ther. 2020;9:265–80.
Al Jumah M, Al Malik Y, AlKhawajah NM, et al. Family planning for people with multiple sclerosis in Saudi Arabia: an expert consensus. Mult Scler Int. 2021;2021:6667006.
Canibaño B, Ali M, Mesraoua B, et al. Severe rebound disease activity after fingolimod withdrawal in a pregnant woman with multiple sclerosis managed with rituximab: a case study. Case Rep Womens Health. 2019;25:e00162.
Hellwig K, Tokic M, Thiel S, et al. Multiple sclerosis disease activity and disability following discontinuation of natalizumab for pregnancy. JAMA Netw Open. 2022;5(1):e2144750.
Liliemark J. The clinical pharmacokinetics of cladribine. Clin Pharmacokinet. 1997;32:120–31.
Acknowledgements
Funding
Merck Serono, Lyon, France, an affiliate of Merck KGaA, Darmstadt, Germany (CrossRef Funder ID: https://doi.org/10.13039/100009945) funded editorial support (see below) and the journal’s Rapid Service Fee. No other funding was received.
Medical Writing and/or Editorial Assistance
A medical writer (Dr Mike Gwilt, GT Communications) provided editorial assistance, funded by Merck Serono, Lyon France, an affiliate of Merck KGaA, Darmstadt, Germany.
Author Contributions
Pierre Clavelou, Giovanni Castelnovo, Valérie Pourcher, Jerome De Sèze, Patrick Vermersch, Ali-Frederic Ben-Amor, Carine Savarin and Gilles Defer contributed equally to the conception, design, content and interpretation of data in the article, participated in its drafting and critical revision for intellectual content, approved the final version for submission and acknowledge that they are accountable for all aspects of the article.
Disclosures
Pierre Clavelou has received honoraria, and contributions to meeting from Almirall, Biogen, Janssen, Merck, Novartis, Roche, Sanofi-Genzyme and Teva. Giovanni Castelnovo received fees for consulting and speaking from Biogen, Abbvie, Merck, Novartis, Roche, Sanofi Genzyme, Merz and Celgene BMS. Jerome De Sèze has received fees for consultancy, advisory board and clinical trials from UCB, Novartis, Biogen, Merck, Teva, Genzyme/ Sanofi, Roche, Alexion, BMS/Celegene, Janssen and Horizon Therapeutics. Gilles Defer has received personal compensation for scientific advisory boards for Biogen, Novartis, BMS, Genzyme, Merck Serono, Roche and Teva and has received speaker honoraria and travel grants from Merck Serono, Biogen, Novartis, BMS, Roche, Genzyme and Teva. Valérie Pourcher has received fees for consultancy, advisory board and speaking trials from Novartis, Biogen, Merck and Roche. Patrick Vermersch received honoraria for contributions to meetings from Biogen, Sanofi-Genzyme, Novartis, Teva, Merck, Roche, Imcyse, AB Science, Almirall and BMS-Celgene and research support from Novartis, Sanofi-Genzyme and Merck. Ali-Frederic Ben-Amor is an employee of Ares Trading SA, Eysins, Switzerland, an affiliate of Merck KGaA, Darmstadt, Germany. Carine Savarin is an employee of Merck Santé, Lyon, France, an affiliate of Merck KGaA, Darmstadt, Germany.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data availability
No repository of data is available for this review article.
Author information
Authors and Affiliations
Corresponding author
Additional information
The original online version of this article was revised to correct few values in Table 1.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Clavelou, P., Castelnovo, G., Pourcher, V. et al. Expert Narrative Review of the Safety of Cladribine Tablets for the Management of Relapsing Multiple Sclerosis. Neurol Ther 12, 1457–1476 (2023). https://doi.org/10.1007/s40120-023-00496-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-023-00496-3