Abstract
Introduction
Alzheimer’s disease (AD), a neurodegenerative disorder that progresses from mild cognitive impairment (MCI) to dementia, is responsible for significant burden on caregivers and healthcare systems. In this study, data from the large phase III CLARITY AD trial were used to estimate the societal value of lecanemab plus standard of care (SoC) versus SoC alone against a range of willingness-to-pay (WTP) thresholds from a healthcare and societal perspective in Japan.
Methods
A disease simulation model was used to evaluate the impact of lecanemab on disease progression in early AD based on data from the phase III CLARITY AD trial and published literature. The model used a series of predictive risk equations based on clinical and biomarker data from the Alzheimer’s Disease Neuroimaging Initiative and Assessment of Health Economics in Alzheimer’s Disease II study. The model predicted key patient outcomes, including life years (LYs), quality-adjusted life years (QALYs), and total healthcare and informal costs of patients and caregivers.
Results
Over a lifetime horizon, patients treated with lecanemab plus SoC gained an additional 0.73 LYs compared with SoC alone (8.50 years vs. 7.77 years). Lecanemab, with an average treatment duration of 3.68 years, was found to be associated with a 0.91 increase in patient QALYs and a total increase of 0.96 when accounting for caregiver utility. The estimated value of lecanemab varied according to the WTP thresholds (JPY 5–15 million per QALY gained) and the perspective employed. From the narrow healthcare payer’s perspective, it ranged from JPY 1,331,305 to JPY 3,939,399. From the broader healthcare payer’s perspective, it ranged from JPY 1,636,827 to JPY 4,249,702, while from the societal perspective, it ranged from JPY 1,938,740 to JPY 4,675,818.
Conclusion
The use of lecanemab plus SoC would improve health and humanistic outcomes with reduced economic burden for patients and caregivers with early AD in Japan.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This study is the first to assess the societal value of lecanemab in individuals with early Alzheimer’s disease (AD), their families, and society in Japan. |
Treating individuals with early AD provides substantial societal value from both healthcare payer and societal perspectives, across different willingness-to-pay (WTP) thresholds in Japan. |
Although a broad range of thresholds were considered, a WTP threshold of JPY 15 million per quality-adjusted life year (QALY) appears appropriate for assessing the societal value of lecanemab. This threshold is supported by recent literature and rather conservative given the enormity of the AD burden. |
Understanding the maximum amount society is willing to pay for a new breakthrough treatment for AD is crucial and warrants further research in Japan to allocate healthcare resources properly. |
The estimated lifetime clinical, economic, and societal value of lecanemab provides a foundation for healthcare policy and decision-making in Japan. |
Introduction
Alzheimer’s disease (AD), a neurodegenerative disorder that progresses from mild cognitive impairment (MCI) to dementia, is responsible for approximately 60% of all dementia cases worldwide [1] and 67.6% of dementia cases in Japan [2]. More than 55 million people worldwide have dementia, and this number is projected to reach 74.8 million and 131.5 million by 2030 and 2050, respectively [3, 4]. In Japan, more than five million people had dementia in 2018 [5], and as a result of population aging, the prevalence of dementia among those aged 65 years or older in Japan is anticipated to surpass 25% by 2045 [6].
The clinical manifestation of AD progresses from normal cognition to MCI, followed by dementia stages [7]. As AD advances, the associated cognitive and functional impairments worsen, creating a significant economic burden on healthcare systems, caregivers, and society. This burden has been shown to increase in proportion to the severity of the disease [3, 8]. In Japan, the healthcare costs of patients with MCI are lower than those of patients with mild AD [9]. In 2018, the total healthcare costs of patients with AD, including AD drug costs, were JPY 1073 billion. Additionally, the public long-term care costs for patients with AD, total productivity losses of family caregivers, and informal care costs for caregivers were JPY 4783 billion, JPY 1547 billion, and JPY 6772 billion, respectively [10]. Costs were higher for patients with more severe disease, including long-term care and drug costs [10]. These estimates further emphasize the significant economic burden of AD on caregivers and the healthcare system.
The neuropathological hallmarks of AD include the accumulation of abnormal protein deposits in the brain, namely beta-amyloid (Aβ) plaques and neurofibrillary tangles. The two commonly used biomarkers for diagnosing and monitoring AD are positron emission tomography (PET) imaging and cerebrospinal fluid (CSF) analysis. These biomarkers serve as significant indicators of the presence and advancement of the disease and are essential tools for both diagnosing and monitoring AD development and progression [7, 11]. PET imaging estimates the amount of amyloid and tau in the brain, while CSF analysis measures the soluble biomarkers in the CSF. Disease-modifying therapies (DMTs) that can halt or slow the progression of disease by altering the underlying pathological mechanisms of AD have been gaining increasing attention. Studies are now focusing on developing treatments that target various primary and intermediate mechanisms [12]. Currently, most clinical trials (82%) are investigating agents targeting the main pathological features of AD (i.e., Aβ plaques and neurofibrillary tangles) to modify disease progression [13].
The efficacy of lecanemab, a humanized IgG1 monoclonal antibody targeting amyloid protofibrils, was recently evaluated in a large phase III clinical trial (CLARITY AD; NCT03887455) [14]. The trial involved an 18-month, multicenter, double-blind, placebo-controlled study of lecanemab’s therapeutic potential in patients aged 50–90 years with early AD with the evidence of Aβ pathology confirmed by PET imaging or CSF measurement. The trial results demonstrated that treatment with lecanemab (10 mg/kg every 2 weeks) resulted in significant reductions in brain amyloid levels and slower clinical decline in cognition and function scales (Clinical Dementia Rating Scale-Sum of Boxes [CDR-SB] and Alzheimer’s Disease Composite Score [ADCOMS]) compared to the placebo group, after the 18-month trial duration [14].
Earlier research has examined and reported on the long-term health outcomes and societal value of lecanemab treatment through simulation [15, 16]. The objective of this study was to assess the long-term societal value of lecanemab in early AD in Japan. Data from the large phase III CLARITY AD trial were utilized along with a range of willingness-to-pay (WTP) thresholds, as no specific WTP threshold had been determined in Japan through a survey. However, a benchmark threshold has been established for formal cost-effectiveness evaluations to support decision-making [17].
As a result of the progressive and debilitating nature of AD, which places a significant burden on quality of life, daily function, caregivers, and healthcare systems, higher WTP thresholds may be acceptable for assessing the value of treatment compared to standard thresholds. This research explicitly considered the severity of the disease when determining the WTP thresholds, in accordance with the latest developments in economic evaluation, which suggest a threshold of up to five times the annual per capita consumption in AD [18]. In this study, an evidence-based disease simulation model was employed to compare lecanemab plus standard of care (SoC) vs. SoC alone from a healthcare payer and societal perspective in Japan [19]. The analysis utilized data from the phase III CLARITY AD trial and recently published Japanese-specific literature.
Methods
Model Overview
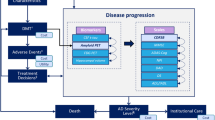
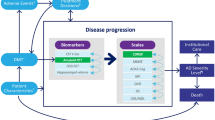
The patient-level AD Archimedes condition-event (AD ACE) simulator was used to estimate the potential impact of lecanemab on disease progression based on data from the CLARITY AD trial. AD ACE was developed according to the International Society of Pharmacoeconomics and Outcomes Research guidelines [20], and a literature review of ongoing clinical trials of AD exploring DMTs and economic modeling AD studies, and has been validated in previous studies. A full description of AD ACE, including the model structure, equations, and validation, is available elsewhere [15, 16, 21, 22].
AD ACE employs a comprehensive approach to estimate the impact of DMTs and interventions on AD progression by considering the intricate interplay between the neuropathological features of AD, such as the levels of Aβ and tau biomarkers, and the clinical manifestations of AD, such as cognitive, behavioral, functional, and dependency deficits measured by patient-level clinical scales [19, 22]. This approach enables a comprehensive assessment of the efficacy of DMTs in slowing down or halting the progression of AD, taking into account the multifaceted nature of the disease.
For patients with early-stage AD, AD ACE uses predictive equations derived from longitudinal data collected by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) [23]. These equations consider the intricate relationships between the clinical features of AD, such as cognitive and functional impairment, and the neuropathological disease features, such as the levels of Aβ and tau, as measured by specific biomarkers. The ADNI data include measurements of CSF Aβ1–42 and total tau (t-tau) protein levels, brain cell metabolic activity assessed by fluorodeoxyglucose (FDG)-PET, and hippocampal volume measured by magnetic resonance imaging (MRI). Although ADNI data can be used to model AD progression, it may not effectively capture the more severe stages of AD. Therefore, for patients with more advanced stages of the disease, AD ACE estimates these relationships on the basis of cognitive and behavioral scales obtained from the Assessment of Health Economics in Alzheimer’s Disease II (AHEAD) study [24, 25]. By making this adjustment, the accuracy of the model is enhanced, and it becomes more representative of the spectrum of disease severity observed in individuals with AD. As patients progress to the moderate stage of AD dementia, indicated by a Mini Mental State Examination (MMSE) score of less than 15, the disease severity reaches a stage of moderately severe to severe AD. At this point, the model shifts from utilizing ADNI-based equations to AHEAD-based equations to capture the full natural trajectory of the disease. Despite being derived from different data sets, this approach allows the model to accurately simulate all stages of AD severity. Moreover, the ADNI and AHEAD equations have been found to offer similar and consistent predictions of disease progression across the spectrum of mild-to-moderate AD [19, 22]. Following the transition from ADNI to AHEAD equations, a thorough evaluation was conducted to ensure that the predicted measures remained consistent and compatible [19, 22]. Moreover, as patients continue to progress to the more severe stages, AD ACE captures the transition from a community care setting to an institutional care setting. The detailed structure and equations of AD ACE have been previously published [15, 16, 22, 26].
The outcomes estimated by AD ACE include direct and indirect outcomes of AD over a lifetime horizon, from either the healthcare payer or societal perspectives. The key health-related outcomes include a patients’ life years (LYs) and quality-adjusted life years (QALYs), while the key economic outcomes include total costs. The costs associated with patients receiving care in both community and institutional settings included both medical and public caregiving costs, from the perspective of the healthcare payer, while only medical costs were considered from a “narrower” healthcare payer’s perspective. Furthermore, informal care costs for caregivers were separately added from the societal perspective. The public medical and caregiving costs are calculated on the basis of the full amount, including the co-payment of 10% to 30% typically borne by the patient. The QALY outcomes are further stratified into patient QALYs, caregiver QALYs loss, and QALY loss caused by adverse events (AEs) such as amyloid-related imaging abnormalities-edema/effusion (ARIA-E). A 2% annual discount rate consistent with the cost-effectiveness recommendations in Japan was applied to all health and economic outcomes [27].
In the base-case analysis, the model population comprised patients with early AD defined as MCI or mild dementia with confirmed Aβ pathology. A total of 260 individual patient profiles from ADNI, meeting the inclusion criteria of the CLARITY AD trial, were selected as the input cohort. The model population consisted of patients aged between 50 and 85 years, an MMSE score of at least 22, and a 1.1 amyloid PET standardized uptake value ratio (SUVr) [28]. The mean baseline characteristics of the patients included in the model were highly similar to those of the patients in the treatment and placebo arms of the CLARITY AD trial (Table 1). To capture the disease trajectory of AD and the treatment effect of lecanemab, 2000 patients were randomly sampled with replacement from the selected 260 ADNI individual patient profiles and simulated separately on the lecanemab plus SoC arm and SoC alone arm using AD ACE. To evaluate the robustness of the results under different assumptions, various scenario analyses were conducted, including alternative stopping rules and dosing regimens. Additionally, to assess the treatment timing effect, patient subsets were defined by their baseline CSF level of t-tau, and the impact of treatment on the neurodegeneration level was estimated.
The CLARITY AD trial was conducted in accordance with the International Council for Harmonization guidelines and the ethical principles of the Declaration of Helsinki. The trial was approved by the institutional review board or independent ethics committee at each center, and all the participants provided written informed consent [14]. The sponsor, Eisai, supplied both the treatment (lecanemab) and placebo in the trial [14]. An independent data and safety monitoring board consisting of experts in Alzheimer’s disease and statistics reviewed unblinded safety data during the trial [14]. An independent medical monitoring team, whose members were unaware of the trial-group assignments, reviewed ARIA, infusion-related reactions, and hypersensitivity reactions. Clinical assessment raters were unaware of the safety assessments and the trial-group assignments [14].
This assessment relies on previously conducted studies and does not involve any new studies with human participants or animals conducted by the authors. The model parameters were primarily informed by published literature or the results of the CLARITY AD trial.
Model Inputs
Clinical Inputs
Disease Progression
The natural progression of AD among patients in the SoC arm was estimated using equations derived from longitudinal patient-level data obtained from ADNI in early stages of AD [23], and from AHEAD in the more severe stages [24, 25]. Clinical Dementia Rating-Sum of Boxes (CDR-SB) thresholds were used in AD ACE to determine the severity of disease in patients at baseline and over time as follows: AD-induced MCI, < 4.5; mild AD, ≥ 4.5 to < 9.5; moderate AD, ≥ 9.5 to < 16; and severe AD, ≥ 16 [29]. Therefore, the proportion of patients with AD-induced MCI in at the start of the AD ACE simulation (i.e., CDR-SB scores < 4.5) should be comparable to that observed at baseline in the CLARITY AD trial (i.e., CDR-Global scores = 0.5).
Mortality
Mortality across all severity levels of AD was calculated by applying hazard ratios (HRs) of age-specific mortality to the natural probability of death due to aging in the general population in Japan [30]. The excess mortality hazard for patients with mild to severe dementia (as defined by their MMSE scale) in the base-case setting was derived from a large multicenter cohort study conducted in Japan (Table 1) [30]. Scenario analyses were conducted to examine alternative HRs based on relevant literature [31, 32].
Institutionalization
The probability of patients transitioning from community care to institutional care based on disease severity was informed by two sources: care need levels reported in national statistics based on public long-term care insurance claim data [33], and a report by Asada et al. on the distribution of care need levels classified by CDR severity level [2]. In a scenario analysis, the risk of institutionalization by AD severity level was estimated using alternative prevalence-based institutional data at each disease severity level [34].
Treatment Effect and Dosing of Lecanemab
The equations utilized by AD ACE to estimate the relationship between disease biomarkers and treatment effect (i.e., patient outcomes) are based on the amyloid PET SUVr level as a predictor. Considering lecanemab is a monoclonal antibody against amyloid protofibrils, it is assumed that the treatment effect is mediated through PET amyloid levels serving as a surrogate endpoint [35, 36]. Based on the estimated amyloid PET SUVr outcomes of a simulated patient, AD ACE can predict the lifetime disease progression trajectory of the simulated patient and estimate the extent to which health-economic outcomes, such as LYs, QALYs, and total costs, are influenced by the treatment effect of lecanemab on the amyloid PET SUVr level.
To ensure AD ACE can accurately estimate the treatment effect based on the amyloid PET SUVr level, a calibration approach was used, as the primary outcome of the CLARITY AD trial was the mean change from baseline in the CDR-SB score at 18 months. The calibration process involved adjusting the treatment effects on amyloid PET SUVr until the model result for CDR-SB matched the target values from the CLARITY AD trial. In AD ACE, the treatment effect is estimated over time; therefore, the amyloid PET SUVr level was calibrated at each time interval such that a calibrated reduction in one time interval would impact the values of the parameters (i.e., amyloid PET SUVr level, CDR-SB score, other AD biomarkers, and AD-related scales) in subsequent time intervals. To appropriately calibrate the model at each time interval, data from the CLARITY AD trial (i.e., change in the amyloid PET SUVr level from baseline) were analyzed to determine the mean amyloid PET SUVr reductions to apply at each time interval. The reductions in amyloid PET SUVr resulted in predictions of CDR-SB that closely matched the changes from baseline in the CDR-SB observed during the first 18 months after treatment initiation in the trial (Fig. 1).
To extrapolate the treatment effect of lecanemab beyond the 18-month duration of the CLARITY AD trial, AD ACE utilized data from a model-based simulation study reporting the continued effect of long-term lecanemab treatment on amyloid PET data [37]. This process continued until the average level of simulated amyloid matched that observed in individuals with normal cognition in the ADNI data set [23]. The cognitively normal mean amyloid level in patients who continued to receive lecanemab was maintained through an additional reduction in their amyloid PET levels. The assumptions underlying the calibration process were validated by clinical experts; only the estimated amyloid PET SUVr levels over time were adjusted by the calibration, without any impact on the default equations used by AD ACE.
For the base-case analysis, it was assumed that patients received lecanemab intravenously at 10 mg/kg every 2 weeks. The CLARITY AD study showed, using a conventional mixed model for repeated measures (MMRM), that the bi-weekly administration of lecanemab 10 mg/kg slowed the clinical decline by 27% on the CDR-SB score after 18 months. As MMRM cannot provide confidence intervals for percent reductions from baseline, the sensitivity analyses assessed the uncertainty in the treatment effect of lecanemab by applying a ± 15% variation, which is consistent with the results across key randomization strata from the CLARITY AD trial [14] and a recent study reporting percent reductions in CDR-SB based on the application of various statistical approaches [38]. Alternative dosing regimens were considered in scenario analyses to determine their impact on treatment effect during the maintenance phase. These scenario analyses were based on a prior model-based simulation study that evaluated the long-term treatment effect of a maintenance dosing regimen beyond 18 months. The study showed less frequent dosing could be used to prevent reaccumulation of amyloid and maintain the treatment effect [37]. In each analysis, the estimated amyloid reductions were recalibrated by adjusting those obtained in the base-case analysis.
Treatment Discontinuation
The risk of discontinuation in the CLARITY AD trial was 18.8% (169 participants) in the lecanemab arm and 15.6% (140 participants) in the placebo arm across the 18-month trial period [14]. Participants from the trial discontinued treatment for various reasons, including loss to follow-up, withdrawn consent, or another unknown reason [14]. In total, 6.9% and 2.9% of the participants in the lecanemab and placebo arms discontinued treatment because of AEs, respectively [14]. For the base-case analysis, we used a 13% annual risk of discontinuation, which was determined on the basis of the observed 18.8% risk over 18 months in the CLARITY AD trial’s lecanemab arm. It was also assumed that progression to a moderate stage of AD would result in treatment discontinuation, which was defined by a CDR-SB score ≥ 9.5. After treatment discontinuation, calibrated amyloid level reductions were no longer applied in subsequent time intervals. Instead, risk equations for natural disease progression were employed to estimate the rate of disease progression based on changes in amyloid levels. As a result, patients experienced a residual benefit from the treatment after discontinuation. Although patients experienced a small residual benefit from the treatment after discontinuation, this benefit gradually decreased over time. Eventually, the level of benefit diminished to the point where patients were in a similar state to those who had not received treatment.
The scenario analyses assessed an annual discontinuation risk of 10% and 20% as well as alternative treatment stopping rules involving a fixed treatment duration of 1.5, 3, and 5 years, respectively.
Adverse Events
In the CLARITY AD trial, there was no significant difference between the lecanemab group and the placebo group in the incidence of death or serious adverse events [14]. Deaths occurred in 0.7% of the trial participants in the lecanemab group and 0.8% of those in the placebo group [14]. No deaths were considered by the investigators to be related to lecanemab or occurred with ARIA. Serious AEs occurred in 14% and 11.3% of the trial participants in the lecanemab group and placebo group, respectively. The most frequently reported serious AEs included infusion reactions (1.2% vs. 0% in the lecanemab arm vs. the placebo arm), ARIA-E (0.8% vs. 0), atrial fibrillation (0.7% vs. 0.3%), syncope (0.7% vs. 0.1%), and angina pectoris (0.7% vs. 0%). The overall incidence of AEs was similar in the two groups. The most common AEs (affecting > 10% of the participants) in the lecanemab group were infusion-related reactions, ARIA with cerebral microhemorrhages, cerebral macrohemorrhages, or superficial siderosis, ARIA-E, headache, and falls. Infusion-related reactions were mild to moderate (grades 1–2), occurred following the first infusion (75%), and resolved following prophylactic treatment. ARIA-E was observed in 113 cases in the lecanemab arm; of these, 25 cases were symptomatic and reported experiencing headache, confusion, and visual disturbance. However, most ARIA-E cases were asymptomatic, occurred primarily within the first 3-month period, and resolved rapidly within 4 months.
In this study, an incidence rate of 12.6% for ARIA-E during the first year was considered based on the CLARITY AD trial. Of those, only 22% were considered symptomatic. Since the frequency of treatment interruptions due to ARIA-E was low in the CLARITY AD trial, it was assumed that ARIA-E AEs did not typically result in treatment discontinuation.
Cost Inputs
The study evaluated costs for patients and their caregivers in both community and institutional care settings, accounting for medical, public caregiving, and informal care costs. Since no single study was able to provide all the cost data required for the analysis, data from multiple sources were gathered in order to construct the model. Medical costs incurred by patients with MCI in community care were informed by a claims database analysis for patients with MCI in Japan [9], while medical costs related to patients in mild to severe AD stages were extracted from a cost-effectiveness analysis study [10, 39]. Medical costs for patients in community and institutional care settings were assumed to be identical. The costs of public caregiving for patients in community and institutional care settings were estimated from the Ministry of Health, Labor, and Welfare (MHLW) report [33] supplemented by published literature [2]. Informal costs of caregivers (by disease-severity) in community care locations were estimated from published literature [2, 40], while no informal costs in the institutional care settings were considered in the analysis.
Patients receiving lecanemab incurred additional costs, including fixed administration costs per visit, as well as monitoring costs assumed to be the equivalent of the cost of two MRI scans in the first year. The unit cost per administration and cost per MRI were obtained from the National Health Insurance (NHI) medical fee database [41]. The cost of using symptomatic treatments, cholinesterase inhibitors and memantine, were included in the analysis. The total cost was estimated considering the distribution of symptomatic patients [42] using cholinesterase inhibitors and memantine in each disease stage and their respective unit costs [42]. The study also incorporated diagnostic and screening costs, such as CSF and PET scans. The cost data were obtained from the NHI medical fee database [41] and the reimbursement proposal submitted by an academic society to MHLW [41, 43], as PET scans are currently not reimbursed in the NHI system. The total cost associated with diagnostic tests was calculated as a weighted average assuming equal weights for CSF and PET scans.
Costs associated with AEs were stratified on the basis of the symptomology of the patients. The cost for symptomatic ARIA-E events was estimated assuming an average cost of receiving three IV steroid infusions (500 mg methylprednisolone per day), either in an inpatient or outpatient setting, followed by a 7-day course of oral steroids (25 mg prednisolone per day) in an outpatient setting [41]. There were no costs associated with asymptomatic ARIA-E events.
Table 2 provides a summary of the cost categories included in the analysis.
Utilities
Patient utilities for Japan were stratified by disease severity and care setting (community versus institutional). In community and institutional care settings, patients utilities were estimated from a cross-sectional study that collected EQ-5D-5L data from elderly patients admitted to nursing homes or residential facilities in Japan [44], and supplemented by a cost-effectiveness analysis study for mild to moderate AD [24]. In the scenario analyses, alternative values for patient utilities based on the CDR-Global scores obtained from Neumann et al. [45] and Landeiro et al. [46] were explored. All patients who experienced an ARIA-E event were assigned a disutility value of 0.09 for a period of 12 weeks, which is the same disutility value associated with a mild migraine [47]. Each patient was assumed to have one caregiver with caregiver disutilities obtained from a previous study [48].
Results
Base-Case Analysis
Over a lifetime horizon, patients treated with lecanemab plus SoC gained an additional 0.73 LYs compared with SoC alone (8.50 years vs. 7.77 years). The mean duration of lecanemab treatment was 3.68 years. The treatment was associated with a significant improvement in the patients’ QALYs by 0.91, and when the caregiver utility was considered, the total QALYs increased by 0.96.
The analysis revealed that patients receiving lecanemab plus SoC incurred significantly lower total medical costs, excluding drug acquisition costs, compared to those receiving SoC alone from the narrow healthcare payer’s perspective. Specifically, the reduction was JPY 95,104. When public caregiving costs were considered, the reduction increased to JPY 1,152,772, indicating the substantial cost-saving benefits of lecanemab plus SoC treatment. The cost reduction increased to JPY 1,989,509 compared to SoC alone when caregiver medical and informal care costs were included in the societal perspective. These findings highlight the potential savings associated with the lecanemab plus SoC treatment, not only from the healthcare payer’s perspective but also from a broader societal perspective.
It was shown that lecanemab plus SoC treatment resulted in a significant decrease in per-patient community and residential care costs, leading to a total decrease of JPY 174,719 in patient care costs from the narrow healthcare payer’s perspective. From the healthcare payer’s perspective, the per-patient community care costs decreased by JPY 646,779, and the per-patient residential care costs decreased by JPY 585,608, resulting in a total decrease of JPY 1,232,387 in patient care costs. From the societal perspective, where caregiver informal care costs were considered, the per-patient community care costs decreased by JPY 1,483,516, and the per-patient residential care costs decreased by JPY 585,608, leading to a total decrease of JPY 2,069,124 in care-related costs associated with lecanemab plus SoC treatment.
Treatment with lecanemab plus SoC was associated with an additional cost related to the management of ARIA-E of JPY 973 per patient and additional monitoring costs of JPY 31,553 per patient. When a range of WTP thresholds from JPY 5 to 15 million per QALY gained was considered, the estimated annual value of lecanemab varied from the narrow healthcare payer’s perspective, ranging from JPY 1,331,305 to JPY 3,939,399, from the healthcare payer’s perspective, ranging from JPY 1,636,827 to JPY 4,249,702, and from the societal perspective, ranging from JPY 1,938,740 to JPY 4,675,818. The base-case results are summarized in Table 3.
Scenario Analysis
Scenario analyses explored the impact of alternative population subgroups, time horizons, treatment stopping rules, input sources, and treatment dosing on the model results. Table 4 presents incremental LYs, QALYs, total costs per patient, and the value of lecanemab based on a WTP threshold of JPY 15 million per QALY. Overall, treating the disease at a younger age and earlier stages leads to higher incremental QALYs, lower costs, and greater societal value as it can prevent disease progression for a longer period of time.
In a younger patient group with a mean baseline age of 70, the value of lecanemab increased by 6% in both healthcare payer and societal perspectives. However, when the mean baseline age was raised to 75, the value of lecanemab decreased by 9% and 10% in the healthcare payer and societal perspectives, respectively. Comparing scenarios with and without symptomatic treatment at baseline, it was found that the value of lecanemab slightly decreased when symptomatic drugs were not used. The analysis showed that apolipoprotein E4 (APOE4) carrier status was associated with a decrease in the value of treatment, with a 4% decrease in both perspectives, while non-carriers showed an increase in value by 2% and 3% in the healthcare payer and societal perspectives, respectively.
The model compared different CSF t-tau levels at baseline and found that lower levels were associated with increased value compared to the base case. For patients in the first CSF t-tau quintile at baseline, the value of treatment increased by 16% in the healthcare payer’s perspective and 20% in the societal perspective, while incremental QALYs increased and incremental costs decreased significantly. However, as higher baseline CSF t-tau levels were considered, incremental QALYs declined and incremental costs increased relative to the base case, leading to a decrease in value in both perspectives. When the patient population was restricted to only the fifth CSF t-tau quintile at baseline, the model showed a 17% decrease in value of treatment in the healthcare payer’s perspective and 18% in the societal perspective.
Shorter time horizons were associated with significantly decreased QALYs, increased costs, and decreases in the value of lecanemab treatment in both perspectives. When a time horizon of 5 years was considered, the model estimated a decreased value of 84% in the healthcare payer and 78% in the societal perspective. A time horizon of 10 years was associated with a higher value than the 5-year time horizon but was still lower compared with the base case.
Using lower mortality HRs based on published literature resulted in an increased value of treatment compared to the base case [31, 49]. Additionally, two scenarios using alternative patient utility sources were conducted. The use of utilities from Neumann et al. [49] led to decreased incremental QALYs in both perspectives (0.73 vs. 0.91 [healthcare payer]; 0.77 vs. 0.96 [societal]), resulting in a decreased value of treatment (reduction of 19% for healthcare payer and 27% for societal). On the other hand, the utilities obtained from Landeiro et al. [46], a systematic literature review of health-related quality of life in patients with AD, showed higher QALYs and a less significant decrease in value (reduction of 10% in the payer and 9% in the societal perspective).
Alternative stopping rules showed that incremental QALYs increased with longer time on treatment and was associated with lower incremental costs. The value was more closely aligned with the base case when patients were allowed to stay on treatment for an extended period. In a scenario that tested an additional 5 years of lecanemab treatment, incremental QALYs remained similar to the base case, while incremental costs increased, resulting in a 5% decrease in value from both perspectives. Shorter stopping rules were associated with further decline in value. The study also explored alternative discontinuation rates of 0% and 6.9%, which estimated higher incremental QALYs and lower incremental costs in both scenarios, but with a lower value. Additionally, a scenario that tested lower alternative costs from Nakanishi et al. [50] showed that the value decreased by 6% in the healthcare payer’s perspective and 8% in the societal perspective compared to the base-case results.
The supplementary material presents scenario analyses that investigate the impact of using WTP thresholds of JPY 5, 7.5, and 10 million per QALY on study outcomes.
Sensitivity Analysis
Sensitivity analyses were conducted by varying key model parameters to test their impact on the results. The results of the one-way sensitivity analyses are presented in Fig. 2.
The analysis showed that the discount rate had the most significant impact on the societal value of lecanemab at a WTP threshold of JPY 15 million per QALY. When a discount rate of 0% was applied, the predicted value of lecanemab decreased by 12%, whereas it increased by 14.5% when a discount rate of 4% was applied. Thus, the value of lecanemab exhibited a negative correlation with the discount rate. Additionally, the study found that patient utility for moderate and severe AD and the mortality HR were also negatively associated with the value of lecanemab.
The patient utilities for individuals with mild AD in the community care setting (0.82–0.99) were found to have a substantial impact on the model results, resulting in a change of − 4.6% and 8.6% compared to the base case, respectively. The patient utilities for mild AD, care costs for moderate and severe AD, treatment efficacy, and discontinuation demonstrated a positive correlation with the model results. However, the remaining parameters tested had a minimal effect on the value compared to the base case. The results of the one-way sensitivity analysis are shown in Fig. 2.
The sensitivity analysis results are consistent using a WTP threshold of JPY 5 million per QALY, and these findings are presented in the supplementary material.
Discussion
The value of lecanemab plus SoC compared with SoC alone was evaluated from both the healthcare payer and societal perspectives in Japan using the AD ACE disease simulator. The clinical inputs for the analysis were based on data obtained from the large phase III CLARITY AD trial. The flexibility of the AD ACE model allowed various scenario analyses to explore alternate data input and assumptions, including different treatment stopping rules and dosing regimens, baseline biomarkers, and specific patient subgroups.
The phase III CLARITY AD trial results demonstrate the significant clinical benefits of lecanemab treatment in individuals with early AD. Treatment with lecanemab is expected to clear Aβ plaques, modify disease biomarkers, and slow clinical decline in individuals with early AD [14]. The study found that the treatment had a disease-modifying effect, which was directly linked to the duration of therapy and expanded over time. In this study, however, the treatment effect was assumed to remain constant while patients received lecanemab and throughout the follow-up period. Treatment discontinuation was allowed if patients experienced AEs or progressed to moderate or severe AD dementia. This assumption aligned with the guidance and recommendations of the Alzheimer’s Association working group, which comprises experienced and internationally recognized clinicians and researchers [51]. The group re-evaluated the definition of meaningful benefits or slowing of AD while prioritizing the needs of patients and their families. According to their recommendations, long-term treatment that maintains modest effectiveness levels, as observed in clinical studies, and lasts beyond the typical 18-month phase III Alzheimer’s trial can result in larger, more significant, and noticeable cumulative benefits over time [51]. As the treatment effect of lecanemab increased with time during the 18-month CLARITY AD trial, our modeling assumption may be overly conservative.
In the base-case analysis, a cohort of patients with early-stage AD, including those with MCI due to AD or mild AD dementia, who were treated with lecanemab plus SoC gained 0.91 QALYs (0.96 QALYs for patients and caregivers combined) compared to those receiving SoC alone. Total care costs (excluding drug acquisition costs) from the healthcare payer’s perspective decreased by JPY 1,152,772 (societal, JPY 1,989,509) for those treated with lecanemab plus SoC. As a result of slower disease progression, patients required less intensive care throughout their lives and could stay in their communities for longer before requiring residential care, leading to reductions in residential and community care costs by JPY 585,608 and JPY 646,779 (JPY 1,483,516 from the societal perspective), respectively, for patients treated with lecanemab plus SoC compared to those receiving only SoC alone. When WTP thresholds ranging from JPY 5 to 15 million per QALY gained were applied, the estimated annual value of lecanemab varied between JPY 1,331,305 and JPY 3,939,399 from the narrow healthcare payer’s perspective, between JPY 1,636,827 and JPY 4,249,702 from the healthcare payer’s perspective, and between JPY 1,938,740 and JPY 4,675,818 from the societal perspective. The study’s findings suggest that lecanemab treatment provides substantial societal value, given the significant clinical, economic, humanistic, and social burdens associated with AD. This value was observed from both healthcare payer and societal perspectives, across different WTP thresholds in Japan. Considering that experts in the field advocate for a WTP threshold that is five times higher for severe AD [18] because of its debilitating nature and significant societal costs, the WTP threshold of 15 million per QALY applied in this research appears to be conservative.
The delay in disease progression attributed to lecanemab based on clinical trial data was projected to result in more time spent in earlier stages of AD and in the community rather than in residential care, resulting in greater quality of life for patients and caregivers. This also led to reduced community and residential care costs and presented significant value from the payer and societal perspectives. Ultimately, societal WTP is a critical factor in assessing the value of a new treatment and allocating healthcare resources. However, no existing research in Japan investigates the maximum amount society is willing to pay for a new breakthrough treatment for AD dementia. This is challenging and further study is necessary to determine this factor.
Scenario analyses demonstrated that lecanemab can be most impactful when treatment initiates at a younger age and in earlier stages of disease, as demonstrated by scenarios altering baseline age and CSF t-tau levels. Estimated QALYs gained in the healthcare payer’s perspective ranged from 0.10 to 1.27 and 0.12 to 1.34 in the societal perspective, with the largest gain in patients with MCI due to AD and a mean baseline age of 65 years. Scenario analyses also found that restricting the model population to carriers of APOE4 reduced the value of lecanemab by 4% in both perspectives as the disease began earlier and progressed faster in these patients as a result of increased genetic risk. Overall, longer treatment durations were closely associated with better outcomes for patients and higher payer and societal value, as patients had more time to experience the treatment effect of lecanemab.
One of the strengths of this research is its comprehensive approach to evaluating the societal value of lecanemab in the Japanese healthcare system. The study utilized epidemiological data specific to Japan, including age-specific mortality rates in the general population and excess mortality hazard among patients with AD dementia from a large multicenter cohort study [30], providing a more accurate representation of the burden of disease and mortality rates in Japan. By incorporating these factors into the analysis, the study’s findings can be more directly applied to the Japanese healthcare system, providing important insights for healthcare decision-makers. The inclusion of costs and utility values specific to Japanese subjects further strengthens the study’s analysis. The use of WTP thresholds across a range of values also adds to the study’s robustness. This approach allows for a more comprehensive assessment of the value of lecanemab and enables healthcare decision-makers to weigh the benefits against the costs. Moreover, the study employs the latest published data from the phase III CLARITY AD trial, which is a large, well-designed study that provides a high level of evidence. By utilizing these data, the study provides findings that are more reliable and applicable to real-world scenarios.
Overall, the study’s comprehensive approach, the use of specific data and thresholds, and the integration of the latest evidence are all strengths that contribute to the validity and usefulness of the study’s findings.
However, some limitations need to be addressed. Firstly, the study employs amyloid level as a surrogate endpoint to predict the effect of lecanemab on the key trial outcomes, namely CDR-SB. If this assumption is proven to be unfounded, the outcome estimates in this research could be biased [52]. However, the validity of this assumption was supported by the results of the CLARITY AD trial, which demonstrated that lecanemab significantly reduced brain amyloid levels and slowed cognitive and functional decline in individuals with early AD [14]. There is potential uncertainty around various crucial parameters, such as mortality rates, costs, utility, and institutionalization risk, which may impact the analysis results. Nonetheless, our study includes scenario and sensitivity analyses to minimize prediction uncertainty regarding model outcomes. For some model inputs, such as caregiver disutility, Japan-specific data was not accessible, and therefore, data from other countries were utilized for these analyses. Additionally, longer clinical data and real-world observational study would help validate the current study findings. Finally, while alternative studies using ADNI as the primary data source for disease progression have highlighted the role of regional Aβ and tau deposition in AD and genetic factors underlying the disease, the restrictive inclusion criteria and lack of diversity in ethnocultural cohorts may have limited external validity [53]. Recent studies, however, show similar disease progression profiles among individuals with early AD in the ADNI cohort in North America and the J-ADNI (Japanese Alzheimer’s Disease Neuroimaging Initiative) cohort [54, 55]. This similarity is particularly evident in cognitive, clinical, and functional measures, including changes in CDR-SB, which show almost identical progression profiles in terms of scores and rates of changes [54, 55]. These findings suggest that populations in Japan and North America share comparable characteristics, transcending ethnicity and geography, and that the findings from ADNI can be generalized to the Asian population.
Overall, this research aimed to assess the societal value of lecanemab in individuals with early-stage AD, their families, and society. Although some components of the care burden associated with AD can be quantified, such as healthcare expenditures, others, such as the quality of life, emotional support, and dependence, cannot be easily measured. These elements are essential to patient management and represent a significant challenge to determining their true value [56]. Therefore, a comprehensive approach is necessary to evaluate the full societal value of lecanemab.
Conclusion
The analysis suggested that the use of lecanemab plus SoC can improve the health and humanistic burden with a lower economic burden for patients and caregivers with early AD in Japan compared with SoC alone. The study demonstrates the potential economic and societal value of lecanemab from a payer and societal perspective for Japan and can be used to help guide healthcare decision-making for AD.
References
Huang L-K, Chao S-P, Hu C-J. Clinical trials of new drugs for Alzheimer disease. J Biomed Sci. 2020;27(1):1–13.
Asada T. Prevalence of dementia and response to life dysfunction due to dementia in urban areas, Health Labour Sciences Research Grants, Dementia Countermeasures Comprehensive Research Project 2011-2012 Research Report. 2013.
Prince MJ, Wimo A, Guerchet MM, Ali GC, Wu Y-T, Prina M. World Alzheimer Report 2015—the global impact of dementia: an analysis of prevalence, incidence, cost and trends. Alzheimer's Disease International, 2015. 84 p. https://www.alzint.org/u/WorldAlzheimerReport2015.pdf. Accessed 15 Mar 2023.
World Health Organization. Dementia 2023. https://www.who.int/en/news-room/fact-sheets/detail/dementia. Accessed 20 Feb 2023.
Fukuda H, Ono R, Maeda M, Murata F. Medical care and long-term care expenditures attributable to Alzheimer’s disease onset: results from the LIFE Study. J Alzheimers Dis. 2021;84(2):807–17.
Nakahori N, Sekine M, Yamada M, Tatsuse T, Kido H, Suzuki M. Future projections of the prevalence of dementia in Japan: results from the Toyama Dementia Survey. BMC Geriatr. 2021;21(1):1–10.
2022 Alzheimer's disease facts and figures. Alzheimers Dement. 2022;18(4):700–89. https://doi.org/10.1002/alz.12638.
Takechi H, Kokuryu A, Kuzuya A, Matsunaga S. Increase in direct social care costs of Alzheimer’s disease in Japan depending on dementia severity. Geriatr Gerontol Int. 2019;19(10):1023–9.
Shibata K, Ariyoshi K, Azuma M, Shiromi K, Hioki S, Ishii M. Challenges of defining healthcare costs for people with mild cognitive impairment (MCI) based on the claim database analysis in Japan. Alzheimers Dement. 2021;17:e055092.
Ikeda S, Mimura M, Ikeda M, Wada-Isoe K, Azuma M, Inoue S, et al. Economic burden of Alzheimer’s disease dementia in Japan. Journal of Alzheimer's Disease. 2021;81(1):309–19.
Frisoni GB. Structural imaging in the clinical diagnosis of Alzheimer’s disease: problems and tools. J Neurol Neurosurg Psychiatry. 2001;70(6):711–8. https://doi.org/10.1136/jnnp.70.6.711.
Cummings J, Fox N. Defining disease modifying therapy for Alzheimer’s disease. J Prev Alzheimers Dis. 2017;4(2):109–15. https://doi.org/10.14283/jpad.2017.12.
Cummings J, Fox N. Defining disease modifying therapy for Alzheimer’s disease. J Prev Alzheimers Dis. 2017;4(2):109.
van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9–21.
Tahami Monfared AA, Tafazzoli A, Ye W, Chavan A, Zhang Q. Long-term health outcomes of lecanemab in patients with early Alzheimer’s disease using simulation modeling. Neurol Ther. 2022;11(2):863–80.
Tahami Monfared AA, Tafazzoli A, Chavan A, Ye W, Zhang Q. The potential economic value of lecanemab in patients with early Alzheimer’s disease using simulation modeling. Neurol Ther. 2022;11(3):1285–307.
Hasegawa M, Komoto S, Shiroiwa T, Fukuda T. Formal implementation of cost-effectiveness evaluations in Japan: a unique health technology assessment system. Value Health. 2020;23(1):43–51. https://doi.org/10.1016/j.jval.2019.10.005.
Lakdawalla DN, Phelps CE. Health technology assessment with risk aversion in health. J Health Econ. 2020;72:102346. https://doi.org/10.1016/j.jhealeco.2020.102346.
Kansal AR, Tafazzoli A, Ishak KJ, Krotneva S, ADNI Collaboration. Alzheimer’s disease Archimedes condition-event simulator: development and validation. Alzheimers Dement (N Y). 2018;4:76–88.
Caro JJ, Briggs AH, Siebert U, Kuntz KM. Modeling good research practices—overview: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force—1. Med Decis Mak. 2012;32(5):667–77.
Tahami Monfared AA, Tafazzoli A, Ye W, Chavan A, Deger KA, Zhang Q. A simulation model to evaluate the potential impact of disease-modifying treatments on burden of illness in Alzheimer’s disease. Neurol Ther. 2022;11(4):1609–23.
Tafazzoli A, Weng J, Sutton K. Validating simulated cognition trajectories based on ADNI against trajectories from the National Alzheimer’s Coordinating Center (NACC) dataset. 11th Clinical Trial on Alzheimer’s Disease (CTAD). 2018.
Alzheimer's Disease Neuroimaging Initiative. 2017. https://adni.loni.usc.edu/. Accessed June 2021.
Getsios D, Blume S, Ishak KJ, Maclaine GD. Cost effectiveness of donepezil in the treatment of mild to moderate Alzheimer’s disease. Pharmacoeconomics. 2010;28(5):411–27.
Guo S, Getsios D, Revankar N, et al. Evaluating disease-modifying agents: a simulation framework for Alzheimer’s disease. Pharmacoeconomics. 2014;32(11):1129–39.
Small GW, McDonnell DD, Brooks RL, Papadopoulos G. The impact of symptom severity on the cost of Alzheimer’s disease. J Am Geriatr Soc. 2002;50(2):321–7.
Council. TCSIM, Center for Outcomes Research and Economic Evaluation for Health, National Institute of Public Health (C2H). Guideline for preparing cost-effectiveness evaluation to the Central Social Insurance Medical Council Ver 3.0. 2022. https://c2h.niph.go.jp/tools/guideline/guideline_en.pdf. Accessed Mar 2023.
Swanson CJ, Zhang Y, Dhadda S, et al. A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer’s disease with lecanemab, an anti-Aβ protofibril antibody. Alzheimer’s Res Ther. 2021;13(1):1–14.
O’Bryant SE, Waring SC, Cullum CM, et al. Staging dementia using Clinical Dementia Rating Scale Sum of Boxes scores: a Texas Alzheimer’s Research Consortium study. Arch Neurol. 2008;65(8):1091–5.
Takata Y, Ansai T, Soh I, et al. Cognitive function and 10 year mortality in an 85 year-old community-dwelling population. Clin Interv Aging. 2014;9:1691–9. https://doi.org/10.2147/CIA.S64107.
Wimo A, Handels R, Winblad B, et al. Quantifying and describing the natural history and costs of Alzheimer’s disease and effects of hypothetical interventions. J Alzheimers Dis. 2020;75(3):891–902.
Andersen K, Lolk A, Martinussen T, Kragh-Sørensen P. Very mild to severe dementia and mortality: a 14-year follow-up–the Odense study. Dement Geriatr Cogn Disord. 2010;29(1):61–7.
Ministry of Health Labour and Welfare. Statistics of long-term care benefit expenditures (cases assessed in April 2018). https://www.mhlw.go.jp/toukei/saikin/hw/kaigo/kyufu/2018/04.html. Accessed Mar 15.
Davis M, O’Connell T, Johnson S, et al. Estimating Alzheimer’s disease progression rates from normal cognition through mild cognitive impairment and stages of dementia. Curr Alzheimer Res. 2018;15(8):777–88.
Avgerinos KI, Ferrucci L, Kapogiannis D. Effects of monoclonal antibodies against amyloid-β on clinical and biomarker outcomes and adverse event risks: a systematic review and meta-analysis of phase III RCTs in Alzheimer’s disease. Ageing Res Rev. 2021;68: 101339.
Fletcher E, Filshtein TJ, Harvey D, et al. Staging of amyloid β, t-tau, regional atrophy rates, and cognitive change in a nondemented cohort: results of serial mediation analyses. Alzheimers Dement (Amst). 2018;10:382–93.
McDade E, Cummings JL, Dhadda S, et al. Lecanemab in patients with early Alzheimer’s disease: detailed results on biomarker, cognitive, and clinical effects from the randomized and open-label extension of the phase 2 proof-of-concept study. Alzheimers Res Ther. 2022;14(1):191.
Dhadda S, Kanekiyo M, Li D, et al. Consistency of efficacy results across various clinical measures and statistical methods in the lecanemab phase 2 trial of early Alzheimer’s disease. Alzheimers Res Ther. 2022;14(1):182. https://doi.org/10.1186/s13195-022-01129-x.
Kitamura S, Inoue S, Matsui D, Matsushita Y. Cost-effectiveness analysis for memantine monotherapy in patients with moderate and severe Alzheimer’s disease. Jpn J Geriatr Psychiatry. 2014;25:1361–73.
Sado M, Ninomiya A, Shikimoto R, et al. The estimated cost of dementia in Japan, the most aged society in the world. PLoS ONE. 2018;13(11):e0206508. https://doi.org/10.1371/journal.pone.0206508.
Ministry of Health, Labour and Welfare (MHLW). National Health Insurance (NHI) Medical fee. 2022. Medical Service Fees. https://shirobon.net/medicalfee/latest/ika/r04_ika/, in Japanese. Accessed 15 Mar 2023.
Medical Data Vision. Company website. Analysis conducted by Eisai using MDV analyzer 2019 dataset 2020. https://en.mdv.co.jp/service/web-tools/. Accessed July 28.
Social Insurance Union of Societies Related to Internal Medicine. Proposal for the revision of National Health Insurance (NHI) reimbursement for 2022. https://fa.kyorin.co.jp/naihoren/shinryohoshu2022/suggestion2022_p322.pdf, in Japanese, Accessed 15 Mar 2023.
Ashizawa T, Igarashi A, Sakata Y, et al. Impact of the severity of Alzheimer’s disease on the quality of life, activities of daily living, and caregiving costs for institutionalized patients on anti-Alzheimer medications in Japan. J Alzheimers Dis. 2021;81(1):367–74. https://doi.org/10.3233/JAD-201514.
Neumann PJ, Kuntz KM, Leon J, et al. Health utilities in Alzheimer’s disease: a cross-sectional study of patients and caregivers. Med Care. 1999;37:27–32.
Landeiro F, Mughal S, Walsh K, et al. Health-related quality of life in people with predementia Alzheimer’s disease, mild cognitive impairment or dementia measured with preference-based instruments: a systematic literature review. Alzheimers Res Ther. 2020;12(1):1–14.
Igarashi H, Ueda K, Jung S, Cai Z, Chen Y, Nakamura T. Social burden of people with the migraine diagnosis in Japan: evidence from a population-based cross-sectional survey. BMJ Open. 2020;10(11):e038987. https://doi.org/10.1136/bmjopen-2020-038987.
Mesterton J, Wimo A, Langworth S, Winblad B, Jonsson L. Cross sectional observational study on the societal costs of Alzheimer’s disease. Curr Alzheimer Res. 2010;7(4):358–67.
Neumann PJ, Hermann R, Kuntz K, et al. Cost-effectiveness of donepezil in the treatment of mild or moderate Alzheimer’s disease. Neurology. 1999;52(6):1138–45.
Nakanishi M, Igarashi A, Ueda K, et al. Costs and resource use of community-dwelling patients with Alzheimer’s disease in Japan: 18-month results from the GERAS-J study. Curr Med Res Opin. 2021;37(8):1331–9. https://doi.org/10.1080/03007995.2021.1922369.
Petersen RC, Aisen PS, Andrews JS, et al. Expectations and clinical meaningfulness of randomized controlled trials. Alzheimers Dement. 2023. https://doi.org/10.1002/alz.12959.
Hawkins N, Richardson G, Sutton AJ, et al. Surrogates, meta-analysis and cost-effectiveness modelling: a combined analytic approach. Health Econ. 2012;21(6):742–56. https://doi.org/10.1002/hec.1741.
Veitch DP, Weiner MW, Aisen PS, et al. Using the Alzheimer’s Disease Neuroimaging Initiative to improve early detection, diagnosis, and treatment of Alzheimer’s disease. Alzheimers Dement. 2022;18(4):824–57. https://doi.org/10.1002/alz.12422.
Iwatsubo T, Iwata A, Suzuki K, et al. Japanese and North American Alzheimer’s Disease Neuroimaging Initiative studies: harmonization for international trials. Alzheimers Dement. 2018;14:1077–87.
Yagi T, Kanekiyo M, Ito J, et al. Identification of prognostic factors to predict cognitive decline of patients with early Alzheimer’s disease in the Japanese Alzheimer’s Disease Neuroimaging Initiative study. Alzheimers Dement (N Y). 2019;5(1):364–73.
Igarashi A, Ikeda S. Value assessment of new interventions for Alzheimer’s disease dementia in Japan based on literature review and group interview. Expert Rev Pharmacoecon Outcomes Res. 2022;22(8):1163–70. https://doi.org/10.1080/14737167.2022.2118113.
Ministry of Health, Labour, and Welfare. What's new 2018. https://www.mhlw.go.jp/english/new-info/2018.html. Accessed 15 Mar 2023.
Acknowledgements
We would like to express our gratitude to the trial participants and their families for their generous contributions to this study. Their invaluable support made this research possible.
Funding
Eisai Inc. provided funding for both the study and the journal’s Rapid Service Fee.
Medical Writing and Editorial Assistance
The authors affirm that no medical writing was used in the development of this manuscript.
Author Contributions
Amir Abbas Tahami Monfared and Quanwu Zhang were responsible for the initial study concept and overall study direction and planning. Henri Folse, Ameya Chavan, and Aditya Sardesai contributed to the study concept and design. Weicheng Ye was responsible for developing the model and conducting the analysis. Ataru Igarashi, Mie Kasai Azuma, and Kiyoyuki Tomita provided country-specific data inputs and contextual considerations with respect to the healthcare system in Japan. The manuscript was drafted or revised with contributions from all authors, who consequently read and approved the final version.
Disclosures
Ataru Igarashi received consultant fee from Eisai Co., Ltd. Mie Kasai Azuma and Kiyoyuki Tomita are employees of Eisai Co., Ltd. Quanwu Zhang is an employee of Eisai Inc. Weicheng Ye, Aditya Sardesai, Henri Folse, and Ameya Chavan are current employees of Evidera, a healthcare research firm that provides consulting and other research services to pharmaceutical, device, government, and non-government organizations. Eisai Inc. provided funding to Evidera for conducting the analysis and preparing the manuscript. Amir Abbas Tahami Monfared is an employee of Eisai Inc. He serves as Associate Editor for the Journal of Alzheimer’s Disease and did not receive any fees or honoraria.
Compliance with Ethics Guidelines
This assessment is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. The CLARITY AD trial (ClinicalTrials.gov identifier, NCT03887455) was conducted in accordance with the International Council for Harmonization guidelines and the ethical principles of the Declaration of Helsinki. The trial was approved by the institutional review board or independent ethics committee at each center, and all the participants provided written informed consent. An independent data and safety monitoring board consisting of experts in Alzheimer’s disease and statistics reviewed unblinded safety data during the trial. An independent medical monitoring team, whose members were unaware of the trial-group assignments, reviewed ARIA, infusion-related reactions, and hypersensitivity reactions. Clinical assessment raters were unaware of the safety assessments and the trial-group assignments.
Data Availability
All data generated or analyzed during this study are included in this published article and the supplementary material. Additional details are available from the corresponding author upon request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Igarashi, A., Azuma, M.K., Zhang, Q. et al. Predicting the Societal Value of Lecanemab in Early Alzheimer’s Disease in Japan: A Patient-Level Simulation. Neurol Ther 12, 1133–1157 (2023). https://doi.org/10.1007/s40120-023-00492-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-023-00492-7