Abstract
Introduction
Alzheimer’s disease (AD), a progressive neurodegenerative disease, is the main cause of dementia and one of the leading causes of death for elderly people in the USA. Lecanemab is a humanized IgG1 monoclonal antibody targeting amyloid protofibrils for the treatment of early AD [i.e., mild cognitive impairment (MCI) or mild AD dementia]. In a recent 18-month phase III trial, using a double-blind, placebo-controlled design, lecanemab treatment led to reduced brain amyloid burden and significant improvements in cognitive and functional abilities in individuals with early AD.
Methods
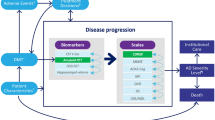
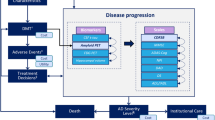
An evidence-based patient-level disease simulation model was updated to estimate the long-term health outcomes of lecanemab plus standard of care (SoC) compared to SoC alone in patients with early AD and evidence of brain amyloid burden, using recent phase III trial data and published literature. The disease progression is described by changes in the underlying biomarkers of AD, including measures of amyloid and tau, and their connection to the clinical presentation of the disease assessed through various patient-level scales of cognition and function.
Results
Lecanemab treatment was estimated to slow the progression of AD to moderate and severe stages and reduce the time spent in these more advanced states. In individuals with early AD, lecanemab plus SoC was associated with a gain of 0.71 quality-adjusted life-years (QALYs), a 2.95-year delay in mean time to progression to AD dementia, a reduction of 0.11 years in institutional care, and an additional 1.07 years in community care as shown in the base-case study. Improved health outcomes were demonstrated with lecanemab treatment when initiated earlier based on age, disease severity, or tau pathology, resulting in estimated gains in QALYs ranging from 0.77 to 1.09 years, compared to 0.4 years in the mild AD dementia subset, as shown by the model.
Conclusion
The study findings demonstrate the potential clinical value of lecanemab for individuals with early AD by slowing down disease progression and prolonging time in earlier stages of disease, which significantly benefits not only patients and caregivers but also society overall.
Trial Registration
ClinicalTrials.gov identifier, NCT03887455.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Alzheimer’s disease (AD) is a neurodegenerative disease that progresses from mild cognitive impairment to mild, moderate, and severe dementia, leading to a significant burden to patients, caregivers, and healthcare systems. |
This study aimed to update the evaluation of lecanemab’s long-term health outcomes using a validated evidence-based disease simulation model and the phase III CLARITY AD trial results in early AD. |
Treatment with lecanemab plus standard of care (SoC) slowed disease progression, extended time in early disease stages, delayed progression to advanced dementia, and improved the quality of life in patients with early AD. |
This study validates our preliminary assessment and provides strong evidence to support the credibility and reliability of our conclusions. |
The results of this study provide healthcare decision makers with a foundation to better understand the potential clinical and socioeconomic value associated with lecanemab. |
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disease that progresses from mild cognitive impairment (MCI) to mild, moderate, and severe dementia [1]. Approximately 60–70% of dementia cases are caused by AD [2], and 6.2 million adults aged 65 years or more currently have AD in the USA. An estimated 13.8 million older adults are projected to have AD by 2060 [3]. In 2019, over 120,000 deaths were attributable to AD, rendering it the sixth-leading cause of death in the USA and the fifth-leading cause of death among those aged 65 years or older [3]. Additionally, care for those living with AD is costly, with an estimated $271.6 billion in unpaid care provided by caregivers and $321 billion in costs related to healthcare, long-term care, and hospital services in 2022 [3].
The diagnosis of AD is based on the presence of the two classic neuropathological hallmarks of AD, namely beta-amyloid (Aβ) plaques and neurofibrillary tangles. Neuroimaging and cerebrospinal fluid (CSF) biomarkers are used to improve diagnostic accuracy and distinguish AD from other dementias. These biomarkers include Aβ, which is correlated with amyloid precursor protein (APP) metabolism and amyloid deposition, total tau (T-tau) which reflects neurodegeneration, and phosphorylated tau, which reflects tangle pathology measurement; they are evaluated using positron emission tomography (PET) and lumbar puncture [3, 4]. Once a diagnosis of AD is confirmed, the standard of care (SoC) includes pharmacological therapies, such as memantine and cholinesterase inhibitors, that are symptomatic in nature and help counterbalance neurotransmitter disturbance [5, 6]. However, the advent of disease-modifying therapies (DMTs) with the ability to impact the pathophysiological mechanisms underlying AD, i.e., amyloid or tau, could have high clinical value in halting or slowing the progression of AD [7]. Currently, over 120 agents, of which 82.5% are DMTs, are under investigation in over 150 clinical trials of treatments for AD [8]. In June 2021, the US Food and Drug Administration approved aducanumab, a monoclonal antibody targeting Aβ plaques, for the treatment of AD. Aducanumab has been shown to reduce the probability of and delay the transition to dementia and institutionalization [9].
Lecanemab, a humanized IgG1 monoclonal antibody targeting amyloid protofibrils (soluble aggregated Aβ), was investigated in Study 201, a phase IIb study exploring the efficacy and safety of lecanemab in individuals with early AD (i.e., MCI due to AD and mild AD dementia) [10]. More recently, the 18-month, double-blind, placebo-controlled, phase III CLARITY AD trial (ClinicalTrials.gov identifier, NCT03887455) was conducted to investigate lecanemab for the treatment of early AD in individuals aged 50–90 years with evidence of amyloid positivity based on PET or CSF testing. In the CLARITY AD trial, the randomization was stratified based on clinical subgroup (MCI due to AD or mild AD dementia), baseline presence or absence of approved AD symptomatic medication (e.g., acetylcholinesterase inhibitors, memantine, or both), apolipoprotein E (ApoE) ε4 carriers or noncarriers, and geographic region. Compared with those receiving placebo, patients treated with a 10 mg/kg dose of lecanemab every 2 weeks exhibited greater reductions in amyloid levels in the brain and less clinical decline on measures of cognition and function, including the Clinical Dementia Rating Scale-Sum of Boxes (CDR-SB) and AD Composite Score (ADCOMS) scales [11].
The long-term health outcomes of lecanemab were recently evaluated based on the efficacy results of the phase IIb Study 201 [12]. In this study, the objective was to present an updated evaluation of the long-term health impacts of lecanemab using the findings from the large, confirmatory, phase III CLARITY AD trial in early AD. To simulate the clinical efficacy of lecanemab treatment on a cohort of patients with early AD and confirmed brain amyloid burden, the previously published evidence-based disease simulation model [12] was utilized. Individual patient profiles within the model were carefully selected to closely match the baseline characteristics of patients in the CLARITY AD trial who received lecanemab treatment. The findings of this study will serve as a validation of our preliminary assessment conducted at the outset of our research. The thorough analysis and evaluation carried out in this study will provide substantial evidence to support the credibility and reliability of our conclusions. This validation will not only instill greater confidence in the accuracy and effectiveness of our approach but also pave the way for further exploration and development in this area of study.
Methods
Model Structure
To evaluate the effects of lecanemab plus SoC on AD disease progression, the previously published AD Archimedes condition-event (ACE) model [13] was used. The AD ACE is a patient-level simulator capturing the pathophysiology and management of AD by simulating disease progression based on alterations in underlying markers, such as CSF Aβ and T-tau protein levels, brain cell metabolic activity based on fluorodeoxyglucose (FDG)-PET, and hippocampal volume based on magnetic resonance imaging, and their association with the clinical presentation of AD based on patient-level cognitive, behavior, function, and dependence scales. Predictive mixed linear equations are utilized to assess alterations in these outcomes over time. These equations are developed based on longitudinal clinical and biomarker data derived from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) [14]. Further information about these equations can be found in a previous publication [13]. The profiles of 1735 patients with normal cognition, MCI, and mild AD dementia from ADNI, including their baseline characteristics, are incorporated into the AD ACE. This enables a predefined subset of patients with specific characteristics, such as age group or disease severity, to be sampled and simulated to investigate disease progression and treatment effect under various scenarios.
To simulate the more severe stages of AD, AD ACE employs equations based on cognitive and behavioral scales from the Assessment of Health Economics in Alzheimer’s Disease II (AHEAD) study [15, 16]. This adjustment enhances the accuracy of the model and makes it more representative across the entire AD continuum and its varying levels of disease severity. AD ACE switches from the ADNI-based equations to the AHEAD-based equations once a patient reaches the moderate AD stage, i.e., a Mini Mental State Examination (MMSE) score < 15 representing a stage of moderately severe to severe Alzheimer’s. The full details of AD ACE, its equations, and validation of its ability to accurately predict AD-related dementia, institutionalization, and mortality were described in detail in previous publications [12, 13, 17,18,19].
Study Population
The CLARITY AD trial and the base-case population for this modeling effort focused on patients with MCI due to AD and mild AD dementia with confirmed Aβ pathology. Therefore, a population of patients with early AD who shared similar characteristics with the CLARITY AD trial [11] population from the ADNI data set [14] was selected. This included a subset of 260 individual patient profiles from the existing 1735 ADNI patients in the AD ACE simulator. These profiles were matched based on the key trial inclusion criteria, which were age range of 50–90 years, Mini-Mental State Examination ≥ 22, Clinical Dementia Rating (CDR) of 0.5, and amyloid PET standard uptake value ratio (SUVr) level ≥ 1.1 [20]. The mean baseline characteristics of the selected profiles highly resembled those of the placebo and 10 mg/kg biweekly lecanemab groups in the CLARITY AD trial (Table 1). To minimize the variability and produce more reliable and robust model results, the analysis was conducted using eight replications of the 260 selected individual patient profiles from the ADNI. Each replication was simulated separately on both the lecanemab plus SoC arm and the SoC alone arm in order to capture the AD natural history, disease trajectory, and treatment effect. Furthermore, we explored patient subsets based on disease severity, treatment stopping rules, and dosing regimens under different scenarios.
One-way sensitivity analyses were further applied to assess the robustness of the results, and parameter uncertainty was assessed in scenario analyses. The population in the scenario analyses comprised a subset of the 260 ADNI profiles from the base-case analysis, and treatment effect in different stages on AD onset and progression. To further determine the effect of treatment on AD-caused neurodegeneration, profiles were grouped by baseline CSF T-tau level.
Clinical Inputs
Disease Progression
The AD ACE modeled AD progression in patients receiving SoC from early AD (using equations based on longitudinal data from ADNI) [14] to more severe stages of AD (using equations from AHEAD) [15, 16]. AD ACE defined the severity of AD based on the CDR-SB thresholds as follows: < 4.5, MCI; ≥ 4.5 to < 9.5, mild AD; ≥ 9.5 to < 16, moderate AD; and ≥ 16, severe AD [21]. At baseline, the proportion of patients with MCI due to AD in AD ACE (i.e., CDR-SB scores < 4.5) was comparable to that in the CLARITY AD trial (i.e., CDR-Global scores = 0.5).
Mortality
Mortality in all stages of AD was explored by utilizing disease severity-specific hazard ratios (HRs) applied to age-specific all-cause mortality in the general US population [22] to reflect the increase in mortality related to age. The risk of general mortality was assumed to be unchanged by MCI due to AD. Therefore, in the base-case analysis, AD ACE used estimates of the HRs of mortality in patients with mild to severe dementia informed by a previously published cohort study that showed an association between severe AD dementia and reduced survival [23], while the HRs used in the scenario analyses were informed by Wimo et al. [24].
Institutionalization
The probability of transitioning from community care to institutional care according to the severity level of AD was determined using estimates based on data from the Consortium to Establish a Registry for Alzheimer’s disease [25]. The risk of institutionalization varied by the disease severity of AD. On the basis of the available literature and limited impact on the outcomes, patients with MCI caused by AD were assumed to not be at risk of institutionalization.
Treatment Effect and Dosing
In the CLARITY AD trial, the change from the baseline CDR-SB score at 18 months was the primary endpoint. However, in the equations incorporated into the AD ACE, the predictor is a surrogate endpoint, i.e., amyloid PET SUVr, because lecanemab directly impacts the PET amyloid level, which, in turn, impacts other outcomes [26, 27]. Thus, AD ACE models the amyloid PET SUVr in a simulated patient and tracks the progression of AD across a lifetime horizon to determine how DMT-induced changes in amyloid PET SUVr impact health outcomes, including the patient’s life-years (LYs) and quality-adjusted life-years (QALYs). The QALY is a metric used to capture the value of health outcomes, by considering both the quality and length of life attributes. It is often used to help inform healthcare policies and resource allocation decisions by identifying interventions that provide the greatest value. A 3% annual discount rate was applied to the outcomes accumulated over a patient’s lifetime based on prior recommendations for analyses in the USA [28].
Since the primary endpoint in the CLARITY AD trial was the CDR-SB score and AD ACE relies on a surrogate endpoint, a calibration process was applied. The calibration process, which relied on the change in amyloid PET SUVr from baseline in the CLARITY AD trial, involved a simulation of approximately 2000 ADNI profiles (260 patient profiles with eight replications) over a lifetime with no treatment discontinuation. During this process, the treatment effects on amyloid PET SUVr were continuously adjusted until the CDR-SB results produced by AD ACE matched those produced by the CLARITY AD trial. The default AD ACE equations were not impacted by the calibration process, which only calibrated the reductions applied to estimated PET SUVr values over time. Since a lifetime time horizon was applied, calibration to reduce amyloid PET SUVr was performed at each time interval, influencing the predicted values of amyloid PET SUVr, CDR-SB, other biomarkers, and other cognitive and functional scales in the subsequent time intervals.
To extend beyond the time horizon of the CLARITY AD trial and extrapolate the effect of continued lecanemab treatment, amyloid PET data from a previous simulation study [29] investigating the long-term treatment effect of lecanemab were applied in the calibration process. The calibration process using these data was performed until the mean amyloid level observed in those with normal cognition in the ADNI data set was reached. An additional reduction in the amyloid level was applied in patients exhibiting normal cognition who continued treatment with lecanemab, following consultation with clinical experts who validated this assumption.
The process successfully resulted in predicted CDR-SB scores closely matching the change from baseline in CDR-SB scores during the first 18 months of the CLARITY AD trial after the initiation of treatment. Figure 1 shows both the short-term (3 years) and long-term amyloid PET and CDR-SB trajectories in both the lecanemab plus SoC arm and SoC alone arm, revealing that the effects of lecanemab plus SoC on the PET SUVr and CDR-SB values compared with those of SoC alone are consistent between the AD ACE model and CLARITY AD trial.
Amyloid PET SUVr and CDR-SB trajectories. Calibration of treatment effect on amyloid level during and beyond trial time horizon. AD ACE = Alzheimer’s disease Archimedes condition-event, CDR-SB = Clinical Dementia Rating Scale-Sum of Boxes, PET = positron emission tomography, SoC = standard of care, SUVr = standard uptake value ratio
In the base-case scenario, patients in the lecanemab plus SoC arm were assumed to receive lecanemab (10 mg/kg intravenous biweekly), which reduced the CDR-SB from baseline by an estimated 27% according to a conventional mixed model for repeated measures (MMRM). In the sensitivity analyses, a ± 15% variation was applied to assess uncertainty based on a recently published study [30] because MMRM cannot provide confidence intervals demonstrating changes from baseline. This is consistent with the subgroup analysis across key randomization strata in the CLARITY AD trial [11]. In the scenario analyses, the effects of different dosing regimens during a maintenance phase were explored based on outcomes reported in a previous study that explored a long-term maintenance dosing regimen extending beyond the trial period (18 months) and showed that the accumulation of amyloid was prevented and the treatment effect was maintained with less frequent dosing during the long-term maintenance phase [29]. In each dosing scenario, the reduction in PET SUVr at each time interval during the maintenance period was adjusted by the estimated amyloid reduction in the base-case analysis.
Treatment Discontinuation
In the base-case analysis, no treatment discontinuation was assumed to assess the impact of lecanemab on clinical outcomes of patients who were stable on treatment. Scenario analyses explored the impact of an annual treatment discontinuation risk of 13.0% based on the discontinuation observed in the CLARITY AD study. Additionally, treatment was discontinued when patients advanced to the moderate stage of AD, characterized by a CDR-SB score ≥ 9.5, as lecanemab’s efficacy has not been assessed in advanced AD stages (i.e., moderate and severe AD dementia). Despite clinical trials showing benefits of continuous treatment with lecanemab, treatment stopping rules based on fixed durations of treatment of 1.5, 3, and 5 years were explored in scenario analyses. This is consistent with recent findings that demonstrated that the treatment effect of lecanemab was maintained after dosing was discontinued over an average of 24 months in the gap period prior to the Study 201 open-label extension [31].
Following treatment discontinuation, mean amyloid reductions were no longer applied, and any estimated changes in amyloid PET SUVr were calculated based on the risk equations reflecting the natural progression of AD. Therefore, the residual benefit of treatment observed after treatment discontinuation gradually diminished over time until the patients reached a state similar to that observed in patients who were did not receive the treatment.
Adverse Events
In the lecanemab arm and placebo arm of the CLARITY AD trial, the incidence rates of serious adverse events (AEs) were 14% and 11.3%, respectively [11]. The most frequently reported serious AEs included infusion-related reactions, amyloid-related imaging abnormalities (ARIA)-edema/effusion (ARIA-E), atrial fibrillation, syncope, and angina pectoris. The rate of AEs was comparable between the two groups. In the lecanemab group, 6.9% of participants discontinued treatment due to AEs, compared to 2.9% in the placebo group. The most common AEs (affecting more than 10% of the participants) in the lecanemab group were infusion-related reactions (26.4% with lecanemab and 7.4% with placebo); ARIA with cerebral microhemorrhages, cerebral macrohemorrhages, or superficial siderosis (ARIA-H; 17.3% with lecanemab and 9.0% with placebo); ARIA-E (12.6% with lecanemab and 1.7% with placebo); headache (11.1% with lecanemab and 8.1% with placebo); and falls (10.4% with lecanemab and 9.6% with placebo) [11]. The reported infusion reactions were mostly mild to moderate; of these, 75% occurred after the first dose and responded to treatment. Of the 898 individuals receiving lecanemab in the trial, 113 reported ARIA-E AEs and experienced headache, vision problems, and confusion. These events were mainly mild to moderate in nature and occurred during the first 3 months of treatment, but resolved rapidly. Therefore, these events only briefly interrupted treatment in the CLARITY AD trial. On the basis of these data, AD ACE applied an ARIA-E AE occurrence rate of 12.6%, 22% of which were considered symptomatic. No ARIA-AE treatment discontinuation was applied, in line with the observations in the clinical trial.
Utilities
The patient utilities were informed by a previously published systematic literature review [32] of studies defining AD severity based on the MMSE, CDR-Global, AD Assessment Scale-Cognitive Subscale, and Global Deterioration Scale. These studies were subjected to a fixed-effect meta-analysis to obtain the patient utilities. To explore uncertainty, the scenario analyses applied CDR-Global score values derived from Neumann et al. [33]. The estimated utilities (by AD severity level) were applied in both the community care setting and the institutional setting. A 1:1 ratio of patient to caregiver was also considered, and caregiver disutilities were informed by a previous study [34]. A disutility of − 0.14, representing the disutility associated with severe headache, was applied to patients experiencing symptomatic ARIA-E for 12 weeks [35].
Compliance with Ethics Guidelines
The CLARITY AD trial was conducted in accordance with the Declaration of Helsinki and the International Council for Harmonization and Good Clinical Practice guidelines, and was approved by the institutional review board or independent ethics committee at each center. All patients provided written informed consent. An independent interim monitoring committee was responsible for oversight and conduct of the interim analyses and response adaptive randomization design to evaluate the safety routinely and review futility analysis results. This assessment is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Results
Base-Case Results
The base-case analysis investigated the potential long-term health outcomes of lecanemab plus SoC in patients with early AD, over a lifetime horizon. Table 2 presents the outcomes obtained from a lifetime simulation of 2000 patients, sampled from the ADNI individual patient profiles, across various levels of disease severity and care settings. In this study, treatment with lecanemab plus SoC was estimated to slow down the progression of the disease, allowing patients to stay longer in the earlier stages of disease. A 27% reduction in clinical decline, as measured by CDR-SB, among patients treated with lecanemab plus SoC was found to result in an 7.5% decrease in the number of patients progressing to mild AD dementia compared with SoC over a patient’s lifetime. The model also predicted that lecanemab treatment prevented 13.7% and 8.8% of the patients from progressing to moderate and severe AD dementia, respectively. Treatment with lecanemab plus SoC led to a 4.9% decrease in the number of patients requiring institutional care and 42.2% discontinued treatment over the study period.
Patient disposition across different levels of AD severity or mortality over their lifetime is presented in Fig. 2. Patients treated with lecanemab plus SoC were estimated to have a lower lifetime probability of transitioning to AD dementia (i.e., mild, moderate, or severe AD dementia) compared with patients treated with SoC alone, over a lifetime horizon. The use of lecanemab decreased the proportion of patients in the mild AD and more severe health states, with an increase in mean time to reach severe AD. With SoC, patients progressed to mild AD in 2.35 years, which is over two times faster than those on lecanemab (5.06 years). Treatment with lecanemab also delayed the mean time to more severe stages of AD by 2–3 years and showed improvement in the average expected time to institutionalization compared with SoC. The median time to event also increased with lecanemab compared with SoC alone, by 0.842 years to mild AD, 1.862 years to moderate AD, 2.031 years to severe AD, and 0.5767 years to institutional care. The model also estimated the median time to event of those alive, predicting lecanemab to increase the time to mild AD by 0.69 years and to moderate AD by 3.13 years.
When evaluating the total time spent in community and institutional care for all patients, the model predicted that patients treated with SoC spent 5.49 and 0.89 years in each setting, respectively. The use of lecanemab extended time in the community setting by 1.07 years and reduced time in institutional care by 0.11 years. On average, patients with MCI spent more time in community care while those with severe AD stayed longer in institutional care.
Considering a discount rate for health outcomes, the model estimated a total of 6.30 LYs for patients treated with lecanemab plus SoC and 3.68 LYs for those treated with SoC alone. The estimated total discounted QALYs for patients treated with lecanemab were 4.39 years, an increase of 0.71 QALYs over SoC, including an estimated loss of 0.002 QALYs due to ARIA AEs.
Scenario Analysis
The scenario analyses measured the impact of alternative population subsets, model settings, and input sources on the study results. The results of the scenario analyses in terms of the impact on QALYs and mean time to advancing to more severe AD stages are presented in Table 3; other detailed outputs from the model are provided in the Supplementary Material.
In a subset of patients with MCI due to AD, the model estimated similar incremental results, with a mean time to AD dementia of 2.55 versus 2.71 years, longer incremental mean time to moderate AD dementia (3.15 versus 2.95 years), and 0.06 additional QALYs over a lifetime compared with the base-case. Patients with mild AD dementia gained fewer total QALYs (0.31 years less) and on average advanced to moderate AD dementia faster than the base-case where most patients had a confirmed diagnosis of MCI at study baseline. In the scenario when lecanemab treatment was initiated in younger patients (average baseline age 65 years) with early AD, the QALY gain was higher (1.03 versus 0.71 years) over a lifetime, and the time to AD progression was longer (3.01 versus 2.71 years) than in the base-case analysis. In patients with MCI due to AD who are younger (mean baseline age of 65) and earlier in their disease trajectory, an additional 0.38 QALYs gained, and a longer mean time to AD dementia can be observed compared with the base-case.
Patients were further divided into groups based on their baseline CSF T-tau levels, and the results suggested that lecanemab treatment was linked to improved clinical outcomes and greater QALY gains in patients with early stages of tau pathology in the lower quintiles. Analysis of different scenarios with shorter model time horizons of 5 and 10 years showed smaller QALY gains (0.13 and 0.46 years, respectively) and a lower delay in mean time to mild and moderate AD dementia compared with the base-case analysis of a lifetime horizon. Adjusting the discount rate to 0% and 5% resulted in an incremental QALY gain of 0.94 and 0.59 years, respectively.
Alternative sources of mortality and patient utilities in the model were evaluated as scenarios. Using HRs from Wimo et al. [24] produced similar incremental QALY gains and a quicker progression to moderate AD dementia (2.29 years) compared with the base-case (2.95 years). Another scenario, using HRs for patients with MCI derived from Wilson et al. [36], resulted in lower overall gains in QALY and a shorter mean time to advance to moderate AD dementia, but a slower progression to mild AD dementia than in the base-case analysis. The use of patient utilities for various levels of AD severity reported in Neumann et al. [33] caused a 0.06 decrease in incremental QALYs compared with the base-case.
The base-case analysis did not consider treatment discontinuation, so the impact of treatment discontinuation and duration was explored as different scenarios. In all scenarios, patients followed the natural history of the disease once they stopped treatment, except for one where the amyloid reduction achieved over 1.5 years of treatment was maintained over a lifetime after treatment discontinuation. Overall, the results indicated that a shorter treatment duration led to fewer predicted QALY gains and a faster progression to mild and moderate AD dementia. An annual discontinuation rate of 13%, matching the observed discontinuation rate in the CLARITY AD trial, resulted in 4.32 QALYs for patients treated with lecanemab plus SoC versus 4.39 for the base-case—a decline of 0.07—as well as shorter mean times to mild and moderate AD dementia. Sensitivity analysis using annual discontinuation rates of 10% and 20% showed similar results to the discontinuation rate observed in the trial, with slightly lower QALYs and shorter times to disease progression compared with the base-case analysis.
Discussion
This study used the AD ACE disease simulator to evaluate the long-term health benefits of treatment with lecanemab plus SoC versus SoC alone, based on the results of the large, confirmatory, phase III CLARITY AD trial [11]. The flexible framework of AD ACE allowed the assessment of different patient characteristics to account for patient and disease heterogeneity, as well as alternative data sources to explore uncertainties and demonstrate robustness of the results in this study.
The CLARITY AD trial presents compelling evidence of the clinical benefits of lecanemab. The treatment effectively clears Aβ plaques, alters other disease biomarkers, and slows the rate of clinical decline in individuals with early AD [11]. Furthermore, the observed treatment effect appears to improve with time on therapy, suggesting a disease-modifying effect. However, it is important to note that our study assumed a constant treatment effect during the on-treatment and follow-up periods. Given the observed expansion of the treatment effect with time on therapy in the CLARITY AD trial, it is possible that our modeling assumption was overly conservative.
The base-case results indicated that, for amyloid-positive patients with MCI due to AD or mild AD dementia, lecanemab plus SoC compared with SoC alone provided a 0.71 additional gain in QALYs, 2.95 years of delay in mean time to progression to AD dementia, 0.11 years reduction in institutional care, and 1.07 additional years in community care. The use of lecanemab was estimated to slow the rate of disease progression, thus prolonging time in earlier stages of the disease and delaying patient progression to moderate and severe AD dementia. The mean time spent in severe stages of AD was also reduced on treatment with lecanemab plus SoC, with fewer patients progressing to later stages of AD during their lifetime. Patients who did not progress, however, were at an increased risk of mortality due to their older age at progression.
Scenario analyses showed that treatment initiation at earlier ages in patients with MCI due to AD and in patients earlier in their tau pathology (first, second, and third quintiles) resulted in significantly improved health outcomes. The estimated gain in QALYs ranged from 0.77 to 1.09 years, compared with 0.4 years in the mild AD dementia subset. These outcomes were consistent with findings from recent studies, which demonstrated that the use of DMTs may be more effective if initiated at earlier stages of dementia [37]. Scenario analyses showed that the study results were dependent on the timeframe of the analysis, as the predicted benefits of lecanemab plus SoC accumulated steadily over time, with better health outcomes for a higher time horizon. Alternate assumptions around treatment stopping rules and discontinuation rate indicated that a longer time on treatment or maintenance of residual effects after treatment discontinuation resulted in improved health outcomes and QALYs gained. This was evident from the lifetime base-case analyses having the highest increase in QALYs of 0.71 years compared with the QALYs gained for alternative treatment discontinuation and stopping rules, ranging from 0.65 to 0.45 years. Selecting alternative data sources to inform the hazard of survival increased incremental QALYs by 0.6–0.75 years. The increase was related to lower HRs reported in Wimo et al. versus the base-case [24]. Excess mortality risk for patients with MCI due to AD resulted in a slightly lower gain in QALYs but an increased delay in mean time to mild AD dementia [36]. Some published studies modeled survival based on baseline patient characteristics and not by disease severity level, which can potentially result in lower survival gains [38, 39].
A key strength of this study was that actual individual patient characteristics were used, rather than mean cohort characteristics, to better capture patient heterogeneity. Disease progression in the model was based on a set of risk equations that explicitly tracked the change in amyloid level and CDR-SB over time. The treatment effect was also modeled by calibrating the reduction in amyloid level to achieve the effects observed during the CLARITY AD trial (Fig. 1); this approach has been published in previous studies [40]. This study updated the previous assessment of long-term health outcomes of lecanemab by incorporating the recently published data from the CLARITY AD trial. To align with the primary outcome of the trial, a calibration process for the treatment effect on amyloid levels was conducted carefully until the treatment effect observed in the model matched the target values from the CLARITY AD study. This process continued beyond the time horizon of the CLARITY AD trial by using another study exploring the effect of continued treatment of lecanemab [31].
There are some potential limitations of the study to highlight. Primarily, the study was based on amyloid PET levels, which served as a surrogate endpoint, assumed to predict the effects of lecanemab on the key trial outcomes, i.e., change in CDR-SB. If the assumption of conditional independence is unwarranted, the estimate of the mean time to more severe AD states and QALY estimates may be biased, leading to an underestimation of uncertainty [41]. The use of a surrogate endpoint that is unreliable could impact future research efforts as well. The results of the phase III CLARITY AD confirmatory trial, however, showed that the use of lecanemab reduced brain amyloid levels and was associated with less decline on clinical measures of cognition and function compared with placebo. Next, while the model was developed using the most robust data sources to inform key parameters, such as the mortality risk, costs, utility, and risk of institutionalization, uncertainty in these parameters continues to exist. Scenario and sensitivity analyses in our study aimed at reducing prediction uncertainty on model outcomes. Furthermore, treatment effects were assessed using both the amyloid level and CDR-SB as the primary outcome, while the behavior, dependence, function, and other domains were not examined. Additionally, disease severity, used to estimate patient utilities and institutionalization risk, was estimated based on CDR-SB. However, this measure of AD global cognition and function has been validated and is widely accepted in literature. Finally, while studies of disease progression using ADNI have highlighted the pivotal role of regional Aβ and tau deposition in AD and have identified genetic factors underlying the disease, the strict inclusion and exclusion criteria and lack of diversity in ethnocultural cohorts may have limited external validity [42]. The key assumptions underlying the model may require further validation based on data derived from clinical trials with long-term follow-up periods and longitudinal real-world evidence. Such evidence will inevitably emerge following the market authorization of lecanemab, enabling the collection of real-world data. In this study, the use of lecanemab was shown to delay AD onset and prolong time in earlier stages of the disease, significantly benefiting not only patients and their caregivers but also society overall.
Conclusion
This study evaluated the long-term health outcomes for individuals with early AD treated with lecanemab. The study showed that treatment with lecanemab plus SoC would result in increased mean survival, greater QALYs gained, and a delay in the onset of AD dementia or the need for institutional care compared with disease management with SoC alone. The strong evidence provided by this study validates our preliminary assessment and reinforces the credibility and reliability of our conclusions. The health outcomes estimated in this study serve as a foundation for healthcare decision- and policymakers to assess the potential clinical, economic, and societal value of lecanemab as a treatment option for patients with early AD.
References
Mank A, van Maurik IS, Rijnhart JJ, et al. Development of multivariable prediction models for institutionalization and mortality in the full spectrum of Alzheimer’s disease. Alzheimers Res Ther. 2022;14(1):1–12.
Huang L-K, Chao S-P, Hu C-J. Clinical trials of new drugs for Alzheimer disease. J Biomed Sci. 2020;27(1):1–13.
Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimers Dement. 2022;18(4):700–89.
Frisoni G. Structural imaging in the clinical diagnosis of Alzheimer’s disease: problems and tools. J Neurol Neurosurg Psychiatry. 2001;70(6):711–8.
Arvanitakis Z, Shah RC, Bennett DA. Diagnosis and management of dementia. JAMA. 2019;322(16):1589–99.
Cummings JL, Tong G, Ballard C. Treatment combinations for Alzheimer’s disease: current and future pharmacotherapy options. J Alzheimers Dis. 2019;67(3):779–94.
Cummings J, Fox N. Defining disease modifying therapy for Alzheimer’s disease. J Prev Alzheimer’s Dis. 2017;4(2):109.
Cummings J, Lee G, Zhong K, Fonseca J, Taghva K. Alzheimer’s disease drug development pipeline: 2021. Alzheimers Dement (N Y). 2021;7(1):e12179.
Herring WL, Gould IG, Fillit H, et al. Predicted lifetime health outcomes for aducanumab in patients with early Alzheimer’s disease. Neurol Ther. 2021;10(2):919–40.
Swanson CJ, Zhang Y, Dhadda S, et al. A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer’s disease with lecanemab, an anti-Aβ protofibril antibody. Alzheimers Res Ther. 2021;13(1):1–14.
van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9–21.
Tahami Monfared AA, Tafazzoli A, Ye W, Chavan A, Zhang Q. Long-term health outcomes of lecanemab in patients with early Alzheimer’s disease using simulation modeling. Neurol Ther. 2022;11(2):863–80.
Kansal AR, Tafazzoli A, Ishak KJ, Krotneva S, Collaboration A. Alzheimer’s disease Archimedes condition-event simulator: development and validation. Alzheimers Dement (N Y). 2018;4:76–88.
Alzheimer’s Disease Neuroimaging Initiative. 2017. https://adni.loni.usc.edu/. Accessed Jun 2021.
Getsios D, Blume S, Ishak KJ, Maclaine GD. Cost effectiveness of donepezil in the treatment of mild to moderate Alzheimer’s disease. Pharmacoeconomics. 2010;28(5):411–27.
Guo S, Getsios D, Revankar N, et al. Evaluating disease-modifying agents: a simulation framework for Alzheimer’s disease. Pharmacoeconomics. 2014;32(11):1129–39.
Tafazzoli A, Weng J, Sutton K. Validating simulated cognition trajectories based on ADNI against trajectories from the National Alzheimer’s Coordinating Center (NACC) dataset of Clinical Trials on Alzheimer’s Disease (CTAD). 2018.
Small GW, McDonnell DD, Brooks RL, Papadopoulos G. The impact of symptom severity on the cost of Alzheimer’s disease. J Am Geriatr Soc. 2002;50(2):321–7.
Tahami Monfared AA, Tafazzoli A, Chavan A, Ye W, Zhang Q. The potential economic value of lecanemab in patients with early Alzheimer’s disease using simulation modeling. Neurol Ther. 2022;11(3):1285–307.
Lynch SY, Irizarry M, Dhadda S, et al. Baseline characteristics for clarity-ad: a phase 3 placebo-controlled, double-blind, parallel-group, 18-month study evaluating BAN2401 in early Alzheimer’s disease (3021). AAN; 2021.
O’Bryant SE, Waring SC, Cullum CM, et al. Staging dementia using clinical dementia rating scale sum of boxes scores: a Texas Alzheimer’s Research Consortium study. Arch Neurol. 2008;65(8):1091–5.
Arias E, Xu J, Kochanek KD. United States life tables, 2016. Natl Vital Stat Rep. 2019;68(4):1–66.
Andersen K, Lolk A, Martinussen T, Kragh-Sorensen P. Very mild to severe dementia and mortality: a 14-year follow-up—the Odense study. Dement Geriatr Cogn Disord. 2010;29(1):61–7.
Wimo A, Handels R, Winblad B, et al. Quantifying and describing the natural history and costs of Alzheimer’s disease and effects of hypothetical interventions. J Alzheimers Dis. 2020;75(3):891–902.
Neumann PJ, Hermann R, Kuntz K, et al. Cost-effectiveness of donepezil in the treatment of mild or moderate Alzheimer’s disease. Neurology. 1999;52(6):1138.
Avgerinos KI, Ferrucci L, Kapogiannis D. Effects of monoclonal antibodies against amyloid-β on clinical and biomarker outcomes and adverse event risks: a systematic review and meta-analysis of phase III RCTs in Alzheimer’s disease. Ageing Res Rev. 2021;68: 101339.
Fletcher E, Filshtein TJ, Harvey D, et al. Staging of amyloid β, t-tau, regional atrophy rates, and cognitive change in a nondemented cohort: results of serial mediation analyses. Alzheimers Dement (Amst). 2018;10:382–93.
Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093–103.
Bateman R, McDade E. Phase 2 lecanemab early Alzheimer’s disease study biomarker results and correlations with clinical outcomes. In: International Conference on Alzheimer’s and Parkinson’s Diseases and Related Neurological Disorder; Barcelona, Spain, 2022.
Dhadda S, Kanekiyo M, Li D, et al. Consistency of efficacy results across various clinical measures and statistical methods in the lecanemab phase 2 trial of early Alzheimer’s disease. Alzheimers Res Ther. 2022;14(1):182.
McDade E, Cummings JL, Dhadda S, et al. Lecanemab in patients with early Alzheimer’s disease: detailed results on biomarker, cognitive, and clinical effects from the randomized and open-label extension of the phase 2 proof-of-concept study. Alzheimers Res Ther. 2022;14(1):191.
Landeiro F, Mughal S, Walsh K, et al. Health-related quality of life in people with predementia Alzheimer’s disease, mild cognitive impairment or dementia measured with preference-based instruments: a systematic literature review. Alzheimers Res Ther. 2020;12(1):154.
Neumann PJ, Kuntz KM, Leon J, et al. Health utilities in Alzheimer’s disease: a cross-sectional study of patients and caregivers. Med Care. 1999;37:27–32.
Mesterton J, Wimo A, Langworth S, Winblad B, Jonsson L. Cross sectional observational study on the societal costs of Alzheimer’s disease. Curr Alzheimer Res. 2010;7(4):358–67.
Xu R, Insinga RP, Golden W, Hu XH. EuroQol (EQ-5D) health utility scores for patients with migraine. Qual Life Res. 2011;20(4):601–8.
Wilson RS, Aggarwal NT, Barnes LL, Bienias JL, Mendes-de-Leon CF, Evans DA. Biracial population study of mortality in mild cognitive impairment and Alzheimer disease. Arch Neurol. 2009;66(6):767–72.
Di Carlo M, Giacomazza D, San Biagio PL. Alzheimer’s disease: biological aspects, therapeutic perspectives and diagnostic tools. J Phys Condens Matter. 2012;24(24): 244102.
Guo S, Getsios D, Revankar N, et al. Evaluating disease-modifying agents: a simulation framework for Alzheimer’s disease. Pharmacoeconomics. 2014;32(11):1129–39.
Ito K, Chapman R, Pearson SD, Tafazzoli A, Yaffe K, Gurwitz JH. Evaluation of the cost-effectiveness of drug treatment for Alzheimer disease in a simulation model that includes caregiver and societal factors. JAMA Netw Open. 2021;4(10): e2129392.
Tahami Monfared AA, Tafazzoli A, Ye W, Chavan A, Zhang Q. Long-term health outcomes of lecanemab in patients with early Alzheimer’s disease using simulation modeling. Neurol Ther. 2022;11(2):863–80.
Hawkins N, Richardson G, Sutton AJ, et al. Surrogates, meta-analysis and cost-effectiveness modelling: a combined analytic approach. Health Econ. 2012;21(6):742–56.
Veitch DP, Weiner MW, Aisen PS, et al. Using the Alzheimer’s disease neuroimaging initiative to improve early detection, diagnosis, and treatment of Alzheimer’s disease. Alzheimers Dement. 2022;18(4):824–57.
Acknowledgements
The authors express their deepest gratitude to the trial participants and their families for their selfless contributions to this study. Their invaluable support was crucial in enabling us to conduct this research. Without their generosity, this study would not have been possible.
Funding
This study and the journal’s Rapid Service Fee was funded by Eisai Inc.
Medical Writing and/or Editorial Assistance
The authors would like to acknowledge Evidera’s medical writer, Ruth Sharf-Williams, for proofreading and editorial services. This was funded by Eisai Inc.
Author Contributions
Amir Abbas Tahami Monfared and Quanwu Zhang contributed to the initial study concept and were responsible for the overall study direction and planning. Henri Folse, Ameya Chavan, and Aditya Sardesai contributed to the study conception and design. Model development and analysis were performed by Weicheng Ye and Elena Aruffo. All authors contributed to the writing and editing of the final manuscript. All authors read and approved the final manuscript.
Disclosures
Amir Abbas Tahami Monfared is an employee of Eisai Inc. He serves as Associate Editor for the Journal of Alzheimer’s Disease and did not receive any fees or honoraria. Quanwu Zhang is an employee of Eisai Inc. Weicheng Ye, Aditya Sardesai, Henri Folse, Ameya Chavan, and Elena Aruffo are current employees of Evidera, a healthcare research firm that provides consulting and other research services to pharmaceutical, device, government, and non-government organizations. Evidera received funding from Eisai Inc. to conduct the study and develop this manuscript.
Compliance with Ethics Guidelines
The CLARITY AD trial (ClinicalTrials.gov identifier, NCT03887455) was conducted in accordance with the Declaration of Helsinki and the International Council for Harmonization and Good Clinical Practice guidelines and was approved by the institutional review board or independent ethics committee at each center. All patients provided written informed consent. An independent interim monitoring committee was responsible for oversight and conduct of the interim analyses and response adaptive randomization design to evaluate the safety routinely and review futility analysis results. This assessment is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
All data used for this study are provided in the manuscript. Additional details are available from the corresponding author on request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Tahami Monfared, A.A., Ye, W., Sardesai, A. et al. A Path to Improved Alzheimer’s Care: Simulating Long-Term Health Outcomes of Lecanemab in Early Alzheimer’s Disease from the CLARITY AD Trial. Neurol Ther 12, 863–881 (2023). https://doi.org/10.1007/s40120-023-00473-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-023-00473-w