Abstract
Based on the results of the pivotal CLARITY study, cladribine tablets were approved for use in the European Union in 2017 as a high-efficacy therapy for highly active relapsing-remitting multiple sclerosis (MS). Cladribine tablets are used as an induction therapy: half of the total dose is given in year 1 and the other half in year 2. In the CLARITY Extension trials, repeating the dose routinely in years 3 and 4, was not associated with significantly improved disease control. However, there is very limited evidence on how to manage people with MS (pwMS) beyond year 4, which is increasingly important because more and more patients are now ≥ 4 years after cladribine treatment. Overall, postapproval data show that treatment with two cladribine cycles effectively controls disease activity in the long term. However, there is general agreement that some pwMS with suboptimal response could benefit from retreatment. This study reviews the practical aspects of using cladribine tablets, summarizes the evidence from clinical trials and real-world studies on the safety and efficacy of cladribine, and proposes a treatment algorithm developed by expert consensus for pwMS previously treated with cladribine. In brief, we propose that additional courses of cladribine tablets should be considered in patients with minimal (no relapses, 1–2 new lesions) or moderate (1 relapse, 3–4 new lesions) disease activity, while significant disease activity (> 1 relapse, > 3 new lesions) or progression should warrant a switch to another high-efficacy treatment (HET). More evidence is needed to improve the treatment guidelines for pwMS who previously received cladribine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The currently approved dose of cladribine tablets is given in two cycles (year 1 and year 2), and routine retreatment in years 3 and 4 does not significantly improve disease control | |
Little evidence is available to provide guidance on how to manage cladribine-treated people with multiple sclerosis (pwMS) beyond year 4 | |
A panel of eight experts in multiple sclerosis used a Delphi approach to develop a treatment algorithm for cladribine-treated pwMS | |
Based on expert consensus, we propose that retreatment with cladribine tablets should be considered in pwMS with minimal or moderate disease activity, while those with significant disease activity should be switched to another highly active disease-modifying agent |
Introduction
Multiple sclerosis (MS) is the most common chronic inflammatory disease of the central nervous system, affecting 2.8 million people worldwide [1, 2]. Although Southeast European countries have been considered to have a moderately high prevalence of MS, recent data indicated a significant increase in disease prevalence in this region. In Croatia, the prevalence of MS is estimated at 144 per 100,000 [3]; in Serbia, at 136 per 100,000 [2]; in Hungary, at 127 per 100,000 [4]; and in Slovenia, at 148 per 100,000 [5]. The exact cause of MS remains unclear, but the pathogenesis is known to involve acute and chronic (“smoldering”) neuroinflammation and various neurodegenerative mechanisms that lead to neuroaxonal damage [6, 7]. Although MS is an incurable disease, numerous disease-modifying treatments (DMTs) are available for people with MS (pwMS) with active disease, which is defined by relapses and/or appearance of new lesions on neuroimaging. All approved DMTs reduce the likelihood of relapses, and accumulating evidence shows that some DMTs may slow disease progression [8]. Most DMTs are used chronically as maintenance therapy, while selected treatments are given for a limited period as induction therapy, such as hematopoietic stem cell transplantation, alemtuzumab, and cladribine. Induction therapies are also referred to as immune reconstitution therapies (IRTs) because they cause an initial immune depletion, which is followed by a gradual regeneration of the immune system that is less likely to mount an autoimmune response [9]. While the risk associated with IRTs is increased in the short term, these treatments might be more effective in early disease control compared with an escalation strategy with maintenance treatments in pwMS with poor prognostic factors [10]. Although IRTs are considered high-efficacy therapies and lead to long-lasting disease control in most pwMS, there are limited data for pwMS on IRTs who experience disease activity and/or progression during follow-up.
The experience with alemtuzumab is longer than that with cladribine tablets. In the 4-year extension of CARE-MS I and II studies, 20–29% of pwMS received a third course, and 4–13% received a fourth course of alemtuzumab, without an effect on the safety profile and with reduced relapse rates, magnetic resonance imaging (MRI) activity, and disease progression [11]. The median interval between the second and third courses of alemtuzumab was approximately 2 years. In the CLARITY Extension trial of cladribine tablets, no benefit was shown for routine retreatment with additional cladribine cycles in years 3 and 4. However, there are no data showing what happens in this subset of pwMS after year 4 or whether additional cladribine cycles after year 4 in those with active disease offer an additional benefit. Because of these gaps and the fact that many pwMS in Southeast Europe are currently entering year 4 since starting cladribine treatment, the aim of this article was to provide a position statement on the management of pwMS treated with cladribine tablets in Southeast Europe.
Cladribine: Background and Practical Considerations
Cladribine is a nucleoside analogue of deoxyadenosine, and it is phosphorylated intracellularly to an active, toxic drug. Lymphocytes are particularly susceptible to cladribine-induced cell death because they have a low capability to dephosphorylate activated cladribine [12]. Because cladribine depletes primarily lymphocytes, it has been termed a selective IRT: the greatest depletion is seen for CD19 + B cells, while the depletion of CD4 + or CD8 + T cells and CD16 + /CD56 + NK cells is less pronounced [13].
Cladribine tablets (Mavenclad®) are approved for adult pwMS with highly active relapsing MS as defined by clinical or imaging features. This definition includes people with secondary progressive MS who have superimposed relapses [11]. Cladribine tablets are used at a cumulative dose of 3.5 mg/kg given in two cycles separated by 12 months. In the pivotal placebo-controlled CLARITY study, cladribine tablets (3.5 mg/kg) reduced the annualized relapse rate by 86%, the risk of sustained disability progression by 33%, and the number of gadolinium-enhancing (Gd +) lesions by 86% [14].
Oral cladribine is approved for use in pwMS with highly active disease. While the definition of highly active disease is still debatable (European Committee for Treatment and Research in Multiple Sclerosis and American Academy of Neurology expert panels have not reached a consensus so far [15]), clinicians need guidance for everyday practice. Sorensen et al. [16] proposed the following definition of highly active disease:
-
1.
Treatment-naïve patients: 1 prior clinical relapse in the last year AND evidence of subclinical MRI activity (Gd + or new or enlarging T2 lesions) in a patient with poor prognostic factors (clinical, MRI, or biomarkers) OR two or more clinical relapses in the last year, with or without MRI activity
-
2.
Patients who previously used DMTs: one prior clinical relapse in the last year with subclinical MRI activity (Gd + or new or enlarging T2 lesions) OR two prior clinical relapses in the last year without MRI activity OR ≥ 1 Gd + lesions or ≥ 2 new or enlarging T2 lesions in the last 12 months.
In addition to pwMS with highly active disease, high-efficacy therapies, such as cladribine, should be considered in patients with poor prognostic indicators of future disability, such as older age, male sex, greater disability at baseline, early cognitive impairment, high T2 lesion load at baseline, high concentrations of neurofilament light chain, infratentorial type of the first relapse, and numerous infratentorial or spinal lesions [15].
Pregnancy and family planning are important considerations when starting any DMTs, including oral cladribine. Cladribine might be advantageous for pwMS who plan to have children because it is given as an induction therapy and no maintenance is needed (no need to discontinue treatment during pregnancy). Previous studies showed that the risk of relapse is reduced during pregnancy, but it increases in the postpartum in women with MS. There is evidence that the use of high-efficacy DMTs (natalizumab, fingolimod) before pregnancy is associated with an increased risk of relapse during pregnancy, likely due to rebound disease activity after treatment discontinuation [17]. The mechanism of action of cladribine in relapsing MS suggests that the relapse risk should be decreased below pretreatment levels both during pregnancy and in the postpartum period. However, this hypothesis should be tested in future studies. Pregnancy is considered safe 6 months or later after the last dose of cladribine (for female pwMS and for female partners of male pwMS). However, cladribine is contraindicated in pregnancy and during breastfeeding.
Apart from a new perspective on family planning, oral cladribine provides other benefits to pwMS, such as a lower treatment burden (only 2 cycles, no need for injections or chronic use), a lower monitoring burden, and improved adherence [18]. In a cohort study of pwMS from the UK, treatment durability at 2 years for cladribine exceeded 90%, which was the highest of all oral DMTs studied [19].
Cladribine: Postapproval Data
The available postapproval data showed that cladribine effectively controls disease activity. In the CLASSIC-MS study, pwMS exposed to cladribine during the CLARITY or CLARITY Extension trials were less likely to require an ambulatory device at a median follow-up of 10.9 years compared with those exposed to placebo [20]. Registry data from a real-world setting also confirmed the sustained effect of oral cladribine. In Italy, 57% of pwMS remained relapse free for 5 years since the last cladribine dose [21]. In another study from Italy, nearly two-thirds of pwMS had no evidence of disease activity (NEDA-3) at 2 years since the start of treatment with cladribine tablets [22]. In Finland, 84% of pwMS remained relapse free for a median of 19 months since the last dose [23]. In Australia, approximately 80% of pwMS did not experience disease progression, and 65% were relapse free for 2 years after the last cladribine dose [24]. In an MSBase registry study, cladribine was associated with lower relapse rates compared with other oral DMTs (fingolimod, teriflunomide, dimethyl fumarate) during a median of 11 to 13 months [25]. A study from two tertiary centers from Germany reported that treatment with cladribine tablets substantially reduced relapse rates and the accumulation of T2 lesions, particularly in treatment-naïve pwMS [26].
Safety
As with all induction therapies, the adverse reactions of oral cladribine are “front loaded,” that is, occur close to active treatment. The risk decreases steadily with time after treatment completion. The mechanism of action of cladribine is lymphocyte depletion, which manifests clinically as lymphopenia. An analysis of pooled data from the CLARITY, CLARITY Extension, and PREMIERE trials showed that the median absolute lymphocyte count returned to a normal range 30 weeks after the last cladribine dose [27]. Although most pwMS have lymphopenia during cladribine treatment, the overall risk of infection was similar as that in the placebo arm, except for herpes zoster, which was more common in the cladribine arm [28]. After treatment, pwMS with grade 3 or 4 lymphopenia must be closely monitored for symptoms of infection [20]. Prophylaxis for herpes zoster should be recommended in pwMS with grade 4 lymphopenia and in those with grade 3 lymphopenia who are also immunocompromised [20].
Before starting cladribine, active and latent infections must be excluded. Screening for infection is needed to rule out hepatitis B and C, tuberculosis (ELISpot or QuantiFERON), HIV, and varicella zoster virus [12]. An assessment for human papilloma virus (cervical smear test, screening for cutaneous warts) and a syphilis test may be considered according to local guidelines [28].
A review of vaccinations is recommended, and vaccinations should be done according to local guidelines. All pwMS without humoral response should be vaccinated against varicella zoster virus before starting cladribine [16]. Cladribine tablets should not be initiated within 4–6 weeks after vaccination with live or attenuated live vaccines. If an inactivated component vaccination is essential for the patient, clinicians should wait for lymphocyte levels to return to the normal range. An advantage of cladribine, as opposed to maintenance immunosuppressive therapies, is that vaccinations can be resumed after immune reconstitution. An expert panel recommended that all pwMS should be vaccinated against coronavirus disease 2019 (COVID-19) as soon as possible, including those with a previous infection [29]. However, cladribine treatment should be started without delay when vaccination against COVID-19 is not possible because the risk of disease worsening seems to be greater that that of infection [29]. The available evidence suggests that pwMS on cladribine treatment do not have an increased risk of severe COVID-19 [30].
In the pivotal CLARITY study, the risk of malignancy was greater in the cladribine group than in the placebo group [31]. However, this difference could be because the risk in the placebo group (no cancer cases) was lower compared to that in the general population. The risk of malignancy in the cladribine group was similar to that observed in the placebo groups in phase 3 trials of other DMTs [31]. Moreover, an analysis of pooled data from the CLARITY, CLARITY Extension, ORACLE MS, and PREMIERE trials showed that the overall incidence rate of malignancy was not significantly different between cladribine and placebo [32].
When starting oral cladribine in pwMS who had previously used DMTs, clinicians must consider various factors to minimize the risk for the patient. For example, when switching from natalizumab, a thorough clinical and neuroimaging evaluation should be done to exclude progressive multifocal leukoencephalopathy [28]. In pwMS who had used DMTs associated with lymphopenia (fingolimod, dimethyl fumarate, anit-CD20 monoclonal antibodies), a washout period should be allowed for lymphocyte repopulation [28]. A rapid elimination protocol should be used in pwMS receiving teriflunomide because spontaneous clearance of this drug can take up to 2 years [33]. Starting cladribine tablets after alemtuzumab requires a washout period of at least 12 months [34]. Although starting oral cladribine in pwMS who previously received first-line injectable DMTs (interferons beta, glatiramer acetate) is considered safe, these drugs can rarely cause adverse reactions that are contraindications to cladribine use, such as lymphopenia and liver or kidney damage [16]. With numerous DMTs approved for use in patients with relapsing MS, starting cladribine in already treated pwMS might be complex, and it seems unfeasible to provide guidance for every possible clinical scenario. We suggest that expert advice should be sought from centers with the greatest experience in oral cladribine use in unusual cases.
The monitoring of pwMS receiving oral cladribine includes clinical, neuroimaging, and laboratory evaluations. Disability assessment and brain MRI should be done at baseline, at 12 and 24 months, and then annually. The lymphocyte count should be measured before each treatment cycle (years 1 and 2) and at months 2 and 6 in each treatment year [12]. The lymphocyte count must be > 1000 cells/mm3 before the first treatment cycle and > 800 cells/mm3 before the second cycle. The monitoring in the first 2 years of treatment is similar to that for most DMTs, but after this period, the monitoring burden for most pwMS who had received cladribine is lower compared with that for pwMS who had received other DMTs.
Cladribine Tablets Beyond Year 4
Currently, there are no relevant data to support the routine administration of additional treatment cycles to all pwMS. In the CLARITY Extension trial [35], the administration of another two cycles of cladribine in years 3 and 4 did not improve clinical or neuroimaging outcomes compared with placebo. NEDA-3 was observed in approximately 30% of pwMS in both groups during years 3 and 4 [36], and > 70% of pwMS in both groups did not reach 3- or 6-month confirmed disability progression by year 5 [37]. Currently, there is no evidence on the use of cladribine in relapsing MS beyond years 3 and 4, with the CLARITY Extension trials investigating the longest treatment schemes so far.
In an observational study among 41 pwMS who were followed for up to 20 years, subcutaneous cladribine at a cumulative dose exceeding that of the approved dose of oral cladribine was associated with stable disease and a favorable safety profile [38].
However, the growing experience with cladribine treatment and data for other IRTs, such as alemtuzumab, suggest that selected pwMS could benefit from retreatment [39]. Further follow-up of pwMS and dedicated clinical trials are needed to justify cladribine retreatment in years 3 and 4.
A longer postapproval experience with alemtuzumab (vs. cladribine tablets) may provide some guidance on when to retreat pwMS who have already received the full treatment course with an IRT. In the 4-year CARE-MS extension trial, > 20% of pwMS received one or more additional courses of alemtuzumab (3 or more in total) because of disease activity [11]. Data from real-world studies including pwMS who received additional alemtuzumab courses as needed showed that about 30–65% of pwMS are free from disease activity [40]. There is evidence that pwMS with active disease despite additional alemtuzumab courses show a limited reduction in CD4 + T cells [41], which are typically depleted after alemtuzumab [42]. However, alemtuzumab also depletes B cells, although with a hyperrepopulation of these cells 6 to 12 months after infusion, which could explain the autoimmune adverse reactions of alemtuzumab [43]. Cladribine selectively depletes central memory T cells and memory B cells. However, although a mild hyperrepopulation of maturing B cells is observed after cladribine treatment, there are no autoimmune adverse reactions [44].
The evaluation of cladribine’s efficacy is essential to decide whether to continue with another cladribine cycle, wait for response, or switch to another treatment. Sorensen et al. [16] suggested that pwMS without improvement or with worsening disease activity in years 1 or 2 are candidates for another high-efficacy DMT. A recent expert opinion paper from Germany proposed six patterns of treatment response to cladribine and provided clinical guidance for each [39, 45]. These patterns are described below:
-
1.
Optimal responders: pwMS who are stable during the 2 treatment years and then at least up to year 4. These pwMS do not need additional DMTs. Most pwMS are expected to be in this category.
-
2.
Delayed responders: pwMS with moderate disease activity during year 1. These pwMS should continue with the second cycle and should not be switched to other DMTs.
-
3.
Nonresponders: pwMS with substantial disease activity (severe relapse or ≥ 2 relapses) or progression (by ≥ 1 Expanded Disability Status Scale [EDSS] point) detected at 3 months to 1 year. In particular, this category includes pwMS who show greater disease activity than before cladribine treatment. These pwMS should be switched to another high-efficacy DMT.
-
4.
Temporary responders: pwMS with stable disease in year 1 and moderate disease activity late in year 2. These pwMS might benefit from additional cladribine treatment, which could include one or two additional cycles as in the CLARITY Extension study. The fourth treatment cycle could be delayed until year 5 if the disease becomes active again.
-
5.
Mid-term responders: pwMS without disease activity in years 1 and 2, but with reappearance of substantial disease activity in years 3 or 4. These pwMS should receive additional treatment cycles with cladribine or be switched to another DMT.
-
6.
Sustained responders: pwMS without disease activity up to year 4. From year 5, a third cladribine cycle could be given with reappearance of substantial disease activity or as a “prophylactic” course at the beginning of year 5. Retreatment with cladribine seems to be particularly justified in these pwMS because of the efficacy in years 1–4. Alternatively, treatment could be de-escalated to a maintenance DMT at the beginning of year 5. Sorensen et al. [16] proposed that pwMS without disease activity in years 3 and 4 or beyond should not be switched to another DMT.
German and Spanish experts suggest that pwMS with substantial breakthrough disease after year 4, defined as a severe relapse requiring plasmapheresis, inflammatory activity on MRI (e.g., ≥ 3 Gd + lesions or ≥ 5 new T2 lesions), or relapse-related disease worsening should be switched to another high-efficacy DMT [45, 46].
The above classification seems useful in various clinical scenarios, but—as the authors pointed out themselves—retreatment with oral cladribine should be done as part of a prospective clinical trial [39].
Methods
In November 2021, a panel of eight neurologists convened a virtual meeting. The aim of the meeting was to reach a consensus on the best-practice approach to the management of cladribine-treated pwMS beyond year 4. Based on the current literature, guideline recommendations for MS treatment [47, 48], and conclusions from the meeting, we propose an algorithm for the management of pwMS treated with cladribine tablets. A consensus on each recommendation was achieved using the Delphi method to minimize bias that can be introduced by group dynamics or dominant personalities. The Delphi method involves anonymous voting, facilitated discussions, group feedback, and statistical analysis of responses [49].
The online Delphi survey was designed to include two rounds. Round 1 consisted of eight recommendations formulated by three members of the panel (MH, JD, GBH). All members of the panel were asked to evaluate the recommendations. The Delphi survey was designed using SurveyMonkey (https://www.surveymonkey.com/) and distributed electronically with personalized links via email. During the process, all responses were anonymized. Participants were asked to rate the extent to which they agreed with each recommendation, with the following response options: 1, strongly agree; 2, agree; 3, neither agree nor disagree; 4, disagree; 5, strongly disagree; 6, other. The last option provided the possibility to enter any comments. Based on the responses received, the recommendations were revised, and the same process was repeated. For this Delphi survey, the general consensus level was set a priori to be 80% of the answers “strongly agree” or “agree.” The Delphi survey was conducted on September 21–26, 2022. In both rounds, the survey was completed by all participants. Ethics committee approval was not required to prepare this position statement, which was based on previously published evidence.
Position Statement
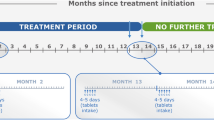
The proposed algorithm for the management of pwMS treated with cladribine tablets is shown in Fig. 1. Evidence from the CLARITY Extension trial indicated that relapses and disability progression occurred in 24.4% and 27.6% of participants, respectively, during years 3 and 4 of the trial. On the basis of these data, we recommend the following:
-
1.
It is of utmost importance to follow pwMS who start treatment with cladribine tablets at least annually. Minimum follow-up should include relapse, EDSS, brain MRI, and lymphocyte count assessment at 2 and 6 months after each cycle. If feasible, additional tests such as the symbol digit modality test, 9-Hole Peg Test (9-HPT), and Timed 25-Foot Walk (T25-FW) test, should be performed annually.
-
2.
In the case of disease activity and/or progression between the first and the second cycle of treatment with cladribine tablets, it is recommended to continue with the second cycle unless significant disease activity or progression occurs, in which case switching to another high-efficacy therapy (HET) with a different mode of action (MOA) should be considered. Factors such as pregnancy planning, comorbidities, and previous DMTs should be considered in the final decision. Furthermore, rebaselining of the MRI (3–6 months after the 1st cycle) should be performed when considering MRI activity.
-
3.
During years 2–4, for pwMS who completed two cycles of cladribine tablets, if there is no disease activity and progression, we recommend annual clinical and MRI follow-up, without additional treatment with cladribine tablets or other DMTs.
-
4.
During years 2–4, if minimal disease activity is present, we recommend continuing annual follow-up or considering additional cycle(s) of cladribine tablets. In the case of significant disease activity or disease progression, switching to another HET with a different MOA should be considered. Other factors, such as the number of new lesions on MRI, the severity of relapse, pregnancy planning, comorbidities, and previous DMTs, should be considered in the final decision.
Proposed algorithm for the management of people with multiple sclerosis treated using cladribine tablets. *If significant disease activity or progression occurs in between two cycles, consider switching to another HET with different MOA. Disease activity: relapse or new/enlarging T2 lesions. Disease progression: 6-month confirmed increase in EDSS by 1.5 points for baseline EDSS = 0, or an increase in EDSS of 1 point for baseline EDSS > 0, or an increase in EDSS by 0.5 point for baseline EDSS > 5.0
There are no data from clinical trials on the management of pwMS treated with cladribine tablets beyond year 4. We formulated our recommendations based on the expert opinion of the authors, published real-world studies, and German and Spanish expert opinion statements.
-
1.
Beyond year 4, if there is no disease activity and progression, we recommend annual clinical and MRI follow-up, without additional treatment with cladribine tablets or other DMTs. If feasible, additional tests, such as the symbol digit modality test, 9-HPT, and T25-FW test, should be performed annually.
-
2.
Beyond year 4, if there is minimal disease activity (defined as 1–2 new T2 lesions), we recommend continuing annual follow-up or considering additional cycle(s) of cladribine tablets. Factors such as pregnancy planning, comorbidities, and previous DMTs should be considered in the final decision.
-
3.
Beyond year 4, if there is moderate disease activity (defined as 1 relapse or 3 to 4 new T2 lesions), we recommend administering additional cycle(s) of cladribine tablets or considering a switch to another HET. Other factors, such as the site of lesions on MRI, the severity of relapse, pregnancy planning, comorbidities, and previous DMTs, should be considered in the final decision.
-
4.
Beyond year 4, if there is significant disease activity defined as > 1 relapse or > 4 new T2 lesions and/or disease progression, switching to another HET with a different MOA should be considered. Other factors, such as pregnancy planning, comorbidities, and previous DMTs, should be considered in the final decision.
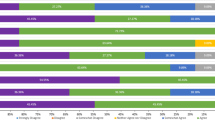
During the second round of the Delphi process, all recommendations received “strongly agree” or “agree” answers from all participants (Fig. 2).
When interpreting this algorithm beyond year 4, a cumulative effect of relapses or new T2 lesions on MRI must be considered. When there is one new lesion on MRI in year 5 and another in year 6, two new lesions should be counted when considering whether to continue monitoring or prescribe additional cladribine cycles or switch to another HET.
Based on the 2021 MAGNIMS-CMSC-NAIMS consensus recommendations on the use of MRI in pwMS, we did not incorporate Gd + lesions into the treatment algorithm because judicious use of gadolinium-based contrast agents for specific clinical purposes is recommended instead of regular administration of gadolinium-based contrast agents during every MRI examination [50].
Future Directions
The premise for using selective IRT with cladribine is that the two treatment cycles should provide sustained control of disease activity. However, in practice, there are pwMS who show signs of disease activity after treatment completion. Currently, there are limited data to make evidence-based decisions. Overall, the CLARITY Extension trial reported that additional treatment cycles in years 3 and 4 did not improve disease control. However, there is reason to believe that a subset of pwMS might benefit from retreatment with oral cladribine (e.g., temporary responders, mid-term responders, and sustained responders). In this position statement, we proposed an algorithm for the management of pwMS treated with cladribine tablets who experience disease activity and/or progression beyond year 4 of treatment. We expect that the proposed algorithm will be easy to use in participating Southeast European countries, but also in other regions where treatment with cladribine tablets is reimbursed. This position statement, however, was agreed upon among panelists from eight countries and therefore might be less applicable in other healthcare systems. Furthermore, the algorithm can serve as a document for negotiations with payers to enable an early and unrestricted treatment of pwMS. It should be noted that the algorithm is based on the expert opinion of the authors, and it gives the treating neurologist and pwMS the freedom to follow an individualized approach, depending on other specific circumstances such as pregnancy planning, comorbidities, or previous DMTs. Prospective clinical trials are needed to collect good-quality data to support the administration of oral cladribine beyond year 4.
References
Reich DS, Lucchinetti CF, Calabresi PA. Multiple Sclerosis. Longo DL (eds). N Engl J Med. 2018;378:169–80.
Coetzee T, Thompson AJ. Atlas of MS 2020: informing global policy change. Mult Scler J. 2020;26:1807–8.
Benjak T, Štefančić V, Draušnik Ž, Cerovečki I, Roginić D, Habek M, et al. Prevalence of multiple sclerosis in Croatia: data from national and non-governmental organization registries. Croat Med J. 2018;59:65–70.
Iljicsov A, Milanovich D, Ajtay A, Oberfrank F, Bálint M, Dobi B, et al. Incidence and prevalence of multiple sclerosis in Hungary based on record linkage of nationwide multiple healthcare administrative data. Nicoletti A, editor. PLoS One. 2020;15:e0236432.
Gustavsson A, Svensson M, Jacobi F, Allgulander C, Alonso J, Beghi E, et al. Cost of disorders of the brain in Europe 2010. Eur Neuropsychopharmacol. 2011;21:718–79.
Lassmann H. Pathogenic mechanisms associated with different clinical courses of multiple sclerosis. Front Immunol. 2019;9:1–14.
Giovannoni G, Popescu V, Wuerfel J, Hellwig K, Iacobaeus E, Jensen MB, et al. Smouldering multiple sclerosis: the ‘real MS.’ Ther Adv Neurol Disord. 2022;15:175628642110667.
Claflin SB, Broadley S, Taylor B V. The effect of disease modifying therapies on disability progression in multiple sclerosis: a systematic overview of meta-analyses. Front Neurol. 2019;9.
Lünemann JD, Ruck T, Muraro PA, Bar-Or A, Wiendl H. Author correction: immune reconstitution therapies: concepts for durable remission in multiple sclerosis. Nat Rev Neurol. 2020;16:125–125.
Prosperini L, Mancinelli CR, Solaro CM, Nociti V, Haggiag S, Cordioli C, et al. Induction versus escalation in multiple sclerosis: a 10-year real world study. Neurotherapeutics. 2020;17:994–1004.
Coles AJ, Arnold DL, Bass AD, Boster AL, Compston DAS, Fernández Ó, et al. Efficacy and safety of alemtuzumab over 6 years: final results of the 4-year CARE-MS extension trial. Ther Adv Neurol Disord. 2021;14:1756286420982134.
Mavenclad - Summary Of Product Characteristics [Internet].
Stuve O, Soelberg Soerensen P, Leist T, Giovannoni G, Hyvert Y, Damian D, et al. Effects of cladribine tablets on lymphocyte subsets in patients with multiple sclerosis: an extended analysis of surface markers. Ther Adv Neurol Disord. 2019;12:175628641985498.
Giovannoni G, Comi G, Cook S, Rammohan K, Rieckmann P, Sørensen PS, et al. A Placebo-Controlled Trial of Oral Cladribine for Relapsing Multiple Sclerosis. N Engl J Med. Massachusetts MedSoc; 2010;362:416–26.
Rotstein D, Montalban X. Reaching an evidence-based prognosis for personalized treatment of multiple sclerosis. Nat Rev Neurol. 2019;15:287–300.
Sørensen PS, Centonze D, Giovannoni G, Montalban X, Selchen D, Vermersch P, et al. Expert opinion on the use of cladribine tablets in clinical practice. Ther Adv Neurol Disord. 2020;13:1–17.
Hellwig K, Verdun di Cantogno E, Sabidó M. A systematic review of relapse rates during pregnancy and postpartum in patients with relapsing multiple sclerosis. Ther Adv Neurol Disord. 2021;14:175628642110510.
Stangel M, Becker V, Elias-Hamp B, Havla J, Grothe C, Pul R, et al. Oral pulsed therapy of relapsing multiple sclerosis with cladribine tablets—expert opinion on issues in clinical practice. Mult Scler Relat Disord. Elsevier B.V.; 2021;54:103075.
Froud J, St John F, Tallantyre, EC , Uzochukwu E, Harding K, Willis M, Anderson V, et al. UK multiple sclerosis registries project: disease modifying treatment durability. ABN VIRTUAL Meet. 2021;P125.
Giovannoni G, Leist T, Aydemir A, Di CEV. Long-term efficacy for patients receiving cladribine tablets in CLARITY/CLARITY extension: primary results from 9–15 years of follow-up in the CLASSIC-MS study. Mult Scler Relat Disord. 2022;59: 103633.
Patti F, Visconti A, Capacchione A, Roy S. Long-term effectiveness in patients previously treated with cladribine tablets: a real-world analysis of the Italian multiple sclerosis registry (CLARINET-MS). 2020;1–10.
Petracca M, Ruggieri S, Barbuti E, Ianniello A, Fantozzi R, Maniscalco GT, et al. Predictors of cladribine effectiveness and safety in multiple sclerosis: a real-world, multicenter, 2-year follow-up study. Neurol Ther. 2022;11:1193–208.
Rauma I, Viitala M, Kuusisto H, Atula S, Sipilä JOT, Ryytty M, et al. Finnish multiple sclerosis patients treated with cladribine tablets: a nationwide registry study. Mult Scler Relat Disord. 2022;61: 103755.
Lizak N, Hodgkinson S, Butler E, Lechner-Scott J, Slee M, McCombe PA, et al. Real-world effectiveness of cladribine for Australian patients with multiple sclerosis: an MSBase registry substudy. Mult Scler J. 2021;27:465–74.
Butzkueven H, Spelman T, Ozakbas S, Alroughani R, Terzi M, Hodgkinson S, et al. Real-world comparative effectiveness and persistence of cladribine tablets and other oral disease-modifying treatments for multiple sclerosis from GLIMPSE: results from the MSBase Registry (P12–4.003). Neurology. 2022;98(8 Sup):P12–4.003
Pfeuffer S, Rolfes L, Hackert J, Kleinschnitz K, Ruck T, Wiendl H, et al. Effectiveness and safety of cladribine in MS: real-world experience from two tertiary centres. Mult Scler J. 2022;28:257–68.
Comi G, Cook S, Giovannoni G, Rieckmann P, Sørensen PS, Vermersch P, et al. Effect of cladribine tablets on lymphocyte reduction and repopulation dynamics in patients with relapsing multiple sclerosis. Mult Scler Relat Disord. 2019;29:168–74.
Giovannoni G, Mathews J. Cladribine tablets for relapsing-remitting multiple sclerosis: a clinician’s review. Neurol Ther Springer Healthcare. 2022;11:571–95.
Rieckmann P, Centonze D, Giovannoni G, Hua LH, Oreja-Guevara C, Selchen D, et al. Expert opinion on COVID-19 vaccination and the use of cladribine tablets in clinical practice. Ther Adv Neurol Disord. 2021;14:1–13.
Giovannoni G, Berger J, Leist T, Jack D, Galazka A, Nolting A, et al. Post-approval safety of cladribine tablets with particular reference to COVID-19 outcomes: an update. Mult Scler J SAGE Publ. 2021;27:134–740.
Pakpoor J, Disanto G, Altmann DR, Pavitt S, Turner BP, Marta M, et al. No evidence for higher risk of cancer in patients with multiple sclerosis taking cladribine. Neurol Neuroimmunol Neuroinflam. 2015;2: e158.
Leist T, Cook S, Comi G, Montalban X, Giovannoni G, Nolting A, et al. Long-term safety data from the cladribine tablets clinical development program in multiple sclerosis. Mult Scler Relat Disord. 2020;46: 102572.
Aubagio—summary of product characteristics [Internet].
Adamec I, Jovanović I, Krbot Skorić M, Habek M. Double immune reconstitution therapy: cladribine after alemtuzumab in the treatment of multiple sclerosis. Eur J Neurol. 2022;29:901–4.
Giovannoni G, Soelberg Sorensen P, Cook S, Rammohan K, Rieckmann P, Comi G, et al. Safety and efficacy of cladribine tablets in patients with relapsing–remitting multiple sclerosis: results from the randomized extension trial of the CLARITY study. Mult Scler J. 2018;24:1594–604.
Giovannoni G, Singer BA, Issard D, Jack D, Vermersch P. Durability of no evidence of disease activity-3 (NEDA-3) in patients receiving cladribine tablets: the CLARITY extension study. Mult Scler J. 2021;1–10.
Giovannoni G, Comi G, Rammohan K, Rieckmann P, Dangond F, Keller B, et al. Long-term disease stability assessed by the expanded disability status scale in patients treated with cladribine tablets 3.5 mg/kg for relapsing multiple sclerosis: an exploratory post hoc analysis of the CLARITY and CLARITY extension studies. Adv Ther. Springer Healthcare; 2021;38:4975–85.
Rejdak K, Zasybska A, Pietruczuk A, Baranowski D, Szklener S, Kaczmarek M, et al. Long-term safety and efficacy of subcutaneous cladribine used in increased dosage in patients with relapsing multiple sclerosis: 20-year observational study. J Clin Med. 2021;10:5207.
Meuth SG, Bayas A, Kallmann B, Kleinschnitz C, Linker R, Rieckmann P, et al. Long-term management of multiple sclerosis patients treated with cladribine tablets: an expert opinion. Expert Opin Pharmacother. 2020;21:1965–9.
Gabelić T, Barun B, Adamec I, Krbot Skorić M, Habek M. Product review on MAbs (alemtuzumab and ocrelizumab) for the treatment of multiple sclerosis. Hum Vaccin Immunother. 2021;17:4345–62.
Rolla S, De Mercanti SF, Bardina V, Horakova D, Habek M, Adamec I, et al. Lack of CD4 + T cell percent decrease in alemtuzumab-treated multiple sclerosis patients with persistent relapses. J Neuroimmunol. 2017;313:89–91.
Rolla S, De Mercanti SF, Bardina V, Maglione A, Taverna D, Novelli F, et al. Long-term effects of alemtuzumab on CD4+ lymphocytes in multiple sclerosis patients: a 72-month follow-up. Front Immunol. 2022;13.
Baker D, Herrod SS, Alvarez-Gonzalez C, Zalewski L, Albor C, Schmierer K. Both cladribine and alemtuzumab may effect MS via B-cell depletion. Neurol Neuroimmunol Neuroinflam. 2017;4: e360.
Moser T, Schwenker K, Seiberl M, Feige J, Akgün K, Haschke-Becher E, et al. Long-term peripheral immune cell profiling reveals further targets of oral cladribine in MS. Ann Clin Transl Neurol. 2020;7:2199–212.
Meuth SG, Bayas A, Kallmann B, Linker R, Rieckmann P, Wattjes MP, et al. Long-term management of multiple sclerosis patients treated with cladribine tablets beyond year 4. Expert Opin Pharmacother. 2022;1–8.
Meca-Lallana V, García Domínguez JM, López Ruiz R, Martín-Martínez J, Arés Luque A, Hernández Pérez MA, et al. Expert-agreed practical recommendations on the use of cladribine. Neurol Ther. 2022.
Montalban X, Gold R, Thompson AJ, Otero-Romero S, Amato MP, Chandraratna D, et al. ECTRIMS/EAN Guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler J. 2018;24:96–120.
Habek M, Adamec I, Barun B, Bašić Kes V, Bogoje Raspopović A, Duka Glavor K, et al. Treatment of relapsing multiple sclerosis—recommendations of the Croatian Neurological Society. Croat Med J. 2022;63:379–88.
Hsu CC, Sandford B. The delphi technique: making sense of consensus. Pract Assess Res Eval. 2007;12:1–8.
Wattjes MP, Ciccarelli O, Reich DS, Banwell B, de Stefano N, Enzinger C, et al. 2021 MAGNIMS–CMSC–NAIMS consensus recommendations on the use of MRI in patients with multiple sclerosis. Lancet Neurol. 2021;20:653–70.
Acknowledgements
Springer Healthcare is not responsible for the validity of the guidelines it publishes.
Funding
The authors received no funding or sponsorship for this study. Medical writing and publication fees were funded by Merck, Zagreb, Croatia.
Medical Writing and Editorial Assistance
Writing and editing assistance in the preparation of this article was provided by Rafał Szot (Proper Medical Writing, Warsaw, Poland) and paid for by Merck, Zagreb, Croatia.
Author Contributions
Study concept and design: Mario Habek, Gregor Brecl Jakob, Jelena Drulović. Acquisition of data: Mario Habek, Jelena Drulovic, Gregor Brecl Jakob, Ivan Barbov, Ljiljana Radulovic, Cecilia Rajda, Konrad Rejdak, Peter Turcani. Analysis and interpretation of data: Mario Habek, Jelena Drulovic, Gregor Brecl Jakob, Barbov, Ljiljana Radulovic, Cecilia Rajda, Konrad Rejdak, Peter Turcani. Drafting of the manuscript: Medical writer, Mario Habek, Gregor Brecl Jakob, Jelena Drulović. Critical revision of the manuscript for important intellectual content: Mario Habek, Jelena Drulovic, Gregor Brecl Jakob, Ivan Barbov, Ljiljana Radulovic, Cecilia Rajda, Konrad Rejdak, Peter Turcani. Administrative, technical, and material support: Mario Habek, Jelena Drulovic, Gregor Brecl Jakob, Ivan Barbov, Ljiljana Radulovic, Cecilia Rajda, Konrad Rejdak, Peter Turcani. All authors read and approved the final manuscript.
Disclosures
Mario Habek: Participated as a clinical investigator and/or received consultation and/or speaker fees from: Biogen, Sanofi Genzyme, Merck, Bayer, Novartis, Pliva/Teva, Roche, Alvogen, Actelion, Alexion Pharmaceuticals, TG Pharmaceuticals. Gregor Brecl Jakob: Participated as a clinical investigator and/or received consultation and/or speaker fees from: Biogen, Sanofi Genzyme, Merck, Novartis, Pliva/Teva, Roche, Lek. Jelena Drulović: serves on scientific advisory boards for Bayer, Biogen, Medis, Merck, Novartis, Roche, Sanofi-Genzyme, Janssen and Teva and have received speaker bureaus for Biogen, Bayer, Merck, Roche, Sanofi-Genzyme, Janssen, Medis, Hemofarm, Medtronic, Zentiva, and Teva. Ivan Barbov: Nothing to disclose. Ljiljana Radulovic: Participated as a clinical investigator and/or serves on scientific advisory boards and/or speaker fees from: Merck, Bayer, Novartis, Biogen, Sanofi Genzyme, Teva, Roche. Cecilia Rajda: Participated as clinical investigator and/or received consultation and/or speaker fees from: Biogen, Merck, Novartis, Roche, Sandoz, Sanofi Genzyme. Konrad Rejdak: has received speaking honoraria and travel grants for participation in scientific meetings, and participated in advisory boards in the past years with Bayer, Biogen, Merck Healthcare KGaA, Darmstadt, Germany, Novartis, Roche, Sanofi-Genzyme, and Teva Pharmaceutical. Peter Turcani: has participated in meetings sponsored by and received honoraria (lectures, advisory boards, consultations) from Bayer, Biogen, Boehringer, Eli Lilly, GlaxoSmithKline, Lundbeck, Merck, Novartis, NovoNordisk, Pfizer, Roche, Sanofi/Genzyme, Teva, and Zentiva. His institution has received financial support by unrestricted research grants (Biogen, Merck, Novartis, Roche, Pfizer, Sanofi/Genzyme Teva) and for participation in clinical trials Bayer, Biogen, Merck, Novartis, NovoNordisk, Roche, Sanofi Genzyme, and Teva.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Information used to obtain the consensus statement is available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Habek, M., Drulovic, J., Brecl Jakob, G. et al. Treatment with Cladribine Tablets Beyond Year 4: A Position Statement by Southeast European Multiple Sclerosis Centers. Neurol Ther 12, 25–37 (2023). https://doi.org/10.1007/s40120-022-00422-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-022-00422-z