Abstract
Alzheimer’s disease (AD) is a debilitating disease leading to great social and economic burdens worldwide. During the past decades, increasing understanding of this disease enables dynamic trials for disease interventions. Unfortunately, at present, AD still remains uncurable, and therefore, developing intervention strategies for improving symptoms and slowing down the disease process becomes a practical focus in parallel with searching for a disease-modifying medication. The aim of this review is to summarize the outcomes of AD clinical trials of non-drug therapies published in the past decade, including cognitive-oriented interventions, physical exercise interventions, brain stimulation, as well as nutrition supplementations, to find out the most effective interventions in the category by looking through the primary and secondary outcomes. The outcomes of the trials could be varied with the interventional approaches, the tested cohorts, the settings of observing outcomes, and the duration of follow-ups, which are all discussed in this review. Hence, we hope to provide crucial information for application of these interventions in real-world settings and assist with optimization of clinical trial designs in this area.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
There are three main types of cognitive-oriented interventions: cognitive training focuses on targeting particular cognitive functions with changes of associated brain regions, cognitive stimulation aims to enhance general cognitive and social functioning of the individual, and individualized cognitive rehabilitation addresses specific functional difficulties and sets realistic goals to help patients and their families in daily life. |
Physical exercise interventions are documented to improve cognition and quality of life (QoL) via improving cardiovascular fitness and well-being of patients with dementia, which is strongly associated with less brain atrophy, less harmful effects of cerebral amyloid on cognition, and reduced risk of dementia. |
Brain stimulation techniques, including deep brain stimulation (DBS), transcranial direct current stimulation (tDCS), repetitive transcranial magnetic stimulation (rTMS), transcranial pulse stimulation (TPS), and intermittent theta burst stimulation (iTBS), have all been tested on patients with Alzheimer’s disease (AD) with varied effectiveness. |
Nutrition interventions, which can be performed by supplementation of a combined formula, specific active ingredients or probiotics, or nutritional guidance, have shown beneficial effects on both cognition and function of patients with prodromal and mild AD. |
Factors such as type of intervention, sample size, targeted cohort, standardized protocol, primary and secondary outcome measures need to be carefully considered when designing randomized controlled trials (RCTs) for further investigations. Interventions on individuals at more severe stages, outcomes of increase in QoL and decrease in caregivers’ burdens are things to be investigated in future studies. |
Introduction
Alzheimer’s disease (AD) is a chronic progressive neurodegenerative disorder which may impair multiple cognitive domains, cause loss of motivation, and disrupt daily activities and social functions, and subsequently affect quality of life (QoL) of both patients and their caregivers and families. It has been estimated that 35 million people and their families live with a diagnosis of AD or related dementia worldwide [1]. Numerous clinical trials have been performed or are ongoing; however, AD is still an untreatable disease and the most common cause of dementia at present. In the past decades, efforts have been made to develop disease-modifying therapies and symptomatic treatments, including therapies targeting amyloid-beta, amyloid-related proteins, tau, or therapies used for improving cognition or other AD-related conditions such as gait disorder and neuropsychiatric and behavioral symptoms. To date, no disease-modifying therapies seem to significantly delay or even reverse the progression of the disease, while symptomatic treatments seem to be more effective in achieving short-term improvements. However, even for the acetylcholinesterase inhibitors (AChEIs), which remain the mainstream of medication for symptom relief, their effects stay within a range, and no additional benefit is added with increased doses or prolonged treatment [2, 3]. Therefore, recent research has indicated that the intervention should be multifocal and multidisciplinary to maximize the beneficial effects.
The repeated failures in the development of pharmacological treatments for AD have aroused increasing interests in developing alternative nonpharmacological interventions, with various major trials, such as the EXERT (https://clinicaltrials.gov/ct2/show/NCT02814526), FINGER(https://clinicaltrials.gov/ct2/show/NCT05109169?term=FINGER&draw=2&rank=1), and POINTER (https://clinicaltrials.gov/ct2/show/NCT03688126) studies underway. Though no general agreement has been reached on these approaches, studies based on the epidemiological findings have emerged as well as practical practice, and some have shown beneficial effects of these management approaches to different extents. Increasing evidence suggests that these nonpharmacological interventions or complementary treatments may be optimal routes to relieve physical and mental symptoms without causing serious side effects [4]. This narrative review comprehensively summarizes clinical trials of non-drug AD therapies published in the past decade, including cognitive-oriented interventions, physical exercise interventions, brain stimulation, as well as nutrition supplementations. We also detail the positive and negative outcomes of these clinical trials, together with the critical factors that may affect the trial outcomes, including the interventional approaches, the tested cohorts, the settings of observing outcomes, and the duration of follow-ups, aiming to point out the advantages and limitations of the trial design, and thereby, to provide valuable information for application of these interventions in clinical practice and assist with optimization of clinical trial designs in the field.
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Search Strategy and Selection Criteria
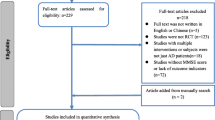
The literature search included terms “Alzheimer’s disease”, “treatment”, “clinical research” or “clinical trial” or “clinical study”. For PubMed, the search strategy is (Alzheimer’s disease) AND (treatment) AND ((clinical research) OR (clinical trial) OR (clinical study)); for Embase, the search strategy is ((clinical research or clinical trial or clinical study) and Alzheimer’s disease and treatment); for Cochrane Library, the search strategy is (Alzheimer’s disease) AND (treatment) AND ((clinical research) OR (clinical trial) OR (clinical study)). Papers published in English between 2010 and 2021 and limited to human studies were collected for subsequent screening. Additional articles were selected on the basis of articles in these searches. Regarding nutritional supplements, an additional search was conducted on ClinicalTrials.gov, showing several ongoing clinical studies, including Mediterranean diet (NCT03841539, NCT04439097), modified Atkins diet (NCT02521818), ketogenic diet (NCT03690193), low-protein diet (NCT05480358) as well as olive oil leaves (NCT04440020). These studies have not been completed and/or the results have not been published yet; thus, they were not included in the present review.
The search results were reviewed by two authors independently, and any discrepancies were evaluated by a third author. Duplicate studies from the same cohort were removed manually using Endnote 20. Original full-text research articles on clinical non-drug intervention in AD were included. Exclusion criteria were drug-related interventions, animal and cell studies, reviews, letters, case reports, commentaries, and protocols. Studies on different disease stages were searched and included; however, studies on prodromal AD did not appear in search results for nonpharmacological interventions other than nutritional interventions. In addition, this review was particularly focused on interventions as treatments or symptom reliefs for AD; thus, articles with a main focus on prevention of disease or reducing disease risks were not included.
Cognitive-Oriented Interventions
Cognitive-oriented interventions are composed of three main types, namely cognitive training, cognitive stimulation, and individualized cognitive rehabilitation. These interventions have received increasing attention in recent years as preventive or enhancing treatment for AD [5]. The randomized controlled trials (RCTs) of cognitive-oriented interventions on individuals with AD published in the last decade are summarized in Table 1.
Cognitive Training
Cognitive training is the first established nonpharmacological intervention that focuses on a particular cognitive function in dementia, e.g., memory, attention, language, or executive functions. It improves dementia-associated cognition by completing some theoretically driven standard tasks to improve or maintain the normal functioning of the targeted cognitive domains as long as possible [6]. For instance, it has been reported that the adaptive chunking training provided to patients with mild AD has led to significant improvements in verbal working memory performance, which was evidenced by reduced task-related activation of the lateral prefrontal and parietal cortex on functional magnetic resonance imaging (fMRI), indicating that chunking-based cognitive training may help maintain cognitive functions in early stage of AD [7]. For another instance, prospective memory refers to remembering to perform an intended action in the future (e.g., taking medications, turning off an oven), and deficits in prospective memory are pronounced in patients with AD even in very mild or preclinical stages [8]. A simple perspective memory encoding strategy has shown effectiveness in restoring prospective memory function in older adults and individuals with very mild AD [9]. In addition, utilizing fMRI echo planar with blood oxygenation level dependent (BOLD) contrast revealed increased activation of the bilateral superior temporal gyrus, the right lentiform nucleus, and thalamus following interventions [10]. The magnitude of activation in the left superior gyrus, precuneus, and left thalamus was correlated with change in cognitive assessments [10]. These fMRI data correlated well with the neuropsychological and neurobehavioral measures, which supports the notion that multidimensional stimulation targeting cognition, behavior, and motor functioning significantly improved cognitive-behavioral status of patients with AD at least partially by restoring neural functioning [10]. Improved cognitive performance, including memory, attention, and language, was also observed after a culture-specific picture-based cognitive training on patients with early AD [11]. Overall, the cognitive training seemed to be effective to the targeted cognitive domains, and its functional effectiveness correlated well with the fMRI data, indicating that fMRI could be included as an observing outcome for this type of clinical study. However, no publications or registered RCTs comparing the effects of cognitive training and donepezil were found. Shortcomings of cognitive training may exist, such as lack of sufficient training provider, the narrow AD cohort that may benefit from the training, and the relatively short duration of effects following training. In addition, the standardized training protocol may not benefit each individual patient, yielding the need for individualized patient management. Furthermore, the effects of cognitive training are relatively difficult to compare across studies for subsequent optimization because of lack of standardized protocols. Resolving or improving these issues requires longitudinal studies and standardized methodologies.
Cognitive Stimulation
Cognitive stimulation therapy (CST) is a model based on the theories and evidence in the Cochrane Review of Reality Orientation database [12]. Cognitive stimulation typically refers to a wide range of group activities and discussions, including reminiscence therapy and reality orientation therapy, aiming to enhance the general cognitive and social functioning of the individual. This approach has been recommended in the 2006 NICE guideline to be used as a routine procedure for those with mild-to-moderate dementia, based on the result showing that this approach was at least as effective as cholinesterase inhibitors (ChEIs) to reduce cognitive decline [13]. CST also helps patients with dementia via enhancing certain cognitive areas, including memory, orientation, language comprehension, coping and adaptation skills, facilitating communication, ensuring sustainability, decreasing anxiety and depression, and consequently improving their QoL [14,15,16]. In addition, CST in combination with emotion recognition rehabilitation has generated better outcomes in emotion identification as well as precision and speed of processing compared to the CST only group [17].
Reminiscence therapy is a non-specific stimulation treatment that uses all the senses as memory triggers (e.g., audiovisual materials) to help individuals with dementia remember events, people, and places from their past lives, verbally or nonverbally, alone or with a group, in order to increase the adaptation to the present time [18]. This is because individuals with dementia often preserve remote memories, so they are usually able to recall events from youth or even childhood, but not from earlier on the same day (ecmnesia). Positive memories, specific subjects for sessions, and a general summarization and evaluation for the closing session are highly recommended for reminiscence therapy [19]. A 12-week reminiscence therapy for patients with mild-to-moderate AD showed increased cognitive levels (assessed by standardized Mini Mental Test) but decreased depressive emotions (assessed by Geriatric Depression Scale, GDS) [20]. In addition, a computerized process-based cognitive training combined with reminiscence therapy has demonstrated positive effects on cognition (assessed by Mini-Mental State Examination, MMSE) and functional abilities (assessed by instrumental activities of daily living, ADL) in patients with mild AD [21]. The clinical trial of a reminiscence therapy on a Chinese cohort is still ongoing [22]. For reminiscence therapy, audiovisual materials are often used to trigger the retrieval of autobiographical memories. Familiar music seems to be a privileged stimulus to influence emotions and memory in patients with AD or dementia, and musical memory appears to be a relative preservation in AD [23, 24]. On the basis of this evidence, music-based therapies alone [25] or in combination with visual stimulation [26] have been actively studied in clinical trials and resulted in positive outcomes in cognition, emotion, and behavior. Furthermore, the most significant improvements in emotion and behaviors of mild-to-moderate AD cases have been observed when using 40 Hz for rhythmic sensory stimulation [26].
On the other hand, CST incorporates the positive aspects of reality orientation therapy, which presents patients with continuous memory and orientation information related to personal issues and living environment, and uses such repeated stimulation to person, time, and place in both group and individual settings. During each session, the individual is encouraged to discuss topics related to recent events, personal interests, or daily routines [27]. The RCT has revealed that a mindfulness stimulation may maintain cognitive function for donepezil-treated patients with AD, which was equivalent to cognitive stimulation and superior to muscle relaxation, and thus could be used as a nonpharmacological treatment for AD [28]. In another RCT, the 6-month reality orientation combined with AChEIs improved cognitive outcomes whereas AChEIs only did not, suggesting the potentiality of reality orientation as a valuable complementary intervention for AD-related dementia [29]. The problem is that CST appears to benefit cognition only in a short-term manner (e.g., during the intervention period), but not in a long-term manner (e.g., 6 or 24 months after intervention) [30]. The long-term benefits of maintenance of CST are being tested on patients with early and mild-stage dementia [31].
Individualized Cognitive Rehabilitation
Initially developed through work with younger brain-injured patients, cognitive rehabilitation has been increasingly discussed and applied in the field of dementia. Cognitive rehabilitation refers to an individualized interventional program which addresses specific functional difficulties and sets realistic goals to help patients and their families in daily life. The rehabilitation program focuses mainly on developing compensatory strategies for impairment and improving the individual’s performance in daily situations to some extent, rather than on cognitive performance itself, and main caregivers are often involved in the studies as well [6, 32]. Therefore, the instrumental ADL, the goal performance, the individual’s satisfaction, and the caregiver’s burden are usually the primary measures for this type of intervention [33,34,35]. In a parallel-group trial comparing cognitive training, reminiscence therapy, and individualized cognitive rehabilitation to usual care, only individualized cognitive rehabilitation, but not the other two, significantly lowered functional disability and delayed institutionalization [36]. In addition, the manual-guided cognitive rehabilitation intervention was superior to standardized cognitive training in non-cognitive domains such as QoL rather than cognitive functions [37]. Furthermore, the individualized cognitive rehabilitation also helps patients and their caregivers mentally or psychologically. In a preliminary open trial, the cognitive-behavioral intervention reduced patients’ depression and caregivers’ distress simultaneously, and such a reduction was maintained at the 3-month follow-up after intervention, although the results need to be further confirmed because of the small sample size and lack of controls [38]. Additionally, further cognitive rehabilitation studies should pay close attention to the aberrant motor behaviors (AMB), which refer to aimless movement without a specific purpose and leads to early institutionalization and caregivers’ burden in patients with AD. In another 4-week home-based trial of cognitive rehabilitation for learning/relearning an instrumental ADL, patients with AD in the intervention group had increased AMB [39], which required further investigations in larger-scaled trials. The intervention may be provided in various ways. In the comparison of three cognitive rehabilitation programs, positive outcomes (decreased depressive symptomology, improved instrumental ADL) have only been observed in the groups with Memo+ (a paper and pencil memory training program) and SenseCam (a wearable camera used as a passive external memory aid), but not in the group with written diary (a personal journal) [33], indicating the importance of selecting appropriate ways to deliver the intervention. Though early diagnosis and intervention is believed to be critical and recommended for individuals with AD, early psychosocial intervention including education, counseling, and social support did not seem to benefit the patient’s disease progression or delay the patient’s institutionalization compared to controls [40].
Assessments of Outcomes
Firstly, the evaluation and re-evaluation of status of different cognitive domains should be a major part of the outcome assessments. MMSE, Alzheimer’s Disease Assessment Scale-Cognitive subscale (ADAS-Cog), and Cambridge Cognitive Examination (CAMCOG) are the most commonly used cognitive assessments in these scenarios. MMSE is a widely used test of multiple cognitive domains including orientation, attention, memory, language, and visual-spatial skills. ADAS-Cog is the FDA-approved cognitive measure for drug RCTs. CAMCOG is a standardized instrument for measuring the extent of cognitive impairment or dementia which has been used for decades. Apart from these global measures, changes in specific cognitive domains, including attention, memory, language, executive function, processing speed, etc., should also be carefully examined. For instance, the Trail Making A&B tests are mainly used to assess visual scanning/processing speed and executive functions, respectively.
Secondly, in addition to traditional assessments for cognitive domains, the measurement of behavioral disturbances is also critical for the evaluation of trial outcomes, since these symptoms affect up to 90–98% of patients with AD in all stages of the disease [41] and often lower QoL of patients and their caregivers [42]. Thus, when designing clinical trials of cognitive rehabilitation for AD, assessing changes of behavioral and psychological symptoms of dementia (BPSD), especially AMB, needs to be taken into consideration. Unlike other BPSD such as depression and delusions, which were reported to be improved after cognitive interventions, AMB was observed to be worsened [39]. Several studies have shown that AMB symptoms are correlated with the severity of cognitive impairment, and tend to increase throughout the natural course of AD [43, 44]. It is common in patients with AD and leads to early institutionalization and caregivers’ burden. The worsening in AMB observed in patients with AD may be explained at least partly by increased anxiety during intervention and, therefore, it is necessary to integrate relaxing components into cognitive intervention programs, such as listening to soothing music and aromatherapy [45], which may help the patients to cope better with the disease- or intervention-induced stress. Several hypotheses have been developed to explain the increase of AMB during the cognitive intervention for AD, among which the reduced stress-threshold model [46] provides a most plausible explanation. The psychological symptoms can also be evaluated by other useful tools such as the Neuropsychiatric Inventory (NPI), or depression- and anxiety-specific assessments, e.g., Cornell Scale for Depression in Dementia (CSDD) and Hamilton Depression Rating Scale (HDRS).
Thirdly, since AD is a chronic progressive disease and requires long-term clinical management, we also need to pay attention to the QoL of both the patients and their caregivers. Apart from the questionnaire of QoL and the carer’s burden, factors that may affect QoL, including Barthel Index (BI) for daily activities, ADL, delay of institutionalization, should also be evaluated.
Limitations
The inconsistency of the results is one of the biggest problems when developing these cognitive-based approaches into clinical practice. The discrepancies were largely due to the differences in sample size, methodological heterogeneity in different studies, and notably the bias in intervention protocols, follow-up strategies, outcome measures, which also impede drawing solid conclusions. Additionally, it is largely unknown whether severe AD cases will achieve any benefits from this type of nonpharmacological intervention.
Therefore, we need to highlight the need for additional well-designed RCTs with larger sample sizes and outcome measures beyond the direct cognitive outcomes evaluated simultaneously. A summary of total time of interventional sessions and measures of outcomes (1. cognitive domains; 2. neuropsychological measures; 3. QoL & ADL) of reviewed trials, whereby information is available in the original article, is shown in Fig. 1.
Summary of total time of interventional sessions and measures of outcomes. Total time of interventional sessions can be calculated for ten reviewed trials in Table 1 owing to availability of the information in the original article, including two trials of cognitive training (red), six trials of cognitive stimulation (green), and two trials of cognitive rehabilitation (purple). The average time of intervention is 42 h for cognitive training, 12 h for cognitive stimulation, and 15 h for cognitive rehabilitation. The assessment of outcomes includes (1) cognitive domains (left flask); (2) neuropsychological measures (middle flask); (3) QoL & ADL (right flask). Each trial has been put into these three flasks on the basis of their outcome measurements
Physical Exercise Interventions
It has been well documented that poor physical function and muscle strength coexist in patients with dementia, which is associated with adverse events such as gait abnormalities, risk of falls, fall-related fracture, increased susceptibility to injury, and other comorbidities. In addition, recent studies suggested that walking might be considered as a cognitive process and that cognitive dysfunction, such as executive dysfunction, was associated with gait disturbances and subsequent falls in patients with AD [47]. Therefore, physical exercises have been proposed to be an effective type of intervention for improving physical health and well-being of patients with dementia, based on the observational evidence showing that higher levels of physical activity and cardiorespiratory fitness are strongly associated with less brain atrophy, less harmful effects of cerebral amyloid on cognition, and reduced risk of dementia [48,49,50,51]. Though the mechanisms remain largely unclear, one study has shown that a 12-month exercise regimen effectively prevented falls not only in mild AD but also in advanced AD [52]. Physical exercises not only improve physical health of patients with AD but also bring additional benefits by reducing BPSD [53] and enhancing performance in ADL [54]. Furthermore, epidemiological research suggests exercise training as a nonpharmacological approach to protect against AD [55,56,57], which is evidenced by changes in brain structures such as increased hippocampus size [58] and upregulation of brain neurogenesis [59].
Physical exercise intervention for AD can be performed by specific exercise program (often aerobic exercise) with moderate-to-high intensity, or exercise based on individuals own exercise habits with low intensity; the intervention can be either home-based or group-based, either single-task, dual-task, or multitask training. The RCTs of physical exercise interventions on individuals with AD published in the last decade are summarized in Table 2.
Different Types of Physical Exercise Interventions
Aerobic exercise offers a low-cost, low-risk, widely available intervention that may have disease-modifying effects. A 26-week aerobic exercise training with moderate intensity improved functional ability compared to non-aerobic exercise in older adults with probable AD [60], while another 16-week aerobic exercise training with moderate-to-high intensity was superior to usual care in the improvement of cardiorespiratory fitness, single-task and dual-task physical performance, and exercise self-efficacy in patients with mild AD with high attendance [61, 62]; however, neither intervention exerts markedly positive effects on cognitive domains. Different results have been observed in another two trials, in which 3-month aerobic exercise training improved cognitive function and QoL in patients with mild AD [63, 64]. The cognitive improvement, as demonstrated by improved Stroop color naming test and Trail Making A&B tests, was likely to be achieved by improving brain energy metabolism via increasing ketone utilization while maintaining brain glucose uptake [64]. One large-scale RCT for observing the effectiveness of 6-month moderate-intensity cycling intervention on global cognition of mild-to-moderate AD is still ongoing, and vascular biomarkers for cardiorespiratory fitness will be measured simultaneously [65]. Not just in older adults without cognitive decline [66], exercise also reduced the number of falls in those with advanced AD [52, 67]. Home-based exercise with physical and cognitive stimuli combined in one program has demonstrated transfer effects to ADL, cognitive and physical performance in patients with AD [68], and more importantly home-based exercise appeared to contribute more benefits in executive function than group-based exercise [52, 67]. A 12-week dual-task training of patients with AD has shown improvements in multiple aspects including cognitive functions, depressive status, aerobic fitness, etc., which were associated with an increase in brain oscillation (the electrical activity of the cerebral cortex recorded by EEG) and decrease in theta/alpha ratio [69], indicated as improved cerebral blood flow, cognitive function, and occipital gray matter density [70]. In addition, improved balance and increased muscle strength in the same study is especially important, as balance and mobility impairments in patients with AD are associated with the risk of falling and reduced QoL [69]. Exercise based on individuals own habits is an easier, safer, and feasible way to deliver physical intervention to patients with AD. For instance, regular walking is effective to benefit individuals with sundown syndrome [71], which is regarded as the appearance or increase in neuropsychiatric symptoms in the late afternoon, evening, or at night, with a prevalence of approximately 66% in community-dwelling patients with AD [72]. Even lower levels of weekly energy expenditure may be associated with health benefits, but not with cognitive improvements, for older patients with mild-to-moderate AD [73].
Intensity and Duration of Intervention
Physical exercise intervention is a promising approach to maintain or improve body fitness and functional ability for patients with mild AD, which may in turn improve ADL performance and QoL, and reduce fall risks. However, it remains uncertain whether physical exercise is beneficial for cognition in AD. Beneficial cognitive outcomes were seen in some but not all of the reported clinical trials, and often existed during the intervention period, but not in the long term. As a result of the heterogeneity of reported training methods (e.g., aerobic training on treadmill, cycling, home-based physical activities), training intensities (mild, moderate to high), durations (3, 4, 6, 12, 24 months), and outcome measures (global cognition and specific domains), and small sample size that limits the power to detect significant group effects, it is hard to draw a solid conclusion about optimized protocols of this type of intervention. Therefore, large-scale comparative studies are needed to explore the most effective type of exercise intervention, the necessary amount of exercise, the most sensitive and significant outcome measures, and strong evidence for physical activity affecting patients’ cognition. Moreover, cohorts with severe AD or early-onset familial AD should also be studied for the possibility of using this type of intervention.
Brain Stimulation
Brain stimulation techniques, including deep brain stimulation (DBS), transcranial direct current stimulation (tDCS), repetitive transcranial magnetic stimulation (rTMS), transcranial pulse stimulation (TPS), and intermittent theta burst stimulation (iTBS), can regulate cognitive functions in a variety of neuropsychiatric diseases. Previous studies have shown some promising effects of brain stimulation in the treatment of AD. The RCTs of brain stimulation on individuals with AD published in the last decade are summarized in Table 3.
Deep Brain Stimulation (DBS)
DBS is an invasive neurosurgical technique that modulates neuron activity by using internal pulse generators connected to electrodes in specific areas of the brain. DBS of the fornix or nucleus basalis of Meynert seemed to be safe and well tolerated in patients with AD in an observatory period of 12 months [74,75,76], without any obvious nutritional side effect [76]. Within 12 months post implantation, although mean hippocampal atrophy was significantly slower [75], cognitive functions were not significantly different between positive-treated and sham-treated groups, and cerebral glucose metabolism was only found to be increased at 6 months but not at 12 months post implantation [74].
Transcranial Direct Current Stimulation (tDCS)
tDCS is a non-invasive technique and works by inducing a low direct current in the cortical area of interest. During the procedure, small electrodes are placed on the scalp above the targeted brain area. This stimulation facilitates cortical excitability and thereby neuroplasticity [77]. Compared to other brain stimulation techniques, tDCS is relatively simple, safe, portable, and inexpensive to administer. These merits are very important for tDCS to be administered for a long time at home. An increasing number of clinical studies have reported the potential of tDCS in enhancing cognition of individuals with cognitive impairment. A short period (10 days) of stimulation at the left temporal cortex failed to improve verbal memory function significantly [78], whereas a longer period (6 months) of stimulation at the dorsolateral prefrontal cortex improved global cognition and language function [79]. It is noteworthy that in advanced AD cases, anodal tDCS appeared to be able to maintain the level of neuropsychological performance and slow down the progression of AD both for the short-term (10 days) and long-term (over 8 months) intervention [80].
Repetitive Transcranial Magnetic Stimulation (rTMS)
The rTMS technique has been reported as a safe and non-invasive tool to regulate and balance the activity of neural cells. When applied repetitively, rTMS can either stimulates (e.g., using 5–20 Hz stimulation) or suppresses (e.g., using ≤ 1 Hz stimulation) excitability of cortical neurons by producing magnetic fields to modulate the synaptic activities of neuronal circuits across specific brain networks [81]. Most of the studies have chosen the dorsolateral prefrontal cortex (DLPFC) as the target brain region because of its neuroplasticity and critical role in cognition [82]. A 6-week rTMS intervention significantly improved global cognitive functions as well as memory and language levels especially in the mild stage of AD [83]. The improved visual recognition memory functions and clock drawing scores after left lateral parietal rTMS were associated with elevated peripheral BDNF levels and antioxidant capacity [84]. rTMS is often administered with cognitive training, and previous reports found that patients with mild-to-moderate AD treated with a combined therapy of cognitive training and real rTMS showed excellent safety profile and high adherence, as well as more benefits in cognition than those receiving cognitive training and sham rTMS [85,86,87].
Transcranial Pulse Stimulation (TPS)
TPS is a novel ultrasound sonication technique for clinical applications, which is based on single ultrasound pulses (3 µs) repeated every 200–300 ms. Ultrasound is the first technique that allows non-invasive DBS and can be reliably targeted [88]. This also enables a controllable stimulation of a target brain region without affecting other brain areas. The neuropsychological scores improved significantly after TPS treatment in patients with probable AD, which was associated with upregulation of memory network and could last up to 3 months [89]. In the follow-up study, the pre-to-post analysis revealed a significant improvement in neuropsychological test battery, which was associated with a reduced cortical atrophy within AD critical brain regions [90]. For future research on the application of TPS in patients with AD, a standard brain sonication concept must be developed, including standardizations of local skull thickness for the inclusion criteria. Cognitive training should also be considered as a combined treatment with TPS in future studies.
Intermittent Theta Burst Stimulation (iTBS)
iTBS is a novel brain stimulation technique that enables elevated cortical excitability more rapidly than conventional rTMS [91]; however, research of its application in the treatment of AD is very limited. There was only one pilot study published within the reviewed period, showing that iTBS of the left DLPFC of patients with AD improved association memory (AM) and other cognitive performance including memory, attention, executive, and language functions, which were negatively correlated with connectivity of the DLPFC and right precuneus [92]. Blinded RCTs with a larger sample size are needed to confirm these results and identify optimized treatment parameters.
Limitations and Future Research Directions of Brain Stimulation in Patients with AD
The long-term effects of brain stimulation on cognitive performance of patients with AD are still largely unclear, and further research to quantify these effects is of clear value. Further research could focus on following up individuals who benefit from this type of treatment throughout a period of up to 1 year after the intervention course to work out the longevity of the effects, and on exploring the time course of efficacy. Additionally, further research on more severe patients may be valuable as well, in which a higher stimulation intensity relative to motor threshold may be required to increase efficacy. Finally, the exact neurobiological mechanisms of brain stimulation remain to be further elucidated.
Nutrition Supplementations
Diet is an important modifiable risk factor for mild cognitive impairment (MCI) and dementia [93], and a nutrient intervention in MCI showed beneficial effects on brain atrophy [94]. The nutrient intervention is unlikely to produce a swift effect in a short period of time; therefore, clinical trials in this area often take several months or even years to observe the outcomes. The RCTs of nutritional interventions on individuals with AD published in the last decade are summarized in Table 4.
A 24-month nutrient supplementation (a combined formula including docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA), vitamin B12, B6, C, E, and folic acid, etc.) for patients with prodromal AD resulted in beneficial effects on cognition and function and hippocampal atrophy measures (secondary outcome), but not on neuropsychological test battery (primary outcome) [95]. When administrating a nutrient supplementation (Souvenaid) of similar ingredients, a significant increase in the exploratory memory outcome was observed in addition to good tolerability, safety, and high intake adherence [96, 97]. Moreover, a combined formula has also been administered in another large-scale trial, and the cognitive and behavioral performance of the active-treated group improved over 3–6 months and was maintained during open-label extensions [98, 99]. These critical nutrient ingredients have also been tested separately in different studies. For instance, as part of the OmegAD study, the cognitive function of patients with mild-to-moderate AD was preserved with the increase of plasma omega-3 fatty acid (ω3 FA) levels [100], and a 6-month supplementation of dietary DHA led to an increased plasma concentration of DHA and EPA, as well as an upregulated global DNA hypomethylation in peripheral blood leukocytes (PBLs), which may influence inflammatory and other critical processes during AD progression [101]. However, the daily supplementation of EPA- and DHA-enriched omega-3 polyunsaturated fatty acids (PUFAs) did not seem to have obvious benefits in cognition or mood for individuals with cognitive impairment or dementia [102]. Low serum folate levels can alter inflammatory reactions in AD; therefore, supplementation of folic acid is beneficial to patients with AD at different disease stages [103]. Cognitive improvement was only detected in ApoE4 noncarriers after a 3-month administration of medium-chain triglyceride-containing food [104]. Supplementation with Macushield (including meso-zeaxanthin, lutein, and zeaxanthin) has merely improved visual function but not cognitive function in patients with AD [105]. Supplementation of scallop-derived purified plasmalogen has improved cognitive function in mild AD [106], while supplementation of soy isoflavones has not [107]. On the basis of the association between changes in microbiota and cognitive behaviors through the gut–brain axis, probiotics have been added into the nutritional supplementations and showed positive outcomes in cognitive improvements as indicated by elevated MMSE score and changed metabolic status [108, 109], suggesting their potential as a nonpharmacological intervention for patients with mild AD. In addition to the aforementioned nutrients or their critical components, amino acids are also being used to ameliorate the cognitive impairment. Previously in a clinical trial of middle-aged and older adults, seven essential amino acids (Leu, Phe, Lys, Ile, His, Val, Trp) showed beneficial effects on the improvement of cognitive and psychosocial functioning [110]. More recently, oral administration of combined metabolic precursors (CMAs), consisting of NAD+ and glutathione precursors, improved cognitive functions in patients with AD as indicated by significantly decreased ADAS-Cog score on day 84 compared to baseline (preprint: https://doi.org/10.1101/2021.07.14.21260511).
Apart from nutrient formula supplementation, nutritional guidance is a different type of intervention, which is particularly important for home-dwelling patients with AD and their families to maintain appropriate nutritional status, improve QoL, and prevent falls. The guidance with high feasibility has inspired these studied AD families to make positive changes in and positive attitudes toward diets and nutrition [111], thus improving QoL and reducing rate of falls without any change in body weight [112].
Limitations and Future Directions
Several factors must be carefully considered when designing future clinical trials for the nonpharmacological interventions. Firstly, like pharmacological treatments, the non-drug interventions may tend to be influenced by some confounding factors, e.g., participants’ “everyday” and “clinical day” variables [113], as well as cultural differences [114]. These hidden variables may have the potential to obscure the perceived efficacy of an intervention. Some statistical approaches such as stratification and multivariate modeling are utilized to minimize the potential influence of confounding variables; however, these approaches require large sample sizes to obtain sufficient statistical power. Alternatively, adopting pair-matched, case-controlled studies may also reduce the influence of confounders. Therefore, large sample trials or carefully designed pair-matched studies may be needed to eliminate the distorted effects.
Secondly, as cognitive impairment in dementia is progressive because of neurodegeneration or aging dystrophy, the evaluation of efficacy in the short-term period may emphasize the superficial initial results. For instance, when short-term cognition was detected to be improved following a comprehensive intervention, such benefits disappeared during a long-term follow-up period of 24 months [30]. The same situation was seen for physical exercise interventions, where cognition (indicated by MMSE) was not improved at a 2-year follow-up visit [73]. Regarding the nutritional interventions (Table 4), beneficial cognitive outcome was observed in a follow-up period of less than 6 months, but often disappeared when follow-up was longer than 6 months. Although overwhelming, the reality here should trigger us to rethink the direction of developing non-drug interventional strategies for patients with AD. Therefore, when designing future studies, the effects of interventions should be confirmed in the long-term course with placebo or sham stimulations.
Thirdly, it is noteworthy that some nonpharmacological treatments may include a time-dependent dilution (so-called placebo effects) as a resolution of reactive neuropsychiatric symptoms against temporary psychosocial conflicts, which may secondarily improve the QoL in individuals with dementia. Thus, these invisible hidden effects must be critically reconsidered to avoid drawing an incorrect conclusion. Other factors, such as study duration, sample size, and study design may affect the placebo response [115]. As time-dependent effects play a crucial role in drawing a conclusion on effectiveness of an intervention, and data from current clinical trials may not be sufficient to draw a firm conclusion, placebo-controlled studies should be performed wherever possible in the future. In addition, utilization of a control database with model predictions conditioned for baseline severity may also be considered as an option to evaluate placebo response. Hopefully, more conclusions would be approached for these non-drug interventions for AD.
Conclusions
The clinical management of patients with AD and dementia is a significant public health concern because of the lack of effective treatments for AD at the moment, especially lack of disease-modifying therapies. The research and development of effective nonpharmacological interventions for controlling the symptoms of patients with AD and relieving the heavy burden posed on our society is therefore of great importance. The exploration for nonpharmacological intervention of AD is still an ongoing and expanding area of interest. Performing these interventions requires practical considerations, as these processes are usually continuous particularly when long-term effects on patients and extra benefits for carers are expected. The aims of clinical trials are to develop effective strategies that will maximize tolerance and efficacy, and minimize risks the intervention itself may bring. Therefore, several aspects should be considered, including type of intervention, sample size, targeted cohort, standardized protocol, primary and secondary outcome measures, etc. In addition to cognitive improvements, the next step is to focus on maintaining independence in daily life, increasing the QoL, and releasing the caregivers’ burdens.
Abbreviations
- AChEIs:
-
Acetylcholinesterase inhibitors
- AD:
-
Alzheimer’s disease
- ADAS-Cog:
-
Alzheimer’s Disease Assessment Scale-Cognitive subscale
- ADL:
-
Activities of daily living
- AMB:
-
Aberrant motor behavior
- BI:
-
Barthel Index
- BPSD:
-
Behavioral and psychological symptoms of dementia
- CAMCOG:
-
Cambridge cognitive examination
- ChEIs:
-
Cholinesterase inhibitors
- CST:
-
Cognitive stimulation therapy
- DBS:
-
Deep brain stimulation
- DHA:
-
Docosahexaenoic acid
- EPA:
-
Eicosapentaenoic acid
- fMRI:
-
Functional magnetic resonance imaging
- GDS:
-
Geriatric Depression Scale
- iTBS:
-
Intermittent theta burst stimulation
- MCI:
-
Mild cognitive impairment
- MMSE:
-
Mini-Mental State Examination
- NPI:
-
Neuropsychiatric inventory
- ω3 FA:
-
Omega-3 fatty acid
- PBLs:
-
Peripheral blood leukocytes
- PUFAs:
-
Polyunsaturated fatty acids
- QoL:
-
Quality of life
- RCT:
-
Randomized controlled trial
- rTMS:
-
Repetitive transcranial magnetic stimulation
- tDCS:
-
Transcranial direct current stimulation
- TPS:
-
Transcranial pulse stimulation
References
Wimo A, Guerchet M, Ali GC, et al. The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimers Dement. 2017;13(1):1–7.
Bohnen NI, Kaufer DI, Hendrickson R, et al. Degree of inhibition of cortical acetylcholinesterase activity and cognitive effects by donepezil treatment in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2005;76(3):315–9.
Homma A, Atarashi H, Kubota N, et al. Efficacy and safety of sustained release donepezil high dose versus immediate release donepezil standard dose in Japanese patients with severe Alzheimer’s disease: a randomized double-blind trial. J Alzheimers Dis. 2016;52(1):345–57.
Scales K, Zimmerman SandMiller SJ. Evidence-based nonpharmacological practices to address behavioral and psychological symptoms of dementia. Gerontologist. 2018;58(1):S88–102.
Bahar-Fuchs A, Clare LB. Cognitive training and cognitive rehabilitation for mild to moderate Alzheimer’s disease and vascular dementia. Cochrane Database Syst Rev. 2013;6:3260.
Clare L, Woods RT, Moniz Cook ED, et al. Cognitive rehabilitation and cognitive training for early-stage Alzheimer’s disease and vascular dementia. Cochrane Database Syst Rev. 2003;4:3260.
Huntley JD, Hampshire A, Bor D, et al. Adaptive working memory strategy training in early Alzheimer’s disease: randomised controlled trial. Br J Psychiatry. 2017;210(1):61–6.
McDaniel MA, Shelton JT, Breneiser JE, et al. Focal and nonfocal prospective memory performance in very mild dementia: a signature decline. Neuropsychology. 2011;25(3):387–96.
Shelton JT, Lee JH, Scullin MK, et al. Improving prospective memory in healthy older adults and individuals with very mild Alzheimer’s disease. J Am Geriatr Soc. 2016;64(6):1307–12.
Baglio F, Griffanti L, Saibene FL, et al. Multistimulation group therapy in Alzheimer’s disease promotes changes in brain functioning. Neurorehabil Neural Repair. 2015;29(1):13–24.
Bajpai S, Tripathi M, Pandey RM, et al. Development and validation of cognitive training intervention for Alzheimer’s disease (CTI-AD): a picture-based interventional program. Dementia (London). 2020;19(4):1203–19.
McAulay JA. Delivery of cognitive stimulation therapy for people with dementia in an inpatient setting (innovative practice). Dementia (London). 2020;19(7):2513–20.
Spector A, Thorgrimsen L, Woods B, et al. Efficacy of an evidence-based cognitive stimulation therapy programme for people with dementia: randomised controlled trial. Br J Psychiatry. 2003;183:248–54.
Berg-Weger M, Tebb S, Henderson-Kalb J, et al. Cognitive stimulation therapy: a tool for your practice with persons with dementia? J Am Med Dir Assoc. 2015;16(9):795–6.
Aguirre E, Hoare Z, Streater A, et al. Cognitive stimulation therapy (CST) for people with dementia–who benefits most? Int J Geriatr Psychiatry. 2013;28(3):284–90.
Lok N, Buldukoglu KE. Effects of the cognitive stimulation therapy based on Roy’s adaptation model on Alzheimer’s patients’ cognitive functions, coping-adaptation skills, and quality of life: a randomized controlled trial. Perspect Psychiatr Care. 2020;56(3):581–92.
Garcia-Casal JA, Goni-Imizcoz M, Perea-Bartolome MV, et al. The efficacy of emotion recognition rehabilitation for people with Alzheimer’s disease. J Alzheimers Dis. 2017;57(3):937–51.
Woods B, Spector A, Jones C, et al. Reminiscence therapy for dementia. Cochrane Database Syst Rev. 2005;2:1120.
Stinson CK. Structured group reminiscence: an intervention for older adults. J Contin Educ Nurs. 2009;40(11):521–8.
DuruAsiret G, Kapucu S. The effect of reminiscence therapy on cognition, depression, and activities of daily living for patients with Alzheimer disease. J Geriatr Psychiatry Neurol. 2016;29(1):31–7.
Barban F, Annicchiarico R, Pantelopoulos S, et al. Protecting cognition from aging and Alzheimer’s disease: a computerized cognitive training combined with reminiscence therapy. Int J Geriatr Psychiatry. 2016;31(4):340–8.
Li M, Lyu JH, Zhang Y, et al. The clinical efficacy of reminiscence therapy in patients with mild-to-moderate Alzheimer disease: study protocol for a randomized parallel-design controlled trial. Medicine (Baltimore). 2017;96(51): e9381.
Baird A, Samson S. Music and dementia. Prog Brain Res. 2015;217:207–35.
Jacobsen JH, Stelzer J, Fritz TH, et al. Why musical memory can be preserved in advanced Alzheimer’s disease. Brain. 2015;138(Pt 8):2438–50.
Fraile E, Bernon D, Rouch I, et al. The effect of learning an individualized song on autobiographical memory recall in individuals with Alzheimer’s disease: a pilot study. J Clin Exp Neuropsychol. 2019;41(7):760–8.
Clements-Cortes A, Ahonen H, Evans M, et al. Short-term effects of rhythmic sensory stimulation in Alzheimer’s disease: an exploratory pilot study. J Alzheimers Dis. 2016;52(2):651–60.
Spector A, Orrell MB. Cognitive stimulation therapy (CST): effects on different areas of cognitive function for people with dementia. Int J Geriatr Psychiatry. 2010;25(12):1253–8.
Quintana-Hernandez DJ, Miro-Barrachina MT, Ibanez-Fernandez IJ, et al. Mindfulness in the maintenance of cognitive capacities in Alzheimer’s disease: a randomized clinical trial. J Alzheimers Dis. 2016;50(1):217–32.
Camargo CH, Justus FF, Retzlaff G. The effectiveness of reality orientation in the treatment of Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2015;30(5):527–32.
Balietti M, Giuli C, Fattoretti P, et al. Effect of a comprehensive intervention on plasma BDNF in patients with Alzheimer’s disease. J Alzheimers Dis. 2017;57(1):37–43.
Brown H, D’Amico F, Knapp M, et al. A cost effectiveness analysis of maintenance cognitive stimulation therapy (MCST) for people with dementia: examining the influence of cognitive ability and living arrangements. Aging Ment Health. 2019;23(5):602–7.
Clare L, Wilson BA, Carter G, et al. Relearning face-name associations in early Alzheimer’s disease. Neuropsychology. 2002;16(4):538–47.
Silva AR, Pinho MS, Macedo L, et al. It is not only memory: effects of SenseCam on improving well-being in patients with mild Alzheimer disease. Int Psychogeriatr. 2017;29(5):741–54.
Regan B, Wells Y, Farrow M, et al. MAXCOG-maximizing cognition: a randomized controlled trial of the efficacy of goal-oriented cognitive rehabilitation for people with mild cognitive impairment and early Alzheimer disease. Am J Geriatr Psychiatry. 2017;25(3):258–69.
Muniz R, Serra CM, Reisberg B, et al. Cognitive-motor intervention in Alzheimer’s disease: long-term results from the Maria Wolff trial. J Alzheimers Dis. 2015;45(1):295–304.
Amieva H, Robert PH, Grandoulier AS, et al. Group and individual cognitive therapies in Alzheimer’s disease: the ETNA3 randomized trial. Int Psychogeriatr. 2016;28(5):707–17.
Brueggen K, Kasper E, Ochmann S, et al. Cognitive rehabilitation in Alzheimer’s disease: a controlled intervention trial. J Alzheimers Dis. 2017;57(4):1315–24.
Garcia-Alberca JM. Cognitive-behavioral treatment for depressed patients with Alzheimer’s disease. An open trial. Arch Gerontol Geriatr. 2017;71:1–8.
Brunelle-Hamann L, Thivierge SM. Impact of a cognitive rehabilitation intervention on neuropsychiatric symptoms in mild to moderate Alzheimer’s disease. Neuropsychol Rehabil. 2015;25(5):677–707.
Koivisto AM, Hallikainen I, Valimaki T, et al. Early psychosocial intervention does not delay institutionalization in persons with mild Alzheimer disease and has impact on neither disease progression nor caregivers’ well-being: ALSOVA 3-year follow-up. Int J Geriatr Psychiatry. 2016;31(3):273–83.
Craig D, Mirakhur A, Hart DJ, et al. A cross-sectional study of neuropsychiatric symptoms in 435 patients with Alzheimer’s disease. Am J Geriatr Psychiatry. 2005;13(6):460–8.
Gomez-Gallego M, Gomez-Amor J-G. Determinants of quality of life in Alzheimer’s disease: perspective of patients, informal caregivers, and professional caregivers. Int Psychogeriatr. 2012;24(11):1805–15.
Gonfrier S, Andrieu S, Renaud D, et al. Course of neuropsychiatric symptoms during a 4-year follow up in the REAL-FR cohort. J Nutr Health Aging. 2012;16(2):134–7.
Spalletta G, Musicco M, Padovani A, et al. Neuropsychiatric symptoms and syndromes in a large cohort of newly diagnosed, untreated patients with Alzheimer disease. Am J Geriatr Psychiatry. 2010;18(11):1026–35.
Gauthier S, Cummings J, Ballard C, et al. Management of behavioral problems in Alzheimer’s disease. Int Psychogeriatr. 2010;22(3):346–72.
Smith M, Gerdner LA, Hall GR, et al. History, development, and future of the progressively lowered stress threshold: a conceptual model for dementia care. J Am Geriatr Soc. 2004;52(10):1755–60.
Sheridan PL, Hausdorff JM. The role of higher-level cognitive function in gait: executive dysfunction contributes to fall risk in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2007;24(2):125–37.
Burns JM, Cronk BB, Anderson HS, et al. Cardiorespiratory fitness and brain atrophy in early Alzheimer disease. Neurology. 2008;71(3):210–6.
Tolppanen AM, Solomon A, Kulmala J, et al. Leisure-time physical activity from mid- to late life, body mass index, and risk of dementia. Alzheimers Dement. 2015;11(4):434–43.
Larson EB, Wang L, Bowen JD, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144(2):73–81.
Schultz SA, Boots EA, Almeida RP, et al. Cardiorespiratory fitness attenuates the influence of amyloid on cognition. J Int Neuropsychol Soc. 2015;21(10):841–50.
Ohman H, Savikko N, Strandberg T, et al. Effects of exercise on functional performance and fall rate in subjects with mild or advanced Alzheimer’s disease: secondary analyses of a randomized controlled study. Dement Geriatr Cogn Disord. 2016;41(3–4):233–41.
Thune-Boyle IC, Iliffe S, Cerga-Pashoja A, et al. The effect of exercise on behavioral and psychological symptoms of dementia: towards a research agenda. Int Psychogeriatr. 2012;24(7):1046–57.
Vidoni ED, Perales J, Alshehri M, et al. Aerobic exercise sustains performance of instrumental activities of daily living in early-stage Alzheimer disease. J Geriatr Phys Ther. 2019;42(3):E129–34.
Huang P, Fang R, Li BY, et al. Exercise-related changes of networks in aging and mild cognitive impairment brain. Front Aging Neurosci. 2016;8:47.
Jia RX, Liang JH, Xu Y, et al. Effects of physical activity and exercise on the cognitive function of patients with Alzheimer disease: a meta-analysis. BMC Geriatr. 2019;19(1):181.
De la Rosa A, Olaso-Gonzalez G, Arc-Chagnaud C, et al. Physical exercise in the prevention and treatment of Alzheimer’s disease. J Sport Health Sci. 2020;9(5):394–404.
Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108(7):3017–22.
Liu PZ, Nusslock R. Exercise-mediated neurogenesis in the hippocampus via BDNF. Front Neurosci. 2018;12:52.
Morris JK, Vidoni ED, Johnson DK, et al. Aerobic exercise for Alzheimer’s disease: a randomized controlled pilot trial. PLoS ONE. 2017;12(2): e0170547.
Sobol NA, Hoffmann K, Frederiksen KS, et al. Effect of aerobic exercise on physical performance in patients with Alzheimer’s disease. Alzheimers Dement. 2016;12(12):1207–15.
Hoffmann K, Sobol NA, Frederiksen KS, et al. Moderate-to-high intensity physical exercise in patients with Alzheimer’s disease: a randomized controlled trial. J Alzheimers Dis. 2016;50(2):443–53.
Yang SY, Shan CL, Qing H, et al. The effects of aerobic exercise on cognitive function of Alzheimer’s disease patients. CNS Neurol Disord Drug Targets. 2015;14(10):1292–7.
Castellano CA, Paquet N, Dionne IJ, et al. A 3-month aerobic training program improves brain energy metabolism in mild Alzheimer’s disease: preliminary results from a neuroimaging study. J Alzheimers Dis. 2017;56(4):1459–68.
Li D, Thomas R, Tsai MY, et al. Vascular biomarkers to predict response to exercise in Alzheimer’s disease: the study protocol. BMJ Open. 2016;6(12): e011054.
El-Khoury F, Cassou B, Charles MA, et al. The effect of fall prevention exercise programmes on fall induced injuries in community dwelling older adults: systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;347:6234.
Ohman H, Savikko N, Strandberg TE, et al. Effects of exercise on cognition: the Finnish Alzheimer disease exercise trial: a randomized controlled trial. J Am Geriatr Soc. 2016;64(4):731–8.
Holthoff VA, Marschner K, Scharf M, et al. Effects of physical activity training in patients with Alzheimer’s dementia: results of a pilot RCT study. PLoS ONE. 2015;10(4): e0121478.
Parvin E, Mohammadian F, Amani-Shalamzari S, et al. Dual-task training affect cognitive and physical performances and brain oscillation ratio of patients with Alzheimer’s disease: a randomized controlled trial. Front Aging Neurosci. 2020;12:605317.
Babiloni C, Del Percio C, Boccardi M, et al. Occipital sources of resting-state alpha rhythms are related to local gray matter density in subjects with amnesic mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging. 2015;36(2):556–70.
Shih YH, Pai MC, Lin HS, et al. Effects of walking on sundown syndrome in community-dwelling people with Alzheimer’s disease. Int J Older People Nurs. 2020;15(2): e12292.
Gallagher-Thompson D, Brooks JO 3rd, Bliwise D, et al. The relations among caregiver stress, “sundowning” symptoms, and cognitive decline in Alzheimer’s disease. J Am Geriatr Soc. 1992;40(8):807–10.
Chen KH, Chen HH, Li L, et al. The impact of exercise on patients with dementia: a 2-year follow-up. Medicine (Baltimore). 2020;99(23):e20597.
Lozano AM, Fosdick L, Chakravarty MM, et al. A phase II study of fornix deep brain stimulation in mild Alzheimer’s disease. J Alzheimers Dis. 2016;54(2):777–87.
Sankar T, Chakravarty MM, Bescos A, et al. Deep brain stimulation influences brain structure in Alzheimer’s disease. Brain Stimul. 2015;8(3):645–54.
Noreik M, Kuhn J, Hardenacke K, et al. Changes in nutritional status after deep brain stimulation of the nucleus basalis of Meynert in Alzheimer’s disease-results of a phase I study. J Nutr Health Aging. 2015;19(8):812–8.
Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. Neuroscientist. 2011;17(1):37–53.
Bystad M, Gronli O, Rasmussen ID, et al. Transcranial direct current stimulation as a memory enhancer in patients with Alzheimer’s disease: a randomized, placebo-controlled trial. Alzheimers Res Ther. 2016;8(1):13.
Im JJ, Jeong H, Bikson M, et al. Effects of 6-month at-home transcranial direct current stimulation on cognition and cerebral glucose metabolism in Alzheimer’s disease. Brain Stimul. 2019;12(5):1222–8.
Gangemi A, Colombo BandFabio RA. Effects of short- and long-term neurostimulation (tDCS) on Alzheimer’s disease patients: two randomized studies. Aging Clin Exp Res. 2021;33(2):383–90.
Bauer PR, Kalitzin S, Zijlmans M, et al. Cortical excitability as a potential clinical marker of epilepsy: a review of the clinical application of transcranial magnetic stimulation. Int J Neural Syst. 2014;24(2):1430001.
Lara AH, Wallis JD. The role of prefrontal cortex in working memory: a mini review. Front Syst Neurosci. 2015;9:173.
Zhao J, Li Z, Cong Y, et al. Repetitive transcranial magnetic stimulation improves cognitive function of Alzheimer’s disease patients. Oncotarget. 2017;8(20):33864–71.
Velioglu HA, Hanoglu L, Bayraktaroglu Z, et al. Left lateral parietal rTMS improves cognition and modulates resting brain connectivity in patients with Alzheimer’s disease: possible role of BDNF and oxidative stress. Neurobiol Learn Mem. 2021;180:107410.
Brem AK, Di Iorio R, Fried PJ, et al. Corticomotor plasticity predicts clinical efficacy of combined neuromodulation and cognitive training in Alzheimer’s disease. Front Aging Neurosci. 2020;12:200.
Sabbagh M, Sadowsky C, Tousi B, et al. Effects of a combined transcranial magnetic stimulation (TMS) and cognitive training intervention in patients with Alzheimer’s disease. Alzheimers Dement. 2020;16(4):641–50.
Zhang F, Qin Y, Xie L, et al. High-frequency repetitive transcranial magnetic stimulation combined with cognitive training improves cognitive function and cortical metabolic ratios in Alzheimer’s disease. J Neural Transm (Vienna). 2019;126(8):1081–94.
Legon W, Ai L, Bansal P, et al. Neuromodulation with single-element transcranial focused ultrasound in human thalamus. Hum Brain Mapp. 2018;39(5):1995–2006.
Beisteiner R, Matt E, Fan C, et al. Transcranial pulse stimulation with ultrasound in Alzheimer’s disease—a new navigated focal brain therapy. Adv Sci (Weinh). 2020;7(3):1902583.
Popescu T, Pernet CR. Transcranial ultrasound pulse stimulation reduces cortical atrophy in Alzheimer’s patients: a follow-up study. Alzheimers Dement (N Y). 2021;7(1): e12121.
Huang YZ, Edwards MJ, Rounis E, et al. Theta burst stimulation of the human motor cortex. Neuron. 2005;45(2):201–6.
Wu X, Ji GJ, Geng Z, et al. Strengthened theta-burst transcranial magnetic stimulation as an adjunctive treatment for Alzheimer’s disease: an open-label pilot study. Brain Stimul. 2020;13(2):484–6.
Scarmeas N, Stern Y, Mayeux R, et al. Mediterranean diet and mild cognitive impairment. Arch Neurol. 2009;66(2):216–25.
Smith AD, Smith SM, de Jager CA, et al. Homocysteine-lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: a randomized controlled trial. PLoS ONE. 2010;5(9): e12244.
Soininen H, Solomon A, Visser PJ, et al. 24-month intervention with a specific multinutrient in people with prodromal Alzheimer’s disease (LipiDiDiet): a randomised, double-blind, controlled trial. Lancet Neurol. 2017;16(12):965–75.
Rijpma A, Meulenbroek O, van Hees AM, et al. Effects of Souvenaid on plasma micronutrient levels and fatty acid profiles in mild and mild-to-moderate Alzheimer’s disease. Alzheimers Res Ther. 2015;7(1):51.
Olde Rikkert MG, Verhey FR, Blesa R, et al. Tolerability and safety of Souvenaid in patients with mild Alzheimer’s disease: results of multi-center, 24-week, open-label extension study. J Alzheimers Dis. 2015;44(2):471–80.
Remington R, Bechtel C, Larsen D, et al. A phase II randomized clinical trial of a nutritional formulation for cognition and mood in Alzheimer’s disease. J Alzheimers Dis. 2015;45(2):395–405.
Remington R, Bechtel C, Larsen D, et al. Maintenance of cognitive performance and mood for individuals with Alzheimer’s disease following consumption of a nutraceutical formulation: a one-year open-label study. J Alzheimers Dis. 2016;51(4):991–5.
Eriksdotter M, Vedin I, Falahati F, et al. Plasma fatty acid profiles in relation to cognition and gender in Alzheimer’s disease patients during oral omega-3 fatty acid supplementation: the OmegAD study. J Alzheimers Dis. 2015;48(3):805–12.
Karimi M, Vedin I, Freund Levi Y, et al. DHA-rich n-3 fatty acid supplementation decreases DNA methylation in blood leukocytes: the OmegAD study. Am J Clin Nutr. 2017;106(4):1157–65.
Phillips MA, Childs CE, Calder PC, et al. No effect of omega-3 fatty acid supplementation on cognition and mood in individuals with cognitive impairment and probable Alzheimer’s disease: a randomised controlled trial. Int J Mol Sci. 2015;16(10):24600–13.
Chen H, Liu S, Ji L, et al. Folic acid supplementation mitigates Alzheimer’s disease by reducing inflammation: a randomized controlled trial. Mediators Inflamm. 2016;2016:5912146.
Ohnuma T, Toda A, Kimoto A, et al. Benefits of use, and tolerance of, medium-chain triglyceride medical food in the management of Japanese patients with Alzheimer’s disease: a prospective, open-label pilot study. Clin Interv Aging. 2016;11:29–36.
Nolan JM, Loskutova E, Howard A, et al. The impact of supplemental macular carotenoids in Alzheimer’s disease: a randomized clinical trial. J Alzheimers Dis. 2015;44(4):1157–69.
Fujino T, Yamada T, Asada T, et al. Efficacy and blood plasmalogen changes by oral administration of plasmalogen in patients with mild Alzheimer’s disease and mild cognitive impairment: a multicenter, randomized, double-blind, placebo-controlled trial. EBioMedicine. 2017;17:199–205.
Gleason CE, Fischer BL, Dowling NM, et al. Cognitive effects of soy isoflavones in patients with Alzheimer’s disease. J Alzheimers Dis. 2015;47(4):1009–19.
Tamtaji OR, Heidari-Soureshjani R, Mirhosseini N, et al. Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer’s disease: a randomized, double-blind, controlled trial. Clin Nutr. 2019;38(6):2569–75.
Akbari E, Asemi Z, DaneshvarKakhaki R, et al. Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer’s disease: a randomized, double-blind and controlled trial. Front Aging Neurosci. 2016;8:256.
Suzuki H, Yamashiro D, Ogawa S, et al. Intake of seven essential amino acids improves cognitive function and psychological and social function in middle-aged and older adults: a double-blind, randomized, placebo-controlled trial. Front Nutr. 2020;7:586166.
Puranen TM, Pitkala KH, Suominen MH. Tailored nutritional guidance for home-dwelling AD families: the Feasibility of and Elements Promoting Positive Changes in Diet (NuAD-Trial). J Nutr Health Aging. 2015;19(4):454–9.
Suominen MH, Puranen TM, Jyvakorpi SK, et al. Nutritional guidance improves nutrient intake and quality of life, and may prevent falls in aged persons with Alzheimer disease living with a spouse (NuAD Trial). J Nutr Health Aging. 2015;19(9):901–7.
Liyanage SI, Santos CandWeaver DF. The hidden variables problem in Alzheimer’s disease clinical trial design. Alzheimers Dement (N Y). 2018;4:628–35.
Brodaty H, Mittelman M, Gibson L, et al. The effects of counseling spouse caregivers of people with Alzheimer disease taking donepezil and of country of residence on rates of admission to nursing homes and mortality. Am J Geriatr Psychiatry. 2009;17(9):734–43.
Ito K, Corrigan B, Romero K, et al. Understanding placebo responses in Alzheimer’s disease clinical trials from the literature meta-data and CAMD database. J Alzheimers Dis. 2013;37(1):173–83.
Acknowledgements
Data Availability
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Disclosures
Xianqian Li, Min Ji, Hongmei Zhang, Zunjian Liu, Yujing Chai, Qi Cheng, Yue Yang, Dennis Cordato and Jianqun Gao have nothing to disclose.
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Authors’ Contributions
JG developed the concept of the article, supervised the work and finalized the manuscript. XL, MJ and QC carried out the literature search and selection. XL, MJ wrote the first draft. HZ, ZL, YC and YY reviewed and edited the draft. A/Prof DC critically evaluated the manuscript and performed language check.
Authorship
All named authors met the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Li, X., Ji, M., Zhang, H. et al. Non-drug Therapies for Alzheimer’s Disease: A Review. Neurol Ther 12, 39–72 (2023). https://doi.org/10.1007/s40120-022-00416-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-022-00416-x