Abstract
Cladribine is a disease-modifying selective immune reconstitution oral therapy for adult patients with highly active relapsing multiple sclerosis (RMS). It was approved in the USA in 2019 and in Europe in 2017, thus there are still gaps in existing guidelines for using cladribine tablets in clinical practice. Nine experts with extensive experience in managing patients with multiple sclerosis in Spain identified some of the unanswered questions related to the real-life use of cladribine tablets. They reviewed the available clinical trial data and real-world evidence, including their own experiences of using cladribine, over the course of three virtual meetings held between November 2020 and January 2021. This article gathers their practical recommendations to aid treatment decision-making and optimise the use of cladribine tablets in patients with RMS. The consensus recommendations cover the following areas: candidate patient profiles, switching strategies (to and from cladribine), managing response to cladribine and safety considerations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Cladribine tablets are the first short-course oral therapy indicated for adult patients with highly active relapsing multiple sclerosis (RMS). |
Nine experts agreed on practical recommendations to optimise the use of cladribine tablets in patients with RMS based on their extensive experience in managing patients and available clinical trial data and real-world evidence. |
The consensus recommendations cover the following topics: candidate patient profiles, switching strategies (to and from cladribine), managing response to cladribine and safety considerations. |
Introduction
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system that affects approximately 2.8 million people [1]. MS is most commonly diagnosed in 20–30-year-olds, with women being three times more likely to be affected than men. MS can lead to physical disability, cognitive impairment and decreased quality of life.
Effective management of the disease requires a multidisciplinary approach including disease-modifying therapies (DMT), symptomatic treatments, lifestyle modifications, psychological support and rehabilitation interventions [2]. DMT for relapsing forms of MS reduce the frequency and severity of MS relapses, reduce the development of new areas of damage in the central nervous system (CNS) and slow the accumulation of disability. They have varying mechanisms of action and routes of administration, and include interferons, glatiramer acetate, teriflunomide, sphingosine-1-phosphate receptor modulators, fumarates, different types of monoclonal antibodies and cladribine.
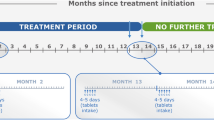
Cladribine is a purine nucleoside analogue. By causing selective B and T cell apoptosis, cladribine interrupts the cascade of immune events central to the pathogenesis of MS [3]. Cladribine tablets (Mavenclad®) are the first short-course oral therapy indicated for adult patients with highly active relapsing multiple sclerosis (RMS) defined by significant clinical or radiological activity [4]. Two courses of cladribine tablets in consecutive years are required to complete the treatment and suppress the disease for 4 years, according to the pivotal studies, and for up to 10 years in some patients according to real-world data [5,6,7]. Each course involves taking cladribine tablets for 4–5 days in two consecutive months.
The efficacy and safety of cladribine were confirmed in phase III trials, CLARITY, CLARITY extension and ORACLE MS, the phase II ONWARD study and the 8-year prospective registry, PREMIERE [5, 8,9,10,11,12]. At 96 weeks, cladribine tablets can reduce the rate of relapse by 58% and the median number of new T1-Gd+ lesions by 86% [8]. Two years after the start of treatment, 91.1% of patients were free of progression in the Expanded Disability Status Scale (EDSS) [9] and after 5 years, 70% were free of progression in the EDSS [13].
Interim data from the CLASSIC-MS phase IV study (that includes patients from CLARITY, CLARITY extension and ORACLE trials), designed to evaluate the long-term efficacy of cladribine tablets in the real-world setting, shows in the CLARITY cohort that over the median follow-up of 10.9 years there was minimal increase in disability, with 78.8% of patients not requiring an ambulatory device (EDSS ≥ 6) at any time since the last parent study dose and 55.8% of patients did not require further DMT treatment after the last dose [14].
Cladribine tablets have been approved worldwide by many different regulatory authorities, including the European Medicines Agency (EMA) in 2017 [15] and the US Food and Drug Administration (FDA) in 2019 [16].
Cladribine has a good tolerability and safety profile. During the first 12 weeks of treatment, patients receiving cladribine tablets and placebo reported similar proportions of serious treatment-emergent adverse events (TEAEs) (2.2% vs. 1.7%) and TEAEs leading to treatment discontinuation (1.6% vs. 1.4%) [17]. Transient lymphopenia is the most common adverse effect (AE) at the recommended dose of 3.5 mg/kg. In general, there is no increased risk for infections with cladribine tablets, except for a higher incidence of herpes zoster [12].
Given the increasing number of approved DMT for RMS in the last few years, physicians need to take into account the patients’ disease severity, prognosis and their circumstances when making treatment decisions [18]. Despite clinical guidelines and detailed drug labels, there are still many unanswered questions related to the real-life use of these treatments.
Nine experts with extensive experience in managing patients with MS in Spain identified some of the gaps in existing guidelines for using cladribine tablets. They reviewed the available clinical trial data and real-world evidence [19,20,21], and discussed their experiences of using cladribine over the course of three virtual meetings held between November 2020 and January 2021.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Participants were asked whether they agreed or not with statements made by a facilitator and to discuss them until they reached consensus. This document gathers the experts’ consensus recommendations which cover the following areas: candidate patient profiles, switching strategies (to and from cladribine), managing response to cladribine and safety considerations. These practical recommendations aim to aid treatment decision-making and optimise the use of cladribine in patients with RMS.
Consensus Recommendations
Profile of Candidates for Treatment with Cladribine Tablets
According to the drug label, cladribine is indicated for adult patients with ‘highly active’ RMS, but the term ‘highly active’ has not been precisely defined and may include patients with a wide range of disease severity [22] defined by clinical or paraclinical tests. Hence, when initiating treatment physicians must consider prognostic factors and other patient intrinsic factors.
The experts agreed that treatment-naïve patients who have experienced two relapses in the last 12 months would be considered good candidates for cladribine. In the case of treatment-naïve patients who have experienced only one relapse, several prognostic factors need to be carefully considered.
Key factors of poor prognosis include demographic factors, such as age at onset of disease (> 40), sex (male) and smoking habit; clinical features, such as relapse severity (defined in Iacobaeus et al. [23]) and poor recovery; and imaging features, such as high lesion load [24], axonal damage (atrophy, black holes) or activity (presence of gadolinium-enhancing lesions or appearance of new lesions in critical areas such as the brainstem/cerebellum and spinal cord) (see also [25] for a review). Other factors such as cognitive impairment and presence of oligoclonal lipid-specific IgM bands in the cerebrospinal fluid should also be considered as factors of poor prognosis [26, 27]. Serum NfL (neurofilament light chain) level is emerging as an important predictive biomarker of MS and may soon also be considered a factor of poor prognosis [28].
Treatment-naïve patients who have experienced more than two relapses in the last 12 months and show signs of highly active disease (with a large number of T2 lesions in the baseline magnetic resonance imaging (MRI), more than three enhancing lesions or high spinal cord injury load) may not be good candidates for treatment with cladribine. Treatment with very high efficacy therapies may be more appropriate to control disease activity in these cases.
In the case of first-line treatment failure, patients’ prognostic factors carry less weight than in treatment-naïve patients. The experts agreed that cladribine is a valid treatment option for patients who have had one relapse after treatment with a moderately effective DMT (such as glatiramer acetate, interferons, teriflunomide, dimethyl fumarate), although the treatment type and other treatment attributes (tolerability, adherence) need to be considered. Switching to cladribine could also be considered for patients who are stable on a moderately effective DMT or sphingosine-1-phosphate receptor modulators that develop tolerability issues or have a medium-term plan to conceive. In the latter case, the change in treatment should be made with caution due to the risk of rebound. If the risk of rebound is high, a highly effective DMT should be considered instead of cladribine.
The experts would not recommend making a change to cladribine tablets in highly active patients who experience AEs with monoclonal antibodies. In this scenario, treatment with cladribine may not be enough to control disease activity.
Because cladribine has an easy, short dosing schedule and offers a good risk–benefit profile with simple monitoring compared with other treatments, there are several patient lifestyle factors that should be taken into account when deciding whether to use this drug. One is the patient’s desire to become pregnant in the short-to-medium term, which is possible 6 months after each course, and preferably once treatment is completed after the second year. Another is the patient’s convenience; cladribine requires few visits to the hospital and is administered at home from the first dose, so it is compatible with the work and personal activity of patients. The convenient dosing regimen of cladribine tablets and the durability of their effect make them a good option for patients with adherence problems, with other comorbidities or who require many drugs, as well as for patients in whom constant immunosuppression is not desired for safety reasons.
In summary, cladribine is a valid treatment option to control RMS in the following treatment-naïve patient profiles:
-
Patients who have had two clinical relapses in the last year and moderate MRI activity
-
Patients who have had one prior clinical relapse with one or two poor prognostic factors (clinical or radiological)
-
Patients who have had one prior clinical relapse and patient lifestyle factors (such as poor adherence and pregnancy planning)
Cladribine is a valid treatment option to control RMS in the following previously treated patients:
-
Patients without marked breakthrough disease who have had one relapse after treatment with a moderately effective DMT
-
Patients who are stable on a moderately effective DMT that develop tolerability issues or wish to become pregnant
-
Patients who are stable on sphingosine-1-phosphate receptor modulators that develop tolerability issues or wish to become pregnant, bearing in mind their risk of rebound
Switching to Cladribine
Several considerations and precautions should be taken before starting cladribine treatment. These are described in the summary of product characteristics (SmPC) and are highlighted here because of their importance. Before starting year 1 treatment with cladribine, the absolute lymphocyte count (ALC) must be in the normal range, a baseline MRI scan within 3 months prior to starting treatment should be available and patients should have antibodies against varicella zoster virus. Active or latent infections (tuberculosis [TB], hepatitis B, hepatitis C, HIV) and other contraindications (pregnancy, renal impairment) should be ruled out.
The experts agreed on some recommendations (additional to the ones on the drug label) when switching to cladribine from another DMT. These are collected in Table 1.
Assessing Response During Treatment with Cladribine
In most cases, unless patients experience a worsening of symptoms or serious safety problems, the efficacy of cladribine should be assessed once two full cladribine courses have been completed (at least 14 months after starting treatment). Data from the CLARITY study indicate that only 2.5% of patients required a change in treatment due to the appearance of disease activity between year 1 and year 2 [8]. In the event of relapse during cladribine treatment, the timing and intensity of the relapse need to be assessed thoroughly as they will influence the treatment strategy.
Activity in Year 1
A relapse after one course of cladribine would not be considered therapeutic failure as the patient would not have completed treatment. These patients should be scanned and monitored closely to assess their clinical evolution. Prior treatment(s) and clinical activity in the previous year should be taken into account when making the decision to complete treatment administration in year 2 or switch to another DMT.
If after the first course of treatment patients have a higher activity than before treatment, perhaps because the patient’s activity was underestimated, the experts would consider switching to another drug. If the activity is caused by a rebound due to prior-treatment washout or occurs nearly a year after the first full course of cladribine, the experts recommend administering the second course. In these circumstances, physicians may consider bringing the second course of cladribine forward slightly (week 48) as was done in the CLARITY trial [8], although this is not mentioned in the SmPC.
Activity in Year 2
A relapse after two courses of cladribine could indicate a suboptimal response and the need for a switch in treatment. However, if the disease activity is observed nearly a year after the second course (months 22–24) or is lower than in year 1, and the ALC is in the normal range (or at least above 800 per mL as stated in the SmPC for the beginning of the second year), the experts would assess the risk–benefit balance and consider the possibility of treating the patient with a third course of cladribine. The decision whether to switch or give another course of cladribine requires careful clinical judgment as well as patient agreement and management of their expectations (Fig. 1).
Patient response and management during the first 24 months of treatment with cladribine. Depending on the degree of clinical and/or radiological activity (y-axis) and when it appears after the first or second course of cladribine (x-axis), physicians should decide whether to give cladribine courses or switch them to a different DMT. Drug-related concerns differ in each scenario as depicted by the different colours in the boxes
Activity in Year 3 or Year 4
If patients who have received two courses of cladribine (complete treatment) have had no disease activity in years 1 and 2 but experience a relapse in year 3 (months 24–36) or year 4 (months 36–48), the experts would consider the possibility of giving a third course of cladribine.
In patients who experience minor clinical and/or radiological activity in year 3 or year 4 and had not experienced any safety or tolerability issues, the experts would consider giving an additional course of cladribine (with the same safety considerations as for starting a second course of cladribine). Safety data of up to four courses of cladribine indicate that there are no unexpected safety issues [5]. If patients experience the same or higher activity than before starting treatment in year 3 or year 4, the experts would consider switching to another DMT. The CLARITY extension study showed that the percentage of patients requiring a change in treatment due to the appearance of disease activity between year 3 and year 4 was 3.1% [5] (Fig. 2).
Management of patients that show significant new disease activity during treatment with cladribine. The decision whether to treat patients with further courses of cladribine or switch them to a different DMT when they show significant new disease activity (clinical and/or radiological) should be made after a risk–benefit assessment. The number of cases is likely to be small. a The CLARITY and CLARITY extension studies showed that the percentage of patients requiring a change in treatment due to the appearance of disease activity between year 1 and 2 was 2.5% and between year 3 and year 4 was 3.1% [5, 8]. b Recent data from the CLASSIC-MS study showed that more than half of the patients (55.8%) who received cladribine in the CLARITY study remained free of additional treatment during a median follow-up of 10.9 years [14]
Activity in Year 5 or Beyond
In patients who have received two courses of cladribine (complete treatment) and have no disease activity until year 5, the experts would consider continuing treatment with cladribine. According to the SmPC there is no contraindication to giving additional courses of cladribine, nor a maximum number of courses. Safety data of up to four courses of cladribine indicates that there are no safety issues.
In year 5 and beyond the monitoring of disease activity must be like that of any patient with MS undergoing treatment. MS specialists should use all possible tools and techniques at their disposal (clinical, radiological, new biomarkers) to establish the benefit of additional courses of cladribine based on previously established criteria.
Switching from Cladribine to Another DMT
When switching to another DMT, physicians should look for the most effective and safe alternative. Data on the outcomes of patients who have switched from cladribine tablets to other DMT are limited and thus guidance on managing this switch is lacking.
Before switching from cladribine to another DMT, a baseline MRI from anytime in the 3 months immediately prior to starting the new treatment should be available, and active or latent infections should be ruled out. Ideally, patients should have a normal ALC to prevent the possible overlap of immunosuppressive effects. Pharmacokinetic interactions are not expected a week after the last dose as cladribine’s estimated terminal half-life is approximately 24 h [4].
The experts recommend starting the new DMT 3–6 months after the last dose of cladribine. The washout period must be short, especially in patients that change therapy as a result of breakthrough disease, but it is necessary for lymphocyte recovery. In some patients with highly active disease, it may not be possible to wait for a normal ALC, and a count of greater than 800 cells/mm3 would be enough for starting a new DMT.
Table 2 collates expert-agreed recommendations for switching from cladribine to another DMT that are additional to the corresponding drug labels.
Cladribine Safety: Recommendations to Maximize the Safety of Treatment with Cladribine
Cladribine tablets have an acceptable and well-characterised tolerability profile in adults with early and advanced RMS. The most frequent AE reported are lymphopenia and decreased lymphocyte count [12]. Cladribine treatment guidelines reduce the incidence of severe and prolonged lymphopenia while preserving durable efficacy.
Cladribine’s dosing schedule offers an opportunity for pregnancy planning for women 6 months after each course. Because cladribine is contraindicated for use during pregnancy, the drug label recommends contraception (for both sexes) for at least 6 months after the last cladribine course. There are many publications on family planning for patients with MS that offer advice on pregnancy prevention methods [30, 31].
In the small number of patients who show persistent lymphopenia or serious opportunistic infections (TB, varicella zoster, human papillomavirus [HPV]) after the first course of cladribine (year 1), the risk–benefit ratio of administering the second course needs to be considered. The experts agreed that these patients should be monitored and, if necessary, switched to another DMT with lower risk of lymphopenia.
To prevent the risk of having to discontinue cladribine as a result of AE, patients should follow the health guidelines provided to the general population, such as routine cancer screening and vaccination schedules. Patients with active TB or hepatitis B or C need to be treated for these infections before starting treatment with cladribine.
The Spanish demyelinating disease study group recommends administering vaccines included in the adult vaccination calendar and some specific vaccines, depending on the patient’s pre-existing immune status, before receiving immunosuppressive treatment to minimize risk of infection during treatment [32]. When immunosuppressive treatment has started, live attenuated vaccines are contraindicated.
If a patient requires a live vaccine after treatment with cladribine, they should wait for the ALC to be within the normal range. Patients should also be tested for high-risk HPV, and pre-treatment vaccination considered. Unlike other MS treatments, cladribine tablets have the advantage that they do not constantly suppress the immune system, which facilitates the vaccination process.
In most cases, inactive vaccines are not contraindicated in patients taking a DMT and there is evidence that their efficacy is similar to that in healthy controls [33, 34]. Preliminary data indicate that seasonal influenza vaccines confer protection to patients with RMS close to cladribine tablets initiation in year 1 and year 2 [35]. Seroprotective antibody levels were maintained or increased for at least 6 months, independent of lymphocyte counts.
Studies to date indicate that the risk of infection and associated morbidity of COVID-19 in patients with MS is similar to that of the population at large [36, 37]. Furthermore, there is no evidence that patients with MS treated with cladribine tablets and who acquire COVID-19 are at more risk of severe disease [38,39,40].
In Spain, currently marketed COVID 19 vaccines (Pfizer-BioNTech, Moderna Oxford-AstraZeneca and Janssen) are inactive, so they are safe for people who are immunocompromised. Several studies have shown that cladribine treatment does not impair the development of protective humoral immunity following administration of the Pfizer–BioNTech COVID-19 vaccine [41,42,43,44]. Moreover, time since last cladribine dose, age, prior therapy, lymphocyte count as well as B and T cell counts had no effect on seropositivity of anti-SARS-CoV-2 IgG antibodies after vaccination [42].
Although, theoretically, COVID-19 vaccines could be given to patients taking cladribine at any point during the course of treatment, whenever possible, experts recommend administering inactive vaccines before initiating cladribine treatment or 3 months after starting treatment to allow lymphocyte recovery. However, there is evidence suggesting that the response to COVID-19 vaccines in patients taking cladribine is independent of lymphocyte count as well as B and T cell counts [42]. In stable patients who are about to start or receive a second course of cladribine, they advise waiting at least 3 weeks after the last dose of an inactivated COVID-19 vaccine to achieve the maximum possible immunity [32].
Cladribine is contraindicated in patients with an active malignancy. In the event of a tumour appearing between the first and second course, a second course should not be given under any circumstances until the tumour has been cleared. Evidence to date indicates that cladribine does not increase the risk of cancer or cause mutagenesis in lymphocytes [12, 45].
Conclusion
Cladribine offers an effective and generally safe long-term therapy for patients with RMS compared with other DMTs. The short dosing schedule and low monitoring requirements make it an attractive therapeutic option for both treatment-naïve patients and those who relapse (or are stable but experience AE) on moderately effective DMT.
Mounting real-world data suggest that starting treatment early can lead to a better response [46] and that a significant proportion of patients do not require further DMT treatment after a full course of cladribine [7]. A better understanding of candidate patient profiles, drug switching (to and from cladribine) and response monitoring strategies could lead to prolonged freedom from disease activity and the prevention of disease progression in more patients.
The recommendations in this document reflect the current status of knowledge. Further understanding of the criteria for failure or suboptimal response and more data on the potential benefits of additional courses of cladribine tablets in patients who relapse in year 3 or beyond will lead to better clinical management plans and outcomes.
References
Multiple Sclerosis International Federation. Atlas of MS 2020. https://www.atlasofms.org/map/global/epidemiology/number-of-people-with-ms. Accessed Mar 25, 2022
McGinley MP, Goldschmidt CH, Rae-Grant AD. Diagnosis and treatment of multiple sclerosis: a review. JAMA. 2021;325:765–79.
Leist TP, Weissert R. Cladribine: mode of action and implications for treatment of multiple sclerosis. Clin Neuropharmacol. 2011;34:28–35.
MAVENCLAD® Summary of product characteristics August 2017. https://www.ema.europa.eu/en/documents/product-information/mavenclad-epar-product-information_en.pdf. Accessed March 25, 2022.
Giovannoni G, Soelberg Sorensen P, et al. Safety and efficacy of cladribine tablets in patients with relapsing-remitting multiple sclerosis: results from the randomized extension trial of the CLARITY study. Mult Scler. 2018;24:1594–604.
Comi G, Cook S, Giovannoni G, et al. Effect of cladribine tablets on lymphocyte reduction and repopulation dynamics in patients with relapsing multiple sclerosis. Mult Scler Relat Disord. 2019;29:168–74.
Giovannoni G, Aydemir A, Verdun Di Cantogno E, Leist T. CLASSIC-MS: long-term efficacy and real-world treatment patterns for patients with relapsing multiple sclerosis who received cladribine tablets in phase III parent trials. Neurology. 2021;96(15 Supplement):1919.
Giovannoni G, Comi G, Cook S, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. NEJM. 2010;362:416–26.
Giovannoni G, Cook S, Rammohan K, et al. Sustained disease-activity-free status in patients with relapsing-remitting multiple sclerosis treated with cladribine tablets in the CLARITY study: a post-hoc and subgroup analysis. Lancet Neurol. 2011;10:329–37.
Leist T, Comi G, Cree B, et al. Effect of oral cladribine on time to conversion to clinically definite multiple sclerosis in patients with a first demyelinating event (ORACLE MS): a phase 3 randomised trial. Lancet Neurol. 2014;13:257–67.
Montalban X, Leist TP, Cohen BA, et al. Cladribine tablets added to IFN-β in active relapsing MS: the ONWARD study. Neurol Neuroimmunol Neuroinflamm. 2018;5: e477.
Cook S, Leist T, Comi G, et al. Safety of cladribine tablets in the treatment of patients with multiple sclerosis: an integrated analysis. Mult Scler Relat Disord. 2019;29:157–67.
Giovannoni G. Long-term disease stability assessed by the expanded disability status scale in patients treated with cladribine tablets in the CLARITY and CLARITY extension studies. ECTRIMS Online Library. 2019;279715:EP1573.
Giovannoni G, Leist T, Aydemir A, Verdun Di Cantogno E, on behalf of the CLASSIC-MS Steering Committee. Long-term efficacy for patients receiving cladribine tablets in CLARITY/CLARITY Extension: primary results from 9–15 years of follow-up in the CLASSIC-MS Study. ECTRIMS 13–15 October 2021.
European Medicines Agency. Mavenclad - summary of EPAR. 2017. https://www.ema.europa.eu/en/medicines/human/EPAR/mavenclad. Accessed Mar 25, 2022.
FDA approves new oral treatment for multiple sclerosis [press release]. US Food and Drug Administration. https://www.fda.gov/news-events/press-announcements/fda-approves-new-oral-treatment-multiple-sclerosis. Accessed March 25, 2022
Oh J, Walker B, Giovannoni G, et al. Treatment-emergent adverse events occurring early in the treatment course of cladribine tablets in two phase 3 trials in multiple sclerosis. Mult Scler J Exp Transl Clin. 2021;7:20552173211024296.
Rotstein D, Montalban X. Reaching an evidence-based prognosis for personalized treatment of multiple sclerosis. Nat Rev Neurol. 2019;15:287–300.
Oreja-Guevara C, García-Merino JA, Saiz A, et al. Recomendaciones de uso de cladribina comprimidos en la esclerosis múltiple recurrente [Recommendations for the use of cladribine tablets in recurring multiple sclerosis]. Rev Neurol. 2019;69:1–9 (Spanish).
Sørensen PS, Centonze D, Giovannoni G, et al. Expert opinion on the use of cladribine tablets in clinical practice. Ther Adv Neurol Disord. 2020;13:1756286420935019.
Meuth SG, Bayas A, Kallmann B, et al. Long-term management of multiple sclerosis patients treated with cladribine tablets: an expert opinion. Expert Opin Pharmacother. 2020;21:1965–9.
Cleveland Clinic. Mellen center approaches: highly active multiple sclerosis. https://my.clevelandclinic.org/-/scassets/files/org/neurological/multiple-sclerosis/14-neu-528-highly-active-ms.ashx?la=en. Accessed Mar 25, 2022.
Iacobaeus E, Arrambide G, Amato MP, et al. 2018 ECTRIMS Focused Workshop Group. Aggressive multiple sclerosis (1): towards a definition of the phenotype. Mult Scler. 2020;26:1352458520925369.
Tintore M, Arrambide G, Otero-Romero S, et al. The long-term outcomes of CIS patients in the Barcelona inception cohort: looking back to recognize aggressive MS. Mult Scler. 2020;26:1658–69.
Langer-Gould A, Popat RA, Huang SM, et al. Clinical and demographic predictors of long-term disability in patients with relapsing-remitting multiple sclerosis: a systematic review. Arch Neurol. 2006;63:1686–91.
Pitteri M, Romualdi C, Magliozzi R, Monaco S, Calabrese M. Cognitive impairment predicts disability progression and cortical thinning in MS: an 8-year study. Mult Scler. 2017;23:848–54.
Villar LM, Masjuan J, González-Porqué P, et al. Intrathecal IgM synthesis is a prognostic factor in multiple sclerosis. Ann Neurol. 2003;53:222–6.
Thebault S, Abdoli M, Fereshtehnejad SM, et al. Serum neurofilament light chain predicts long term clinical outcomes in multiple sclerosis. Sci Rep. 2020;10:10381.
Sacco KA, Abraham RS. Consequences of B-cell-depleting therapy: hypogammaglobulinemia and impaired B-cell reconstitution. Immunotherapy. 2018;10:713–28.
Houtchens MK, Zapata LB, Curtis KM, Whiteman MK. Contraception for women with multiple sclerosis: guidance for healthcare providers. Mult Scler. 2017;23:757–64.
Coyle PK, Oh J, Magyari M, Oreja-Guevara C, Houtchens M. Management strategies for female patients of reproductive potential with multiple sclerosis: an evidence-based review. Mult Scler Relat Disord. 2019;32:54–63.
Otero-Romero S, Rodríguez-García J, Vilella A, et al. Recommendations for vaccination in patients with multiple sclerosis who are eligible for immunosuppressive therapies: Spanish consensus statement. Neurologia. 2021;36:50–60 (English, Spanish).
Ciotti JR, Valtcheva MV, Cross AH. Effects of MS disease-modifying therapies on responses to vaccinations: a review. Mult Scler Relat Disord. 2020;45: 102439.
Williamson EML, Chahin S, Berger JR. Vaccines in multiple sclerosis. Curr Neurol Neurosci Rep. 2016;16:36.
Roy S, Boschert U. Analysis of influenza and varicella zoster virus vaccine antibody titers in patients with relapsing multiple sclerosis treated with cladribine tablets. ACTRIMS 25–27 February 2021.
Berger JR, Brandstadter R, Bar-Or A. COVID-19 and MS disease-modifying therapies. Neurol Neuroimmunol Neuroinflamm. 2020;7:e761.
Möhn N, Konen FF, Pul R, et al. Experience in multiple sclerosis patients with COVID-19 and disease-modifying therapies: a review of 873 published cases. J Clin Med. 2020;9:4067.
De Angelis M, Petracca M, Lanzillo R, Brescia Morra V, Moccia M. Mild or no COVID-19 symptoms in cladribine-treated multiple sclerosis: two cases and implications for clinical practice. Mult Scler Relat Disord. 2020;45: 102452.
Jack D, Nolting A, Galazka A. Favorable outcomes after COVID-19 infection in multiple sclerosis patients treated with cladribine tablets. Mult Scler Relat Disord. 2020;46: 102469.
Cladribine Tablets and COVID-19. https://www.cladribinetabletsinfo.global/en/home/topics-of-further-interest/covid-19.html. Accessed Mar 25, 2022.
Achiron A, Mandel M, Dreyer-Alster S, et al. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther Adv Neurol Disord. 2021;14:1–8.
Grothe C, Steffen F, Bittner S. Humoral immune response and lymphocyte levels after complete vaccination against COVID-19 in a cohort of multiple sclerosis patients treated with cladribine tablets. J Cent Nerv Syst Dis. 2021;13:11795735211060118.
Tortorella C, Aiello A, Gasperini C, et al. Humoral- and T-cell-specific immune responses to SARS-CoV-2 mRNA vaccination in patients with MS using different disease-modifying therapies. Neurology. 2022;98:e541–54.
König M, Lorentzen ÅR, Torgauten HM, et al. Humoral immunity to SARS-CoV-2 mRNA vaccination in multiple sclerosis: the relevance of time since last rituximab infusion and first experience from sporadic revaccinations. J Neurol Neurosurg Psychiatry. 2021. https://doi.org/10.1136/jnnp-2021-327612.
Pakpoor J, Disanto G, Altmann DR, et al. No evidence for higher risk of cancer in patients with multiple sclerosis taking cladribine. Neurol Neuroimmunol Neuroinflamm. 2015;2: e158.
Freedman MS, Leist TP, Comi G, et al. The efficacy of cladribine tablets in CIS patients retrospectively assigned the diagnosis of MS using modern criteria: results from the ORACLE-MS study. Mult Scler J Exp Transl Clin. 2017;3:2055217317732802.
Acknowledgements
Funding
The publication of this article, as well as journal’s Rapid Service Fee, was funded by Merck, S.L.U., Mollet del Valles, Spain, an affiliate of Merck KGaA.
Medical Writing, Editorial, and Other Assistance
This work was funded by an independent medical writing grant from Merck, S.L.U., Mollet del Valles, Spain, an affiliate of Merck KGaA (CrossRef Funder ID: 10.13039/100009945) and the editorial assistance was provided by Monica Hoyos on behalf of Springer Healthcare, Spain.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Conceptualization: Virginia Meca-Lallana, Jose M. García Domínguez, Jaume Sastre. Methodology: Virginia Meca-Lallana, Jose M. García Domínguez, Rocío López Ruiz, Jesús Martín-Martínez, Adrián Arés Luque, Miguel A. Hernández Pérez, José M. Prieto González, Lamberto Landete Pascual, Jaume Sastre. Formal analysis and investigation: Virginia Meca-Lallana, Jose M. García Domínguez, Rocío López Ruiz, Jesús Martín-Martínez, Adrián Arés Luque, Miguel A. Hernández Pérez, José M. Prieto González, Lamberto Landete Pascual, Jaume Sastre. Writing—original draft preparation: Virginia Meca-Lallana, Jose M. García Domínguez, Jaume Sastre. Writing—review and editing: Virginia Meca-Lallana, Jose M. García Domínguez, Rocío López Ruiz, Jesús Martín-Martínez, Adrián Arés Luque, Miguel A. Hernández Pérez, José Mª.Prieto González, Lamberto Landete Pascual, Jaume Sastre. All authors read and approved the final manuscript.
Disclosures
Virginia Meca-Lallana has received consulting or speaking fees from Almirall, Biogen, Genzyme, Janssen, Merck Serono, Novartis, Roche, Terumo, Sanofi, Teva, Celgene and BMS. José M. García Domínguez has received honoraria as speaker, consultant and/or research support from Abbvie, Bristol Myers Squibb, Almirall, Biogen, Janssen, Merck, Roche, Novartis and Sanofi. Rocío López Ruiz has received speaker honoraria and consultant fees from Biogen, Novartis, Merck, Bayer, Sanofi (Genzyme), Roche and Mylan. Jesús Martín-Martínez has received speaking honoraria and personal compensation for participating in advisory boards fees from Sanofi, Biogen, Merck, Brystol Myers Squibb, Roche and Novartis. Adrián Arés Luque has received consultant fees and speaker honoraria from Almirall, Bayer, Biogen, Merck, Novartis, Roche, Sanofi (Genzyme) and Teva. Miguel A. Hernández Pérez has received research support from Biogen, Sanofi, Merck, Novartis, Teva and Roche. José M. Prieto González has received consulting fees from Bayer, Biogen, Daiichi Sankyo, Sanofi (Genzyme), Janssen, Merck, Novartis, Sandoz Iberia, Teva, Roche, Almirall and Celgene; support for participating in conferences from Almirall, Bayer, Biogen, Genzyme, Merck, Novartis, Sanofi, Teva and Roche; and research support from Almirall, Biogen, Novartis, Teva and Sanofi. Lamberto Landete Pascual has received honoraria for participating in advisory boards and scientific and educational activities from Almirall, Bayer, Biogen, Bristol Myers Squibb, Sanofi, Merck, Novartis, UCB Pharma, Roche and Teva. Jaume Sastre-Garriga serves as co-Editor for Europe on the editorial board of Multiple Sclerosis Journal and as Editor-in-Chief of Revista de Neurología. He receives research support from Fondo de Investigaciones Sanitarias (19/950) and has served as a consultant/speaker for Biogen, Celgene/Bristol Meyers Squibb, Genzyme, Novartis and Merck.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. Ethics committee approval was not required.
Data Availability
There are no data, per se, associated with this manuscript. Information extracted from the articles identified from the search can be requested from the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Meca-Lallana, V., García Domínguez, J.M., López Ruiz, R. et al. Expert-Agreed Practical Recommendations on the Use of Cladribine. Neurol Ther 11, 1475–1488 (2022). https://doi.org/10.1007/s40120-022-00394-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-022-00394-0