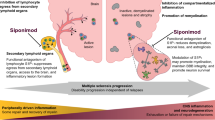
Abstract
Introduction
Siponimod, a potent and selective sphingosine-1-phosphate (S1P1,5) agonist, is the only therapeutic agent that has shown efficacy against disability progression, decline in cognitive processing speed, total brain volume loss, gray matter atrophy and signs of demyelination in patients with secondary progressive multiple sclerosis (SPMS). Although the pathophysiology of progression in SPMS and primary progressive MS (PPMS) is thought to be similar, fingolimod, the prototype S1P1,3,45 agonist, failed to show efficacy against disability progression in PPMS. Differentiating siponimod from fingolimod at the level of their central effects is believed to be the key to a better understanding of the underlying characteristics that could make siponimod uniquely efficacious in progressive MS (PMS).
Methods
Here, we compared the central vs. peripheral dose-dependent drug exposures for siponimod and fingolimod in healthy mice and mice with experimental autoimmune encephalomyelitis (EAE).
Results
Siponimod treatment achieved dose-dependent efficacy and dose-proportional increases in steady-state drug blood levels, with a central nervous system (CNS)/blood drug-exposure ratio (CNS/bloodDER) of ~ 6 in both healthy and EAE mice. In contrast, fingolimod treatments achieved dose-proportional increases in fingolimod and fingolimod-phosphate blood levels, with respective CNS/bloodDER that were markedly increased (≥ threefold) in EAE vs. healthy mice.
Conclusion
If proven to have translational value, these observations would suggest that CNS/bloodDER may be a key differentiator for siponimod over fingolimod for clinical efficacy in PMS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Two members of the sphingosine-1-phosphate receptor (S1P) agonist class have had divergent effects in clinical studies of progressive multiple sclerosis (MS) |
We used animal models to assess the potential mechanisms that might underlie why siponimod reached its primary endpoint (disability accrual) in patients with secondary progressive MS (SPMS) but fingolimod did not meet a similar primary endpoint in patients with primary progressive MS (PPMS) |
What was learned from the study? |
Previous pre-clinical studies have shown that potentially MS relevant CNS-dependent mechanisms show a bell-shaped dose-response curve to S1P agonists |
Siponimod CNS penetration is similar in normal mice and in mice with experimental autoimmune encephalomyelitis (EAE)—an experimentally induced MS-relevant pathology; in strong contrast, the CNS penetration of fingolimod/fingolimod-P is markedly (up to eightfold) increased by the development of EAE |
Although factors such as patient disease characteristics may play a role, the differential CNS penetration in the face of MS-relevant pathology may be an important differentiator for siponimod vs. fingolimod/fingolimod-P which, if translated to the clinic, could help explain the differential therapeutic efficacy of S1P modulators in progressive MS |
Introduction
Siponimod, a potent and selective agonist for the sphingosine-1-phosphate (S1P)-receptor subtypes 1 and 5 (S1P1,5), is approved as an oral disease-modifying therapy (DMT) for the treatment of secondary progressive multiple sclerosis (SPMS) in adult patients with active disease (Europe) [1] and relapsing forms of multiple sclerosis (MS), including clinically isolated syndrome, relapsing–remitting MS (RRMS) and active SPMS in adults (USA) [2]. To date, siponimod is the only DMT that has shown efficacy on slowing disability progression, decline in cognitive processing speed, total brain volume loss, gray matter atrophy and signs of demyelination in patients with SPMS [3,4,5,6,7].
The scientific concept linking S1P receptors to RRMS is now well established and originates from the discovery and development of fingolimod that, upon phosphorylation to fingolimod-phosphate (fingolimod-P), is an S1P1,3,4,5 agonist [8]. As with other SIP modulators, fingolimod is a potent anti-inflammatory agent, and clinical trials of S1P modulators show profound beneficial effects on clinical and magnetic resonance imaging (MRI) measures of MS disease activity and disability progression [9]. Fingolimod was the first S1P modulator approved as an oral agent for the treatment of RRMS, and the therapeutic efficacy of all S1P modulators used to treat MS thus far is believed to be due to their ability to induce sequestration of lymphocytes within secondary lymphoid organs, leading to reduced recirculation of pathogenic lymphocytes into the central nervous system (CNS) [10,11,12]. The underlying molecular mechanisms of this sequestration involve functional antagonism through the internalization and degradation of S1P1 receptors expressed on naive and central memory lymphocytes. This downregulation of S1P1 receptors renders the lymphocytes unresponsive to the S1P gradient responsible for their egress from lymphoid organs [13,14,15,16]. The resulting reduction in lymphocyte counts in blood, which has been seen in all species tested thus far, can be used as a biomarker for pharmacodynamic (PD) effects in humans [14, 17].
In addition to their role in lymphocyte egress, S1P receptors are also expressed on CNS-resident cells, such as oligodendrocytes, astrocytes and microglia [18]. This is of relevance to progressive MS (PMS) where CNS-compartmentalized neuroinflammatory processes have been linked to demyelination, neuroaxonal injury, neurodegeneration and disability progression independent of peripherally driven inflammation [18]. However, in contrast to siponimod, which reduced the risk of 3-month confirmed disability progression by 21% versus placebo in the Phase 3 EXPAND study [7], fingolimod failed to demonstrate significant clinical efficacy in the Phase 3 INFORMS study involving patients with primary progressive MS (PPMS) [19]. To date, no other S1P modulators apart from siponimod and fingolimod have been clinically evaluated in PMS. Hence, understanding the key characteristics that could make siponimod uniquely efficacious for PMS is a major pharmacologic challenge. Experimental observations suggest that both siponimod and fingolimod penetrate the CNS where they can reach S1P receptors on CNS-resident cells and modulate S1P-dependent actions of pathophysiologic relevance [10, 18, 20,21,22,23,24]. This exploratory work also suggests that both molecules share similar in vitro S1P1- and/or S1P5-dependent anti-inflammatory, pro-myelination and neuroprotective properties. The present study investigates the dose-dependent blood and CNS drug levels in healthy mice vs. mice with experimental autoimmune encephalomyelitis (EAE), an experimentally induced MS-relevant pathology [17, 25].
The aim of this study is to generate new insights on the CNS penetration and distribution of siponimod, fingolimod and fingolimod-P that could help explain the different clinical outcomes observed in PMS trials despite similar putative modes of action. The results obtained suggest that the CNS vs. blood drug exposure ratio (CNS/bloodDER) might be a key parameter for explaining the unique central efficacy of siponimod against PMS. Preliminary parts of this work were previously communicated [26, 27].
Methods
Animals
Animal Welfare
In accordance with the Declaration of Helsinki, the welfare of the animals used in these studies was respected. All in vivo studies were performed in accordance with protocols approved by the Cantonal Veterinary Office of Basel and according to regulations defined by the European Community Council Directive for animal protection.
Mice
Adult female C57BL/6J mice (18–19 g; Charles River Switzerland) were group housed and had free access to tap water and standard diet (Maus/Ratte Haltung GLP, KLIBA NAFAG-3890; Provimi Kliba AG, Switzerland). Housing rooms were temperature controlled (22 ± 2 °C) throughout the study period. Animal housing, care and experimental use were conducted according to the Swiss Federal Law for animal protection.
Experimental Protocols
The study measured siponimod, fingolimod and fingolimod-P concentrations, via LC-MS/MS, in blood and brain samples from mice fed with diet loaded with either siponimod or fingolimod at various doses. The use of drug treatments via diet was motivated by animal welfare considerations with the intent of avoiding stressful daily oral gavage. The feasibility of this approach was confirmed by pilot observations [28]. Mice were either healthy or subject to EAE.
-
Healthy mice: After randomization, mice were fed for 5 days with siponimod- or fingolimod-loaded food pellets (prepared as described below) at various doses—0.1, 0.3, 1, 3, 10 or 30 mg drug/kg of food (n = 6 each)—to achieve drug uptakes of about 0.3, 1, 3, 10, 30 and 100 µg/day, respectively (based on a mean daily food uptake of ~ 3 g/day, as estimated during the study). Interim blood samples (~ 50 µl) were collected via saphena vein puncture. At termination, mice were deeply anesthetized with oxygen/isoflurane (97/3, v/v; isoflurane:Forene®, Abbott AG, Switzerland). Terminal blood samples (~ 100 µl) and brains (split into cortex and rest of the brain) were collected for measuring blood lymphocyte counts and drug levels via LC-MS/MS. As mice are known to eat about 70–80% of their food during the night, all terminations were performed early mornings (~ 2 h after start of day light) to ensure that the measured drug levels corresponded to peak drug exposures. Interim blood samples were collected in the evening (prior to the dark period) to measure drug levels that corresponded to trough exposures.
-
EAE mice: EAE was induced in mice as previously described [29]. Briefly, 12-week-old C57BL/6 female mice were randomized into six treatment-groups with comparable body weights (n = 10/group). Siponimod or fingolimod treatments were started on day-0 by introducing siponimod or fingolimod-loaded food pellets at 3, 10 or 30 mg/kg of food (n = 10 each) to achieve drug uptakes of about 10, 30 and 100 µg/day (based on a mean daily food uptake of ~ 3 g/day). On the same day (day-0), mice were immunized under isoflurane anesthesia with recombinant rat MOG protein (MOG28–152 at 6.245 mg/ml; 200 µg/100 µl/mouse via subcutaneous injection on the lower back) emulsified in Complete Freund’s Adjuvant (CFA). Pertussis toxin (100 ng/mouse; Sigma) was administered intraperitoneally on day-0 and day-2. The clinical EAE scores were monitored daily using the well-established scoring system (0: normal appearance; 0.5: distal limp tail; 1: complete tail paralysis; 1.5: hind limb weakness/grid test positive; 2: unilateral partial hind limb paralysis/impaired righting reflex; 2.5: bilateral partial hind limb paralysis; 3: complete bilateral hind limb paralysis; 3.5: fore limb weakness and complete bilateral hind limb paralysis; 4: quadriplegia/moribund; 5: death from EAE). Interim blood samples (~ 50 µl) were collected at various time points, either early in mornings or in evenings, prior to the dark period. All mice were terminated at 4 weeks post-MOG immunization (~ 2 h after start of day light), with terminal blood samples (~ 100 µl) and brain tissues collected for measuring blood lymphocyte counts and siponimod levels via LC-MS/MS.
Preparation of Drug-Loaded Food Pellets
Siponimod hemifumarate or fingolimod (both supplied by Novartis) was loaded to standard diet. The recipe for preparing drug-loaded food pellets has been optimized and validated in house. Briefly, the required amount of drug was added to 300–400 g of dry powdered food (Maus/Ratte Haltung GLP, KLIBA NAFAG-3890; Provimi Kliba AG, Switzerland) and mixed for 10 min using a kitchen mixer. Water was added (50%, w/w) and mixing was continued until a homogeneous dough was obtained, which was then compressed and molded into a dough tube of about 1.8 cm diameter. This tube was cut into 1.5–2-cm-long pellets and dried overnight at 35 ºC in a food dehydrator (Excalibur®). Under these conditions, the pellets obtained were dry, comparable in texture to marketed pellets, and compatible with storage in plastic boxes at room temperature for at least 8 weeks, without showing signs of moisture or mold growth. Random quality control (QC) was performed to assess homogeneity of pellets and stability of the loaded drug over time. Storage times > 8 weeks were not QC-checked, and in fact were excluded. For controls, drug-free pellets were prepared the same way but without drug added.
Drug Treatments via Food-Pellets
Using drug-loaded food pellets, drug intake for each mouse critically depends on their feeding behavior. Hence, monitoring the daily food intake per mouse is key for controlling the success of the drug-treatments. Pellet consumption per mouse cage can easily be monitored by measuring the difference between the weight of pellets distributed in each cage food tray and the weight of pellets left after 2−3 days; in turn, the food intake per mouse can be estimated by dividing the pellet consumption per mouse cage by the number of mice per cage (usually 3–5). Overall, adult healthy mice eat steadily about 3–4 g of food daily, as confirmed in the present study (data not shown). This results in highly reproducible steady-state drug levels established within 2–3 days and lasting until termination, as indicated by pilot work [28]. In EAE mice, pilot observations indicated a severe reduction in food consumption (30–50%) starting at the onset of disease (i.e., 10–12 days post-immunization), most likely due to a reduced access to the pellets in the food trays as a result of the developing hind limb paralysis. An efficacious corrective measure was to ease food access to the paralyzed mice by placing pellets inside the cages.
Analysis of Biologic Samples
Whole-blood lymphocyte counts were measured, from fresh blood samples using the ADVIA 120 Hematology System with MultiSpecies Software (BAYER). In addition, aliquots of blood were stored at − 80 °C until further processing for drug content via LC-MS/MS.
Determination of Drug Concentrations in Blood and Brain Samples by LC-MS/MS
Freshly defrosted brain samples were homogenized on ice with a Wheaton Dounce tissue grinder in 10 mM Tris-hydrochloric acid (Tris–HCl; pH 7.4), 320 mM sucrose and 1 mM EDTA. Protein was precipitated from brain homogenates, and blood samples using a mixture of acetonitrile/methanol/trichloromethane in a 4:3:3 ratio and the supernatants were evaporated to dryness in a SpeedVac concentrator (Thermo Fisher Scientific, Switzerland). The residues were dissolved in methanol containing 5 mM ammonium acetate and 0.2% formic acid. After centrifugation, an aliquot was transferred to HPLC µVials and stored at − 15 °C until analysis. Ten calibration samples (concentration range 0.05–1000 ng/ml) were prepared for each matrix. Three quality control (QC) and recovery control (extract) samples were prepared for each matrix with concentrations of 100 ng/ml for blood and 10 ng/g for brain.
Analyses were performed using an Agilent1290 II UHPLC system, consisting of a binary high-pressure pump with integrated degasser and static mixer, a multisampler and a heated column compartment, coupled to a 6495cQQQ mass spectrometer (Agilent GmBH, Waldbronn, Germany). A MassHunter Workstation was used to control the system and process data.
For separation and detection of siponimod, 2 µl of each sample was injected onto an ZORBAX RRHD Eclipse Plus C18, 2.1 × 50-mm column filled with 1.8-µm particles (Agilent, 959757-902), held at 40 °C. The elution solvents were 0.2% formic acid in water (A) and 0.2% formic acid in acetonitrile (B). For separation a two-step linear gradient from 20 to 50% B within 0.2 min and from 50 to 100% B within 1.7 min was used. The flow was kept constant at 500 µl/min. The column effluent was guided directly to the electrospray Jet Stream source of the triple quadrupole MS with parameters optimized for siponimod. The gas temperature was set to 210 °C at 16 l/min, the nebulizer pressure 25 psi and the sheath gas temperature 350 °C at 12 l/min. Compound and internal standards were detected as their [MH] + ions with the MRM transition 517.3 m/z > 159.0 m/z and 416.2 m/z for siponimod. Data processing was based on compound/internal standard ratio of the extracted ion chromatograms.
For separation and detection of fingolimod and fingolimod-P, 2 µl of each sample was injected onto an Acquity UPLC BEH C18 2.1 × 50 mm column filled with 1.7 µm particles (Waters, 186002350), held at 50 °C. The elution solvents were 5 mM ammonium acetate in water/acetonitrile/methanol 8/1/1 v/v/v, containing 0.02% acetic acid (A) and acetonitrile/methanol 1/1 v/v containing 0.02% acetic acid (B). A linear gradient from 50% B to 100% B within 4.5 min at a flow rate of 200 µl/min was applied. The column effluent was directly guided to the electrospray Jet Stream source of the triple quadrupole MS with parameters optimized for fingolimod and fingolimod-P. The gas temperature was set to 200 °C at 13 l/min, the nebulizer pressure 25 psi and the sheath gas temperature 350 °C at 12 l/min. The fingolimod derivative was detected in positive mode, whereas for fingolimod-P derivative the negative ion mode was selected, using the MRM transitions 434.3 m/z > 255.1 m/z, 374.2 m/z for fingolimod, respectively, 512.2 m/z > 410.3 m/z, 470.3 m/z for fingolimod-P. Data processing was based on compound/internal standard ratio of the extracted ion chromatograms.
For calculation, an external standard method using a not weighted, linear regression was used. Drug concentrations were expressed as ng/ml for liquids and ng/g for solid tissue. The quality parameters of the methods are summarized in Table 1.
Data Analysis
For the EAE study, clinical score comparisons between treatment groups were evaluated daily and compared to vehicle controls. All statistical analyses of clinical score parameters were performed via a Mann-Whitney rank sum test. All statistical analyses were performed using GraphPad Prism version 6 for Windows, (GraphPad Software, USA). Statistical significance was set at p < 0.05.
Results
Siponimod- vs. Fingolimod-Loaded Diet in Healthy Mice
Dose-Proportional Drug Exposures in Blood and CNS
Healthy mice fed with siponimod-loaded diet at 0.1, 0.3, 1, 10 or 30 mg/kg of food exhibited dose-proportional blood levels of approximately 0.006, 0.02, 0.1, 0.5 and 1.4 μM within 2 days, respectively (Fig. 1A). These values are probably indicative of trough levels as the blood samples were collected in the evening. Comparable values, indicative of peak levels, were also measured after 5 days of treatment in blood samples collected early in the morning, suggesting that siponimod blood exposure had already reached its steady state after 2 days. Brain homogenates (both cortex and rest-of-the-brain) had an approximately sixfold higher siponimod dose-proportional level compared to blood levels at steady state, indicating a CNS/bloodDER around 6 (Fig. 1B).
Dose-proportional steady-state siponimod levels in blood/brain of healthy mice. Healthy mice were fed over 5 days with food pellets loaded with siponimod at various concentrations (0.1 to 30 mg per kg of food). Steady-state siponimod concentrations were measured in blood (A) after 2 days post-start of treatment (2 h pre-dark period) and at termination on day 5 (1 h post-dark period). On day 5, siponimod concentrations were also measured in brain tissues (B). Drug concentrations are expressed as nM. All points are mean ± STDV (n = 6/treatment group; not apparent STDVs are smaller than marker size)
Similarly, mice fed with the fingolimod-loaded diet at 0.1, 0.3, 1, 3 and 10 mg/kg of food achieved dose-proportional steady-state blood levels of fingolimod-P within the 0.004, 0.01, 0.04, 0.1 and 0.4 μM ranges, respectively, with ~ twofold lower dose-proportional fingolimod blood levels, i.e., a steady-state fingolimod-P/fingolimod ratio of ~ 2 (Fig. 2A, A’). In the cerebral cortex and rest of the brain, fingolimod-P levels were fourfold higher vs. blood, suggesting a homogeneous CNS/bloodDER around 4 for the active form of the drug. For fingolimod, CNS/bloodDERs of 13 and 6 were observed in the cerebral cortex and rest of the brain, respectively, suggesting major regional differences in drug distribution and/or metabolism (Fig. 2B, B’). Accordingly, the fingolimod-P/fingolimod ratios in the cerebral cortex and rest of the brain were ~ 0.6 and ~ 1.5, respectively, corresponding to 30% and 70% of the extent of fingolimod phosphorylation observed in the blood compartment, respectively.
Dose-proportional steady-state fingolimod and fingolimod-P levels in blood/brain of healthy mice. Healthy mice were fed over 5 days with food pellets loaded with fingolimod at various concentrations (0.1–10 mg per kg of food). Steady-state fingolimod and fingolimod-P concentrations were measured in blood (A/A’) after 2 days post-start of treatment (2 h pre-dark period) and at termination on day 5 (1 h post-dark period). On day 5, fingolimod and fingolimod-P concentrations were also measured in brain tissues (B/B’). Drug concentrations are expressed as nM. All points are mean ± STDV (n = 6/treatment group; not apparent STDV are smaller than the marker size)
Exposure-Dependent Reduction in Blood Lymphocytes
As expected, healthy mice fed over 5 days with siponimod- or fingolimod-loaded diet at various doses exhibited a steady-state drug exposure-dependent reduction in circulating lymphocytes (Fig. 3). A maximal lymphocyte reduction of 75% was achieved with both siponimod and fingolimod food-loading doses > 1 mg/kg and steady-state siponimod and fingolimod-P blood levels > 60 nM and > 35 nM, respectively. The steady-state blood levels required to reduce lymphocyte numbers by 50% (EC50) were estimated to be 18 nM for siponimod (achieved with food-loading dose of 0.3 mg/kg) and 5 nM for fingolimod-P (achieved with fingolimod food-loading dose of 0.1 mg/kg).
Siponimod vs. fingolimod-P blood exposure-dependent reduction in blood lymphocytes. Mean reduction of blood lymphocytes vs. steady-state drug levels observed in healthy mice fed over 5 days with food pellets loaded with either siponimod or fingolimod at various concentrations (0.1–30 mg/kg of food, as indicated beside each point). All points are mean ± STDV (n = 6/treatment group)
Siponimod- vs. Fingolimod-Loaded Diet in EAE Mice
Dose-Proportional Drug Exposures in Blood and CNS
EAE mice fed over 28 days with siponimod-loaded diet at 3, 10 or 30 mg/kg of food exhibited dose-proportional steady-state blood levels within the 0.1, 0.4 and 1.5 μM range, respectively, with a very narrow difference between peak and trough levels (Fig. 4A). These levels, similar to those measured in healthy mice, were maintained over time, indicating that the daily drug intake via food was not impacted by the development of the EAE pathology. At termination, the siponimod levels measured in brain homogenates (cortex and rest-of-the-brain) were dose proportional and indicated a CNS/bloodDER of 6 (Fig. 4B), as seen in healthy mice.
Dose-dependent changes in siponimod levels in blood/brain of EAE mice. Longitudinal changes in siponimod concentrations (expressed as nM) were measured in blood of EAE mice fed with diet loaded with siponimod at 3, 10 or 30 mg/kg of food. Blood samples were collected either mornings (open circle, ~ 2 h post-dark period) or evenings (open triangle, ~ 2 h pre-dark-period), up to termination (A). Reductions in blood lymphocytes (as % from baseline) achieved at termination are indicated (A). Siponimod concentrations in the brain (cortex and rest of the brain, expressed as pmol/g) were also measured at termination (B). All points are mean ± STDV (n = 10/treatment group; when not apparent STDV are smaller than the marker size)
Daily drug intake was also not affected by EAE development in EAE mice fed with the fingolimod-loaded diet. At 3, 10 or 30 mg/kg of food, the fingolimod-P steady-state blood levels were as observed in healthy mice (i.e., within the 0.1, 0.3 and 1.3 μM range, respectively) with associated fingolimod blood levels twofold lower on a dose-proportional basis (Fig. 5A, A’; i.e., a fingolimod-P/fingolimod ratio of ~ 2, as found in healthy mice). In the cerebral cortex, CNS/bloodDERs of 32 and 25 were observed for fingolimod-P and fingolimod, respectively, and corresponding CNS/bloodDERs of 17 and 18 were observed in the rest-of-the-brain (Fig. 5B, B’), suggesting a three to fivefold higher CNS drug exposure compared with healthy mice, with regional differences. Accordingly, the estimated fingolimod-P/fingolimod ratios in the cortex and rest of the brain were ~ 2.5 and ~ 3, respectively, corresponding to levels of fingolimod phosphorylation that were 25% and 50% higher, respectively, compared with the blood compartment, and that were four and twofold higher, respectively, compared with brains from healthy mice.
Dose-dependent changes in fingolimod and fingolimod-P levels in blood/brain of EAE mice. Longitudinal changes in fingolimod and fingolimod-P concentrations (expressed as nM) were measured in blood of EAE mice fed with diet loaded with fingolimod at 3, 10 or 30 mg/kg of food. Blood samples were collected either mornings (open circle, ~ 2 h post-dark period) or evenings (open triangle, ~ 2 h pre-dark-period), up to termination (A/A’). Fingolimod and fingolimod-P concentrations in the brain (cortex and rest of the brain, expressed as pmol/g) were also measured at termination (B/B’). Reductions in blood lymphocytes (as % from baseline) achieved at termination are indicated (A’). All points are mean ± STDV (n = 10/treatment group; when not apparent STDVs are smaller than the marker size)
Prophylactic Efficacy Against the Development of EAE
Siponimod and fingolimod-loaded diet at 3, 10 and 30 mg/kg significantly reduced blood lymphocyte counts by 70–75% (p < 0.05) vs. control diet. As shown in Fig. 6, mice fed siponimod- or fingolimod-loaded diets also demonstrated significantly lower EAE scores vs. mice fed control diet (p < 0.05). Maximal efficacy was observed at the food-loading dose of 10 mg/kg, and increasing the food-loading dose threefold higher to 30 mg/kg did not significantly increase the efficacy against EAE.
Development of EAE Pathology in Mice Affects the CNS/bloodDER of Siponimod and Fingolimod and Fingolimod-P Differently
The dose-dependent correlations between the mean steady-state siponimod, fingolimod and fingolimod-P levels achieved in blood vs. brain homogenates (cortex and rest of the brain) of healthy and EAE mice are compared in Fig. 7. In both siponimod- and fingolimod-fed mice, there was clear dose proportionality of the steady-state levels achieved in blood and CNS. However, Fig. 7A strongly indicates that the development of EAE had no impact on the exposure of siponimod in the blood and CNS exposure vs. that observed in healthy mice (Fig. 7A). This is also demonstrated by the consistent CNS/bloodDER of ~ 6 measured for the cerebral cortex and rest of the brain in both healthy and EAE mice (Fig. 8).
Dose-proportional steady-state drug levels in blood/brain of healthy vs. EAE mice. Correlations between the mean steady-state siponimod (A), fingolimod (B) and fingolimod-P (C) levels achieved in blood vs. brain homogenates (cortex and rest of the brain) of healthy (n = 6/group) and EAE mice (n = 10/group) fed with drug-loaded food pellets at various concentrations. *p < 0.05 vs. healthy mice
In fingolimod-fed mice, the dose-dependent CNS exposures for both fingolimod and fingolimod-P were significantly increased by the development of EAE vs. healthy mice, whereas dose-dependent blood exposure remained similar in EAE compared to healthy mice (Fig. 7B, C). This resulted in marked EAE-dependent increases in CNS/bloodDER of two- to threefold for fingolimod in both cerebral cortex and rest of the brain and of eight to threefold, respectively, for fingolimod-P, further suggesting a particularly high EAE-dependent increase in fingolimod phosphorylation in the cerebral cortex (Fig. 8).
Discussion
The present study suggests that the development of MS-relevant pathology markedly increases, by at least threefold, the CNS exposure of fingolimod and fingolimod-P, but not of siponimod, relative to the drug exposures observed in the blood. It is believed that understanding these results may help to better comprehend the central S1P receptor-dependent mechanisms and perhaps also bring new insights into the reasons why siponimod [7], but not fingolimod [19], achieved clinical efficacy in PMS.
The approach of the present study was to assess the influence of MS-related pathology on the CNS penetration, distribution and subsequent efficacy of siponimod vs. fingolimod. For this, siponimod and fingolimod treatments, via drug-loaded diets, were compared side by side for their dose-dependent changes in central vs. peripheral drug exposures in healthy and EAE mice. As these mice were all females, a gender-dependent confounding factor cannot be excluded. The use of drug-loaded diet was previously shown as a robust alternative to daily oral gavages for achieving dose-proportional steady-state siponimod blood exposures in the mouse [28, 30]. The results from the present study confirm that reproducible dose-proportional increases in steady-state siponimod, fingolimod and fingolimod-P levels in the blood and CNS of both healthy and EAE mice can be achieved through drug-loaded diets. This strongly suggests very low levels of intra- and inter-individual variability in the feeding behavior of healthy mice and also indicates that adding food pellets inside the cages of EAE mice successfully compensated for their reduced access to food trays due to the development of hind limb paralysis. Hence, dose-dependent efficacy could be achieved for both siponimod and fingolimod treatments to reduce circulating lymphocyte counts with in vivo potencies within a similar range (5–18 nM). Also, similar siponimod and fingolimod food-loading doses were required to achieve maximal efficacy for reducing circulating lymphocytes counts (i.e., 1 mg/kg of food) and for reducing the EAE scores vs. controls (i.e., 10 mg/kg of food). Therefore, as expected, siponimod and fingolimod were not noticeably differentiated based on these blood compartment benchmarks.
However, when looking at the CNS compartment benchmarks, major differences between siponimod and fingolimod were observed at the level of CNS penetration, distribution and metabolism. The main findings from the present study relate to the CNS/bloodDER values at steady-state drug exposures in healthy vs. EAE mice. On the one hand, siponimod exhibited a consistent CNS/bloodDER of ~ 6 in the whole brain of both healthy and EAE mice, indicative of a sixfold higher steady-state drug exposure in the CNS vs. blood that was insensitive to the development of EAE. On the other hand, fingolimod displayed more variable CNS/bloodDER values between cortex and rest of the brain of healthy mice (~ 13 and ~ 6, respectively) that were both increased by two- to threefold in EAE mice, indicative of a pronounced regional variability and a two- to threefold EAE-dependent increase in fingolimod steady-state CNS exposure. A marked EAE-dependent increase in fingolimod phosphorylation was also observed, particularly in the cortex, as the CNS/bloodDER values for fingolimod-P in the cortex and rest of the brain were increased by eight- and threefold, respectively. Further evidence supporting an EAE-dependent increase in fingolimod phosphorylation came from the observation that in healthy mice the fingolimod-P/fingolimod ratios measured in cortex and rest of the brain were 70% and 30% lower than in blood, respectively, but in EAE mice were 25% and 50% higher than in blood, respectively.
To our knowledge, this study is the first to report about an EAE-dependent increase in CNS fingolimod and fingolimod-P exposures, although it could be deduced from independent studies reporting a CNS/bloodDER value of ≤ 10 for fingolimod and fingolimod-P in healthy rats [31] and two- to threefold higher values in EAE rats [25]. The reasons for such an EAE-dependent increase in fingolimod and fingolimod-P CNS exposure are unclear. An EAE-dependent reduction in daily drug-intake, due to a reduced feeding behavior of the mice, is most unlikely as it would lead, on the contrary, to lower drug levels in the blood and CNS vs. healthy mice. Changes at the level of drug penetration and distribution throughout the CNS compartment are more probable. They could be the consequence of complex phenomena involving drug lipophilicity, blood-brain barrier (BBB) permeability, pharmacokinetics, in/out transport mechanisms, and drug metabolism [32]. The results from the present study are in favor of an EAE-dependent increase in fingolimod phosphorylation, particularly in the cerebral cortex. This is in line with published work indicating that the enzyme responsible for fingolimod-phosphorylation, i.e., sphingosine kinase-2 (SPHK2) [33, 34] is highly expressed in the cerebral cortext [35] and even overexpressed in response to inflammatory and autoimmune conditions [36]. Another potential option is an EAE-dependent inhibition of specific ATP-binding cassette (ABC) drug-efflux transporters present at the BBB, such as P-glycoprotein (P-gp) and/or multidrug resistance-associated proteins-1 (MRP-1), for which fingolimod was described as a substrate [37, 38] and which are known to be impaired by the development of MS-related pathology [39, 40]. Importantly, whatever the mechanisms leading to the EAE-dependent increase in fingolimod and fingolimod-P CNS exposure, they should be irrelevant in the case of siponimod, as metabolism is not required for its efficacy and it is not a known substrate for active efflux transporters [41].
Conclusion
Overall, the present study suggests that CNS/bloodDER may be considered as a new mechanistic differentiator for siponimod over fingolimod, at least in the EAE mouse model, on top of other differentiators previously identified, such as target selectivity profile, drug metabolism and/or off-target effects. The different target selectivity profile of siponimod vs. fingolimod-P has been well established [17], with siponimod having far lower binding affinities for S1P3 and S1P4 receptors and therefore being expected not to reproduce any potential fingolimod-P-induced S1P3- and S1P4-dependent central effects. However, to date only limited evidence of fingolimod-P-induced S1P3-dependent and pro-inflammatory effects on isolated CNS-resident cells has been described and their biologic relevance is currently unknown [42,43,44,45]. Also well established is the fact that siponimod does not require phosphorylation for on-target efficacy, in contrast to fingolimod [10,11,12, 18], implying that its central effects are not expected to be affected by MS pathology-related changes in local drug-metabolizing enzymatic activity, although evidence on this aspect is lacking. Concerning off-target central effects, several have been described for fingolimod [15, 46], but none has thus far been identified for siponimod.
The limitations of this study include the fact that the clinical relevance of CNS/bloodDER as a differentiation factor between siponimod and fingolimod is currently unknown since EAE models, despite being the most commonly used experimental model to study MS, do not fully reflect MS-related pathologic processes at the level of inflammation, demyelination, axonal loss, gliosis, blood-brain barrier disruption and immune reactions [47, 48]. Available clinical data are currently limited to siponimod and fingolimod drug levels in blood (i.e., 30–40 ng/ml and ≤ 1 ng/ml, respectively) achieved at therapeutic doses (i.e., 2 and 0.5 mg daily, respectively) in healthy volunteers [49,50,51]. New dedicated studies would therefore be required for the comparative longitudinal monitoring of drug levels in CSF and/or brain tissues (most likely via imaging technology) [52, 53] and blood from siponimod- vs. fingolimod-treated PMS patients. Finally, we acknowledge that the different characteristics of the patient populations in the EXPAND [7] and INFORMS [19] trials mean that factors other than pharmacologic profiles may account for the different outcomes observed in these trials. However, we believe the strong evidence for the independent central effects of siponimod, including those on gray matter atrophy and demyelination [3,4,5,6,7], warrants an investigation of any underlying potential mechanisms.
It is, therefore, tempting to make inferences about the potential implications of a clinically validated MS-dependent increase in CNS/bloodDER for fingolimod in contrast to siponimod. In such a case, taken together with recent observations suggesting that the S1P1,5 receptor-dependent central effects (anti-inflammatory, neuroprotective and/or pro-myelination) could follow a non-classical pharmacology characterized by a loss of efficacy at supramaximal drug exposures (i.e., bell-shaped dose–response curves, in contrast to the classical sigmoidal dose-effect relationship on lymphocytes) [54,55,56,57], one could suspect the existence of a CNS/bloodDER upper limit over which S1P modulators start to lose their central efficacy. Hence, a S1P modulator showing an MS-dependent increase in CNS/bloodDER, such as fingolimod, would most likely be more impacted than a molecule with an MS-insensitive CNS/bloodDER, such as siponimod. Preclinical benchmarking with other S1P modulators should help to further assess the differentiation potential of CNS/bloodDER.
References
Mayzent® Summary of Product Characteristics 2020.
Mayzent® US Prescribing Information 2019.
Arnold DL, Cree BAC, Bar-Or A, et al. Magnetization transfer imaging in secondary progressive multiple sclerosis patients treated with siponimod: results from the phase 3 EXPAND study (4037). Neurology. 2020;94:4037.
Arnold DL, Bar-Or A, Cree BAC, et al. Impact of siponimod on myelination as assessed by MTR across SPMS subgroups: post hoc analysis from the EXPAND MRI substudy. Mult Scler J. 2020;26:397–659.
Fox R, Arnold D, Giovannoni G, et al. Siponimod reduces grey matter atrophy in patients with secondary progressive multiple sclerosis: subgroup analyses from the EXPAND study (1130). Neurology. 2020;94:1130.
Fox RJAD, Giovannoni G, Cree BAC, Bar-Or A, Gold R, Benedict RHB, Piani-Meier D, Arnould S, Ritter S, Dahlke F, Karlsson G, Kappos L, Vermersch P. Effect of siponimod on grey matter atrophy in patients with secondary progressive multiple sclerosis: subgroup analyses from the EXPAND study. Eur J Neurol. 2020;27:103–522.
Kappos L, Bar-Or A, Cree BAC, et al. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study. Lancet. 2018;391:1263–73.
Brinkmann V, Billich A, Baumruker T, et al. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov. 2010;9:883–97.
Cohan S, Lucassen E, Smoot K, Brink J, Chen C. Sphingosine-1-phosphate: its pharmacological regulation and the treatment of multiple sclerosis: a review article. Biomedicines. 2020;8:227.
Brinkmann V. FTY720 (fingolimod) in Multiple Sclerosis: therapeutic effects in the immune and the central nervous system. Br J Pharmacol. 2009;158:1173–82.
Brinkmann V, Cyster JG, Hla T. FTY720: sphingosine 1-phosphate receptor-1 in the control of lymphocyte egress and endothelial barrier function. Am J Transplant. 2004;4:1019–25.
Zecri FJ. From natural product to the first oral treatment for multiple sclerosis: the discovery of FTY720 (Gilenya)? Curr Opin Chem Biol. 2016;32:60–6.
Brinkmann V. Sphingosine 1-phosphate receptors in health and disease: mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol Ther. 2007;115:84–105.
Chun J, Giovannoni G, Hunter SF. Sphingosine 1-phosphate receptor modulator therapy for multiple sclerosis: differential downstream receptor signalling and clinical profile effects. Drugs. 2021;81:207–31.
Huwiler A, Zangemeister-Wittke U. The sphingosine 1-phosphate receptor modulator fingolimod as a therapeutic agent: Recent findings and new perspectives. Pharmacol Ther. 2018;185:34–49.
Juif PE, Kraehenbuehl S, Dingemanse J. Clinical pharmacology, efficacy, and safety aspects of sphingosine-1-phosphate receptor modulators. Expert Opin Drug Metab Toxicol. 2016;12:879–95.
Gergely P, Nuesslein-Hildesheim B, Guerini D, et al. The selective sphingosine 1-phosphate receptor modulator BAF312 redirects lymphocyte distribution and has species-specific effects on heart rate. Br J Pharmacol. 2012;167:1035–47.
Cohan SL, Benedict RHB, Cree BAC, DeLuca J, Hua LH, Chun J. The two sides of siponimod: evidence for brain and immune mechanisms in multiple sclerosis. CNS Drugs. 2022;36:703–19.
Lublin F, Miller DH, Freedman MS, et al. Oral fingolimod in primary progressive multiple sclerosis (INFORMS): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2016;387:1075–84.
Behrangi N, Fischbach F, Kipp M. Mechanism of siponimod: anti-inflammatory and neuroprotective mode of action. Cells. 2019;8:24. https://doi.org/10.3390/cells8010024
Bigaud M, Rudolph B, Briard E, et al. Siponimod (BAF312) penetrates, distributes, and acts in the central nervous system: preclinical insights. Mult Scler J Exp Transl Clin. 2021;7:20552173211049170.
Chun J, Kihara Y, Jonnalagadda D, Blaho VA. Fingolimod: lessons learned and new opportunities for treating multiple sclerosis and other disorders. Annu Rev Pharmacol Toxicol. 2019;59:149–70.
Healy LM, Antel JP. Sphingosine-1-phosphate receptors in the central nervous and immune systems. Curr Drug Targets. 2016;17:1841–50.
Hunter SF, Bowen JD, Reder AT. The direct effects of fingolimod in the central nervous system: implications for relapsing multiple sclerosis. CNS Drugs. 2016;30:135–47.
Foster CA, Howard LM, Schweitzer A, et al. Brain penetration of the oral immunomodulatory drug FTY720 and its phosphorylation in the central nervous system during experimental autoimmune encephalomyelitis: consequences for mode of action in multiple sclerosis. J Pharmacol Exp Ther. 2007;323:469–75.
Bigaud M, Perdoux J, Ramseier P, et al. Pharmacokinetic/pharmacodynamic characterization of siponimod (BAF312) in blood versus brain in experimental autoimmune encephalomyelitis mice (P2.2–066). Neurology. 2019;92 (15 Supplement):2.2–066P.
Bigaud M, Tisserand S, Ramseier P, et al. Differentiated pharmacokinetic/pharmacodynamic (PK/PD) profiles for siponimod (BAF312) versus fingolimod. Mult Scler. 2019;25(S2):300–1 (Poster 622).
Dietrich M, Hecker C, Beerli C, et al. Optimising siponimod (BAF312) oral administration for long-term experimental studies in mice. ECTRIMS On Line Library 2018; 229455: ePoster EP1618.
Gentile A, Musella A, Bullitta S, et al. Siponimod (BAF312) prevents synaptic neurodegeneration in experimental multiple sclerosis. J Neuroinflamm. 2016;13:207.
Dietrich M, Hecker C, Martin E, et al. Increased remyelination and proregenerative microglia under siponimod therapy in mechanistic models. Neurol Neuroimmunol Neuroinflamm. 2022;9:e1161.
Meno-Tetang GM, Li H, Mis S, et al. Physiologically based pharmacokinetic modeling of FTY720 (2-amino-2[2-(-4-octylphenyl)ethyl]propane-1,3-diol hydrochloride) in rats after oral and intravenous doses. Drug Metab Dispos. 2006;34:1480–7.
Hammarlund-Udenaes M, Friden M, Syvanen S, Gupta A. On the rate and extent of drug delivery to the brain. Pharm Res. 2008;25:1737–50.
Billich A, Bornancin F, Devay P, Mechtcheriakova D, Urtz N, Baumruker T. Phosphorylation of the immunomodulatory drug FTY720 by sphingosine kinases. J Biol Chem. 2003;278:47408–15.
Zemann B, Kinzel B, Muller M, et al. Sphingosine kinase type 2 is essential for lymphopenia induced by the immunomodulatory drug FTY720. Blood. 2006;107:1454–8.
Blondeau N, Lai Y, Tyndall S, et al. Distribution of sphingosine kinase activity and mRNA in rodent brain. J Neurochem. 2007;103:509–17.
Pyne NJ, Adams DR, Pyne S. Sphingosine kinase 2 in autoimmune/inflammatory disease and the development of sphingosine kinase 2 inhibitors. Trends Pharmacol Sci. 2017;38:581–91.
Cannon RE, Peart JC, Hawkins BT, Campos CR, Miller DS. Targeting blood-brain barrier sphingolipid signaling reduces basal P-glycoprotein activity and improves drug delivery to the brain. Proc Natl Acad Sci USA. 2012;109:15930–5.
Gil-Martins E, Barbosa DJ, Silva V, Remiao F, Silva R. Dysfunction of ABC transporters at the blood-brain barrier: role in neurological disorders. Pharmacol Ther. 2020;213: 107554.
Kooij G, Mizee MR, van Horssen J, et al. Adenosine triphosphate-binding cassette transporters mediate chemokine (C-C motif) ligand 2 secretion from reactive astrocytes: relevance to multiple sclerosis pathogenesis. Brain. 2011;134:555–70.
Kooij G, van Horssen J, de Lange EC, et al. T lymphocytes impair P-glycoprotein function during neuroinflammation. J Autoimmun. 2010;34:416–25.
Bigaud M, Gardin A, Camenisch G et al. Central versus peripheral drug exposure ratio, a key parameter for therapeutic efficacy of S1P receptor modulators in SPMS. Mult Scler J 2021;27:P721.
Chakkour M, Kreydiyyeh S. FTY720P upregulates the Na+/K+ ATPase in HepG2 Cells by activating S1PR3 and inducing PGE2 release. Cell Physiol Biochem. 2019;53:518–31.
Dusaban SS, Chun J, Rosen H, Purcell NH, Brown JH. Sphingosine 1-phosphate receptor 3 and RhoA signaling mediate inflammatory gene expression in astrocytes. J Neuroinflamm. 2017;14:111.
Fischer I, Alliod C, Martinier N, Newcombe J, Brana C, Pouly S. Sphingosine kinase 1 and sphingosine 1-phosphate receptor 3 are functionally upregulated on astrocytes under pro-inflammatory conditions. PLoS One. 2011;6: e23905.
Muller J, von Bernstorff W, Heidecke CD, Schulze T. Differential S1P receptor profiles on M1- and M2-polarized macrophages affect macrophage cytokine production and migration. Biomed Res Int. 2017;2017:7584621.
Zhang L, Wang H. FTY720 in CNS injuries: molecular mechanisms and therapeutic potential. Brain Res Bull. 2020;164:75–82.
Birmpili D, Askar IC, Bigaut K, Bagnard D. The translatability of multiple sclerosis animal models for biomarkers discovery and their clinical use. Int J Mol Sci. 2022;23:11532.
Gharagozloo M, Mace JW, Calabresi PA. Animal models to investigate the effects of inflammation on remyelination in multiple sclerosis. Front Mol Neurosci. 2022;15: 995477.
Shakeri-Nejad K, Aslanis V, Veldandi UK, et al. Effects of therapeutic and supratherapeutic doses of siponimod (BAF312) on cardiac repolarization in healthy subjects. Clin Ther. 2015;37:2489–505.
Park SJ, Felipe CR, Machado PG, et al. Pharmacokinetic/pharmacodynamic relationships of FTY720 in kidney transplant recipients. Braz J Med Biol Res. 2005;38:683–94.
Kovarik JM, Schmouder R, Barilla D, et al. Multiple-dose FTY720: tolerability, pharmacokinetics, and lymphocyte responses in healthy subjects. J Clin Pharmacol. 2004;44:532–7.
Briard E, Rudolph B, Desrayaud S, et al. MS565: a SPECT tracer for evaluating the brain penetration of BAF312 (siponimod). ChemMedChem. 2015;10:1008–18.
Briard E, Orain D, Beerli C, et al. BZM055, an iodinated radiotracer candidate for PET and SPECT imaging of myelin and FTY720 brain distribution. ChemMedChem. 2011;6:667–77.
Bigaud M, Tisserand S, Albrecht P, et al. Siponimod: from understanding mode of action to differentiation versus fingolimod (1536). Neurology. 2020;94:1536.
Bigaud M, Dahlke F, Hach T, et al. Dual mode of action of siponimod in secondary progressive multiple sclerosis: a hypothesis based on the relevance of pharmacological properties. Mult Scler. 2020;26:272 (P0317).
Cuzzocrea S, Doyle T, Campolo M, et al. Sphingosine 1-phosphate receptor subtype 1 as a therapeutic target for brain trauma. J Neurotrauma. 2018;35:1452–66.
Miron VE, Ludwin SK, Darlington PJ, et al. Fingolimod (FTY720) enhances remyelination following demyelination of organotypic cerebellar slices. Am J Pathol. 2010;176:2682–94.
Acknowledgements
The authors are grateful to Daniela Piani-Meier for her scientific and program management support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Novartis Pharma AG, Basel, Switzerland, funded the study and medical writing support for the preparation of this manuscript also funded the journal’s Rapid Service Fee.
Medical Writing and Editorial Assistance
We thank Paul Coyle (Novartis Ireland) for providing medical writing support, which encompassed formatting of the manuscript content, referencing, tables and figures as per the journal guidelines, and incorporating the authors’ revisions and finalizing the draft for submission, all under the direction of the authors.
Author Contributions
Marc Bigaud, Pamela Ramseier, Sarah Tisserand, Meike Lang, Beatrice Urban, Christian Beerli and Goril Karlsson met the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. All authors except Goril Karlsson were involved in generating the study data and were involved in the drafting and review of the manuscript; Goril Karlsson contributed to the study concept and design, and to the interpretation of the results and provided critical review of the manuscript. All authors are responsible for intellectual content and data accuracy.
Prior Presentation
Preliminary parts of this work were previously presented at the American Academy of Neurology Congress, Philadelphia, Pennsylvania, USA, May 2019 [26], and at the ECTRIMS Congress, Stockholm, Sweden, September 2019 [27].
Disclosures
Marc Bigaud, Pamela Ramseier, Sarah Tisserand, Meike Lang, Beatrice Urban, Christian Beerli and Goril Karlsson are employees of Novartis, except for Christian Beerli who has since retired, and all author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Compliance with Ethics Guidelines
In accordance with the Declaration of Helsinki, the welfare of the animals used in these studies was respected. All in vivo studies were performed in accordance with protocols approved by the Cantonal Veterinary Office of Basel and according to regulations defined by the European Community Council Directive for animal protection.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Bigaud, M., Ramseier, P., Tisserand, S. et al. Central Versus Peripheral Drug Exposure Ratio, a Key Differentiator for Siponimod Over Fingolimod?. Neurol Ther 12, 1187–1203 (2023). https://doi.org/10.1007/s40120-023-00487-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-023-00487-4