Abstract
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disorder characterized by progressive deterioration in cognition, memory and activities of daily living. Selective blockade of serotonin-6 (5-HT6) receptors, which are exclusively localized to the central nervous system, is reported to play an important role in learning and memory. Masupirdine is a potent and selective 5-HT6 receptor antagonist with pro-cognitive properties in animal models of cognition.
Methods
The efficacy and safety of masupirdine were evaluated in patients with moderate AD concurrently treated with donepezil and memantine. A total of 564 patients were randomized in a 1:1:1 ratio. The study consisted of a 26-week double-blind treatment period. The primary efficacy outcome was the 11-item cognitive subscale of the Alzheimer’s Disease Assessment Scale (ADAS-Cog 11). Changes from baseline were analyzed using a mixed effects model for repeated measures (MMRM). In exploratory post hoc analyses, patients were subdivided based on the use of memantine dosage forms and memantine plasma concentrations, to evaluate the impact of memantine on the efficacy of masupirdine.
Results
In an exploratory post hoc analysis, less worsening in cognition (ADAS-Cog 11 scores) was observed with masupirdine treatment as compared with placebo in patients whose trough memantine plasma concentrations were ≤ 100 ng/mL.
Conclusions
Although prespecified study endpoints of the phase 2 study were not met, these exploratory post hoc subgroup observations are hypothesis-generating and suggest that the efficacy of masupirdine was adversely affected by concurrent therapy with memantine. Further assessment of masupirdine to determine its potential role as a treatment option for cognitive deficits associated with AD is warranted.
Trial Registration
The study was registered at ClinicalTrials.gov (NCT02580305).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Exploratory post hoc analyses of a masupirdine phase 2 study on cognition were carried out based on the use of memantine dosage forms and memantine plasma concentrations. |
The treatment effect of masupirdine with concomitant treatment with donepezil + Namenda was numerically superior to that with concomitant treatment with donepezil + Namenda XR when compared with placebo. |
Less worsening in cognition was observed with masupirdine treatment as compared with placebo in patients whose trough memantine plasma concentrations were ≤ 100 ng/mL. |
The results suggest potentially important effects of interactions among drugs used to treat Alzheimer’s disease. |
Introduction
Dementia is a syndrome characterized by disturbance of multiple brain functions, including memory, thinking, orientation, comprehension, calculation, learning capacity, language, and judgment. Impairments of cognitive function are commonly accompanied, and occasionally preceded, by deterioration in emotional control, social behavior, or motivation. Alzheimer’s disease (AD), the most common form of dementia, is a progressive and debilitating neurodegenerative disorder characterized by cognitive and memory deterioration in addition to progressive impairment in activities of daily living (ADL), and contributes to 60–70% of cases of dementia. Other types of dementia include vascular dementia, dementia with Lewy bodies, and a group of diseases that contribute to frontotemporal dementia. Approximately 50 million people worldwide are living with AD and other dementias, and an estimated 6.2 million Americans aged 65 years and older were living with AD dementia in 2021 [1]. By 2060, the number of people aged 65 years and older with AD dementia in the United States is projected to reach 13.85 million [2].
Commonly used symptomatic treatments available today for AD dementia do not slow or stop the damage and destruction of neurons that causes AD symptoms and makes the disease fatal. Though these treatment options delay the cognitive decline [3, 4], their effect is modest and use tends to be short-term. The U.S. Food and Drug Administration (FDA) has currently approved six drugs or regimens for the treatment of AD—rivastigmine, galantamine, donepezil, memantine, memantine combined with donepezil, and aducanumab [1]. The last is an anti-amyloid monoclonal antibody that was shown to reduce amyloid plaque burden in the brain; trials show preliminary evidence of slowing of cognitive decline [5]. The modest efficacy and side effects associated with the current treatment options underline the need to develop more effective and safer treatment options.
The serotonin-6 (5-HT6) receptor is a G protein-coupled receptor localized almost exclusively in the central nervous system including the cerebral cortex and hippocampus, and may play role in learning and memory [6,7,8,9,10,11]. Several 5-HT6 receptor antagonists have been evaluated in clinical settings for their potential therapeutic utility in the treatment of cognitive impairment associated with AD either alone or in combination with donepezil. Intepirdine was studied both as monotherapy and in combination with donepezil [12]. As a monotherapy, no benefit of intepirdine was observed on measures of cognition or global impression of change; however, as an adjunct to donepezil, intepirdine treatment was associated with sustained improvement in cognition and ADL compared with treatment with donepezil alone. In a phase 2 clinical trial, the addition of idalopirdine to donepezil showed promising beneficial effects on cognition [13]. However, positive observations from phase 2 studies of intepirdine could not be replicated in phase 3 studies [14]. Similarly, preliminary signs of efficacy for idalopirdine were not confirmed in phase 3 trials [15]. A phase 2 clinical trial evaluating the efficacy and safety of SAM-760 30 mg once daily (QD) for 12 weeks in patients with AD on a stable regimen of donepezil 5 to 10 mg QD was terminated before planned completion, as the study met the futility criteria [16]. The potential utility of 5-HT6 receptor antagonists in the treatment of AD has been evaluated either as monotherapy or as an add-on therapy to donepezil. No previous trial has reported results for the evaluation of a 5-HT6 receptor antagonist as an add-on therapy to both donepezil and memantine, and treatment with memantine was an exclusion for participation in most of the trials.
Masupirdine, a selective 5-HT6 receptor antagonist, modulates neurotransmitters that play an important role in learning and memory [17] and has shown pro-cognitive potential in various behavioral assessments in animal models and in electroencephalography studies. Studies in animal models demonstrated that co-administration of masupirdine with donepezil and memantine resulted in pro-cognitive effects significantly greater than those observed with the combination of donepezil and memantine [17]. Based on the evident potential benefits of masupirdine for cognition in animal models with co-administration of donepezil and memantine, and the wide use of this combination in clinical practice, masupirdine was evaluated as an add-on therapy to donepezil and memantine in patients with moderate AD dementia.
The study did not meet its primary objective, and additional exploratory analyses were conducted to understand the trial outcomes. [18]. An important issue given its unique role in this trial was the impact of memantine on cognitive outcomes. Three different forms of memantine were allowed in study participants: (1) memantine 10 mg twice a day (BID) (Namenda®); (2) memantine 28 mg QD (Namenda XR®); and (3) the fixed combination tablet of memantine 28 mg with 10 mg donepezil QD (Namzaric®). Compliance with donepezil and memantine treatment was monitored by measurement of trough plasma levels that were analyzed after study completion. The analyses revealed differential exposure to memantine. Thus, a post hoc analysis was conducted to evaluate the effect of plasma exposure of memantine on the efficacy of masupirdine.
Methods
The analysis reported here is based on the data from a previously reported randomized, double-blind, placebo-controlled, parallel-group, multicenter study of masupirdine in patients with moderate AD who were on stable doses of donepezil and memantine [18]. Recruitment, enrollment, all characterizations, and analysis of patients in this study were approved by the central or local institutional review boards for all study sites. Informed consent was obtained from all participants at the time of enrollment. All procedures were designed and conducted in accordance with the tenets of the Declaration of Helsinki.
The study recruited ambulatory or ambulatory-aided male or female patients aged between 50 and 85 years (both inclusive) with a diagnosis of probable AD based on the National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA) criteria [19], with moderate severity of dementia, defined as having a Mini-Mental State Examination (MMSE) score of 12 to 20 [20]. A total of 564 patients were randomized in a 1:1:1 ratio to placebo, 50 mg masupirdine, or 100 mg masupirdine treatment for up to 6 months. Study participants were allowed to use one strength of donepezil (10 mg QD) and one of the three forms of memantine: (1) memantine 10 mg BID; (2) memantine 28 mg QD (Namenda XR®); or (3) the fixed combination tablet of memantine 28 mg with 10 mg donepezil QD (Namzaric®). The primary efficacy outcome of the study was the 11-item cognitive subscale of the Alzheimer’s Disease Assessment Scale (ADAS-Cog 11), which has historically been used for evaluating cognitive measures in randomized clinical trials. Secondary efficacy measures were based on the Clinical Dementia Rating Scale—Sum of Boxes (CDR-SB), MMSE, 23-item Alzheimer’s Disease Cooperative Study Activities of Daily Living (ADCS-ADL) scale, and 12-item Neuropsychiatric Inventory (NPI-12).
This exploratory post hoc analysis of the primary study data was conducted to evaluate the impact of memantine regimen and exposure on efficacy of masupirdine with regard to cognition (ADAS-Cog 11 and MMSE) in AD patients. For the purpose of this post hoc analysis, randomized patients were divided in two categories: patients taking memantine 10 mg BID or patients taking memantine 28 mg QD. For further analysis, patients were divided as patients with trough memantine concentrations ≤ 100 ng/mL and > 100 ng/mL at week 26. A responder analysis was carried out to determine the number of responders with active treatment versus placebo.
Statistical Analysis
All statistical tests were two-sided and were performed at the 5% level of significance. The analyses were conducted using either SAS® version 9.3 or GraphPad Prism version 7.3. All efficacy analyses were performed on the modified intent-to-treat (mITT) population. Proportions of responders were subjected to the chi-square test. Adjustments for multiple comparisons were not performed in these post hoc hypothesis-generating analyses.
Results
Patient Demographics and Baseline Characteristics
A total of 564 patients were randomized according to the planned ratio to receive masupirdine 50 mg (190 patients), masupirdine 100 mg (185 patients), or placebo (189 patients). The mITT population comprised 543 patients.
The median age was similar among participants in all three treatment arms (masupirdine 50 mg: 75.0 years; masupirdine 100 mg: 76.0 years; placebo: 74.0 years). The majority of participants (at least 85.8%) were over the age of 65 years, with similar age distribution among participants in the treatment arms. Overall, a slightly higher proportion of female than male participants were enrolled in the study (54.7% female vs. 45.3% male participants). This proportion of female and male participants was similar across all treatment arms. A total of 357 participants (65.7%) used memantine 10 mg BID and 186 participants (34.3%) used either Namenda XR or Namzaric (memantine 28 mg QD), and the treatment formulations were evenly distributed among all three treatment arms.
A summary of patient demographics and baseline disease characteristics is presented in Table 1.
Subgroup Analyses of Primary Endpoint
Treatment Response by Concomitant Medication Use
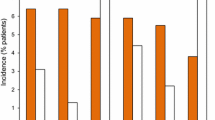
The outcomes on the primary endpoint and subgroup analysis of ADAS-Cog 11 scores by concomitant medication use in the mITT population are presented in Figs. 1 and 2, respectively.
In the mITT population, the treatment effect of masupirdine with concomitant treatment with donepezil + Namenda was numerically superior to that with concomitant treatment with donepezil + Namenda XR when compared with placebo at week 26. In patients who were on donepezil + Namenda, the mean difference in change from baseline in ADAS-Cog 11 scores between masupirdine 50 mg and placebo at week 26 was −1.0 (95% CI −2.6, 0.5; p = 0.192). The mean difference in change from baseline in ADAS-Cog 11 scores between masupirdine 100 mg and placebo was −0.2 (95% CI −1.8, 1.4; p = 0.805). The treatment differences were numerically greater when the analysis was done on the evaluable population (per-protocol population). At week 26, the mean difference between masupirdine 50 mg and placebo was −1.5 (95% CI −3.3, 0.2; p = 0.081) and the mean difference between masupirdine 100 mg and placebo was −0.6 (95% CI −2.3, 1.2; p = 0.517) in patients taking donepezil + memantine (10 mg BID). In patients in the mITT population who were on donepezil + Namenda XR (28 mg QD), at week 26, the mean difference in change from baseline in ADAS-Cog 11 scores between masupirdine 50 mg and placebo was 0.5 (95% CI −1.6, 2.6; p = 0.620). The mean difference between masupirdine 100 mg and placebo was 0.2 (95% CI −2.1, 2.4; p = 0.894). These observations suggest that participants treated with memantine 28 mg, alone or as part of the fixed combination, responded less well to treatment with masupirdine.
Treatment Response by Sex
In the mITT population, the treatment effect of masupirdine was numerically superior in male patients to that in female patients when compared with placebo at week 26. In male patients, the mean difference in change from baseline in ADAS-Cog 11 scores between masupirdine 50 mg and placebo at week 26 was −1.6 (95% CI −3.5, 0.2; p = 0.081). The mean difference in change from baseline in ADAS-Cog 11 scores between masupirdine 100 mg and placebo was −1.2 (95% CI −3.1, 0.7; p = 0.225). In female patients, at week 26, the mean difference in change from baseline in ADAS-Cog 11 scores between masupirdine 50 mg and placebo was 0.3 (95% CI −1.4, 2.0; p = 0.746). The mean difference between masupirdine 100 mg and placebo was 0.7 (95% CI −1.0, 2.4; p = 0.411).
Treatment Response by Memantine Concentration
Based on the observed trends in the effects of masupirdine on cognitive endpoint (ADAS-Cog 11) with respect to memantine dosage forms and the literature indicating differences in memantine exposure with these dosage forms, further analyses were conducted based on memantine exposure levels at week 26.
The median steady-state trough concentration of memantine was 104 ng/mL at week 26 in patients taking memantine 10 mg BID (immediate-release [IR] tablets), whereas it was 130 ng/mL for patients taking Namenda XR, approximately 25% higher in those taking the long-acting formulation.
A summary of subgroup analysis of ADAS-Cog 11 scores by observed memantine plasma concentrations at week 26 in the mITT population is presented in Table 2 and Fig. 3.
Patients with memantine concentrations ≤ 100 ng/mL showed a significant treatment difference in change from baseline in ADAS-Cog 11 scores when comparing the masupirdine 50 mg arm and the placebo arm. The mean difference between masupirdine 50 mg and placebo was −3.08 (95% CI −4.98, −1.18; p = 0.0008) at week 26, favoring masupirdine. The mean difference between masupirdine 100 mg and placebo was −1.98 (95% CI −3.91, −0.05; p = 0.04), favoring masupirdine. In the evaluable population the results were similar to those in the mITT population. The effect size (Cohen’s d) observed with 50 mg masupirdine was 0.52 and with 100 mg masupirdine was 0.35.
Patients with memantine concentrations of > 100 ng/mL did not show any beneficial effect of masupirdine compared with the placebo. At week 26, the least-squares mean difference between the masupirdine 100 mg arm and placebo arm was 1.3 (95% CI −0.6, 3.1; p = 0.180), favoring placebo.
A positive sign was also observed in the MMSE scores in the patients whose memantine concentrations were ≤ 100 ng/mL The mean difference between masupirdine 50 mg and placebo was 0.98 (95% CI −0.03, 1.99; p = 0.0587) at week 26, favoring masupirdine. The mean difference between masupirdine 100 mg and placebo was 1.03 (95% CI 0.02, 2.04; p = 0.0458), favoring masupirdine (Fig. 4).
Responder Analysis
A summary of responder analysis for ADAS-Cog 11 scores in the population with memantine concentrations ≤ 100 ng/mL is presented in Table 3. Patients who showed no deterioration based on the ADAS-Cog 11 during the study were considered responders for this analysis.
In patients with memantine plasma concentrations ≤ 100 ng/mL, the percentage of responders on the ADAS-Cog 11 with masupirdine treatment was 49% versus 23% of participants on placebo. The percentage of responders was slightly higher in the 50 mg masupirdine arm than in the 100 mg masupirdine arm (53 vs. 45%).
Discussion
Masupirdine, a 5-HT6 receptor antagonist, was evaluated in participants with moderate AD (MMSE range of 12–20). In this phase 2 study, based on the potential beneficial effects of co-administration of masupirdine with donepezil and memantine on cognition observed in animal models, and the common use of memantine along with acetylcholinesterase inhibitors (AChEIs) in this population, patients on stable background therapy of donepezil and memantine were recruited. The evaluation of masupirdine is the first of its kind where this class of compounds was evaluated in combination with double background therapy. The phase 2 study with masupirdine did not show a drug–placebo difference on the primary endpoint. In an effort to understand the effects of the combination treatment, an exploratory post hoc analysis was carried out using data generated in a phase 2 study of masupirdine [18]. In the trial, participants were allowed to use either 10 mg BID or 28 mg QD memantine. Due to the variability in the memantine dosage forms used in the trial population, it was hypothesized that the effect of masupirdine would vary with the use of different dosage forms of memantine. Therefore, this post hoc analysis was carried out to understand the influence of memantine with respect to the effects of masupirdine on cognitive decline in patients with moderate AD.
In the phase 2 study, although no statistical significance between drug and placebo effects was observed at the end of 26 weeks of treatment, there were numerical differences observed in the ADAS-Cog 11 scores between the placebo and masupirdine treatment arms. A trend of slower decline was observed at week 26 [18] in patients treated with masupirdine. However when the population was segregated based on memantine use, the difference in the change from baseline scores in ADAS-Cog 11 between placebo and masupirdine was greater in patients taking memantine 10 mg BID than those who were on memantine 28 mg QD.
In a study comparing memantine exposure between 28 mg QD and 10 mg BID, the maximum plasma concentration (Cmax) and area under the concentration–time curve over a 24-h dosing interval (AUC0–24) were 48% and 33% higher for the XR dosage regimen, respectively [21]. In the current study, the median steady-state trough concentrations of memantine were 104 ng/mL at week 26 in patients taking memantine 10 mg BID (IR tablets), whereas it was 130 ng/mL for patients taking Namenda XR, 25% higher. According to the literature and exposure values from the current study, the mean minimum concentration (Cmin) values range from 95 to 105 ng/mL for memantine IR tablets. The differential exposures between memantine dosage forms prompted the post hoc evaluation of the effects of exposure on clinical efficacy variables.
Overall, the results of the post hoc analyses suggest that masupirdine performance was better with less exposure to memantine—those receiving memantine BID and those with lower trough levels of memantine. The effect sizes of 0.52 (50 mg) and 0.35 (100 mg) suggest a clinically meaningful impact of therapy. Effect sizes may be more appropriate for analyses of this type involving small patient populations than p values [22].
Sex may also affect memantine trough levels. When male patients were compared with female patients, the male patients responded better on clinical measures. On review of memantine exposure values, men had lower trough memantine levels than women (104 ng/mL vs. 121 ng/mL).
Serotonin 5-HT6 receptors are expressed on γ-aminobutyric acid (GABA)-ergic spiny neurons of the striatum and are co-localized with glutamic acid decarboxylase in the cerebral cortex and hippocampus [23]. Experimental evidence suggests that 5-HT6 receptor antagonists may modulate cholinergic and/or glutamatergic systems via disinhibition of GABAergic neurons. In agreement with observations in the literature, masupirdine produced significant increases in acetylcholine and glutamate levels [17].
The current study involved combinations of masupirdine with donepezil and memantine, agents acting on two different neurotransmitter systems. Although the increase in acetylcholine by masupirdine is in accord with the mechanism of donepezil, the mechanism of action of memantine is divergent from the glutamate increase observed with masupirdine. Glutamate plays a prominent role in neural circuits involved with synaptic plasticity to shape learning and memory through activation of glutamate receptors, whereas memantine blocks the N-methyl-d-aspartate (NMDA) receptor to stop the current flow through its channels. NMDA receptor antagonists also have varying effects on cognition. Memantine improves memory in AD patients and in some (but not all) studies in animals [24, 25], whereas other NMDA receptor antagonists like MK-801, phencyclidine, and ketamine cause memory deficits.
Studies with idalopirdine and intepirdine in combination with AChEIs showed no clinical benefit. These agents have shown drug/placebo differences in phase 2 trials. The negative trials may have many explanations, including more heterogeneous populations with global trials, unknown adherence to donepezil, and changes in population inclusion parameters between phase 2 and phase 3 trials. Each 5-HT6 receptor antagonist may also have different levels of pharmacologic activity at the receptor level.
While the results here are from post hoc analyses, they suggest potentially important effects of interactions among drugs used to treat AD. These data indicate that further exploration in a prospective clinical trial is warranted to evaluate masupirdine with donepezil alone or with low-dose memantine. From a safety point of view, masupirdine treatment had a good safety and tolerability profile when added to background therapy of donepezil and memantine.
Limitations
This is a post hoc analysis; therefore, the hypothesis generated needs to be tested in a randomized clinical trial.
Conclusions
The use of different memantine products may have affected the potential efficacy of masupirdine in AD patients in the phase 2 trial. These exploratory observations merit further investigation. If proven in a follow-up randomized clinical trial (treatment with masupirdine with a low dose of memantine or without memantine), masupirdine can be used alone or in combination with AchI and/or with low-dose memantine.
References
Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimers Dement 2021;17(3).
Rajan KB, Weuve J, Barnes LL, McAninch EA, Wilson RS, Evans DA. Population estimate of people with clinical Alzheimer’s disease and mild cognitive impairment in the United States (2020–2060). Alzheimers Dement. 2021;17(12):1966–75.
Farlow MR, Cummings JL. Effective pharmacologic management of Alzheimer’s disease. Am J Med. 2007;120(5):388–97.
Alva G, Cummings JL. Relative tolerability of Alzheimer’s disease treatments. Psychiatry (Edgmont). 2008;5(11):27–36.
Budd Haeberlein S, Aisen P, Barkhof F, et al. Two randomized phase 3 studies of Aducanumab in early Alzheimer’s disease. Alzheimer’s Disease J Prev Alzheimers Dis. 2022;9(2):197–210.
Monsma FJ Jr, Shen Y, Ward RP, Hamblin MW, Sibley DR. Cloning and expression of a novel serotonin receptor with high affinity for tricyclic psychotropic drugs. Mol Pharmacol. 1993;43(3):320–7.
Marazziti D, Baroni S, Borsini F, et al. Serotonin receptors of type 6 (5-HT6): from neuroscience to clinical pharmacology. Curr Med Chem. 2013;20(3):371–7.
Helboe L, Egebjerg J, de Jong IE. Distribution of serotonin receptor 5-HT6 mRNA in rat neuronal subpopulations: a double in situ hybridization study. Neuroscience. 2015;310:442–54.
de Jong IEM, Mørk A. Antagonism of the 5-HT6 receptor: preclinical rationale for the treatment of Alzheimer’s disease. Neuropharmacology. 2017;125:50–63.
Ferrero H, Solas M, Francis PT, Ramirez MJ. Serotonin 5-HT6 receptor antagonists in Alzheimer’s disease: therapeutic rationale and current development status. CNS Drugs. 2017;31:19–32.
Lalut J, Karila D, Dallemagne P, Rochais C. Modulating 5-HT4 and 5-HT6 receptors in Alzheimer’s disease treatment. Future Med Chem. 2017;9:781–95.
Maher-Edwards G, Watson C, Ascher J, et al. Two randomized controlled trials of SB742457 in mild-to-moderate Alzheimer’s disease. Alzheimer’s & Dementia Transl Res Clin Interv. 2015;1:23–36.
Wilkinson D, Windfeld K, Colding-Jørgensen E. Safety and efficacy of idalopirdine, a 5-HT6 receptor antagonist, in patients with moderate Alzheimer’s disease (LADDER): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2014;13(11):1092–9.
Lang FM, Mo Y, Sabbagh M, et al. Intepirdine as adjunctive therapy to donepezil for mild-to-moderate Alzheimer’s disease: a randomized, placebo-controlled, phase 3 clinical trial (MINDSET). Alzheimers Dement (N Y). 2021;7(1): e12136. https://doi.org/10.1002/trc2.12136.
Atri A, Frölich L, Ballard C, et al. Effect of idalopirdine as adjunct to cholinesterase inhibitors on change in cognition in patients with Alzheimer disease: three randomized clinical trials. JAMA. 2018;319(2):130–42.
Fullerton T, Binneman B, David W, et al. A Phase 2 clinical trial of PF-05212377 (SAM-760) in subjects with mild to moderate Alzheimer’s disease with existing neuropsychiatric symptoms on a stable daily dose of donepezil. Alz Res Therapy. 2018;10:38.
Nirogi R, Abraham R, Benade V, et al. SUVN-502, a novel, potent, pure, and orally active 5-HT6 receptor antagonist: pharmacological, behavioral, and neurochemical characterization. Behav Pharmacol. 2019;30(1):16–35.
Nirogi R, Ieni J, Goyal VK, et al. Effect of masupirdine (SUVN-502) on cognition in patients with moderate Alzheimer’s disease: a randomized, double-blind, phase-2, proof-of-concept study. Alzheimers Dement (N Y). 2022;8(1):e12307.
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease Neurology. Neurology. 1984;34(7):939–44.
Folstein MF, Folstein SE, McHugh PR. ‘“Mini-mental state”’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98.
Namenda XR (memantine hydrochloride) capsules label https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022525s000lbl.pdf
Friedman LG, McKeehan N, Hara Y, et al. Value-generating exploratory trials in neurodegenerative dementias. Neurology. 2021;96(20):944–54.
Marcos B, Gil-Bea FJ, Hirst WD, et al. Lack of localization of 5-HT6 receptors on cholinergic neurons: implication of multiple neurotransmitter systems in 5-HT6 receptor-mediated acetylcholine release. Eur J Neurosci. 2006;24(5):1299–306.
Parsons CG, Danysz W, Quack G. Memantine is a clinically well tolerated N-methyl-D-aspartate (NMDA) receptor antagonist—a review of preclinical data. Neuropharmacology. 1999;38:735–67.
Witt A, Macdonald N, Kirkpatrick P. Memantine hydrochloride. Nat Rev Drug Discov. 2004;3:109–10.
Acknowledgements
Authors thank the patients and caregivers for their participation in the study. We also thank all study investigators and staff at each clinical site for their support in conducting the study.
Funding
The research was sponsored by Suven Life Sciences Ltd. The journal’s Rapid service fee is also funded by Suven Life Sciences Ltd.
Author contributions
Ramakrishna Nirogi and Vinod Kumar Goyal conceived of the presented idea. Vinod Kumar Goyal, Pradeep Jayarajan, and Vijay Benede drafted the manuscript. All authors were involved in interpreting the results, and preparing and critically reviewing the manuscript.
Prior presentation
Part of the results in this manuscript was presented at CTAD 2019 annual meeting in San Diego during Dec 4–7, 2019 and AAT-AD/PD™ focus meeting (virtual) during Apr 2–5, 2020.
Disclosures
Ramakrishna Nirogi, Vinod Kumar Goyal, Vijay Benade, Ramkumar Subramanian, Jyothsna Ravula, Satish Jetta, Anil Shinde, Santosh Kumar Pandey, Pradeep Jayarajan, Venkat Jasti are employees of Suven life sciences limited. Jeffrey Cummings is supported by NIGMS Grant P20GM109025; NINDS Grant U01NS093334; NIA Grant R01AG053798; NIA Grant P20AG068053; NIA Grant P30AG072959; NIA Grant R35AG71476; Alzheimer’s Disease Drug Discovery Foundation (ADDF); Ted and Maria Quirk Endowment for the Pam Quirk Brain Health and Biomarker Laboratory; and the Joy Chambers-Grundy Endowment.
Compliance with ethics guidelines
The original study protocol was approved by institutional ethics committees / IRBs for all the study sites. The procedures were followed in accordance with good clinical practices, which have their origin in declaration of Helsinki. All of the patients provided written informed consent before any screening procedure was performed.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available as this is proprietary information of Suven life sciences limited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Nirogi, R., Goyal, V.K., Benade, V. et al. Effect of Concurrent Use of Memantine on the Efficacy of Masupirdine (SUVN-502): A Post Hoc Analysis of a Phase 2 Randomized Placebo-Controlled Study. Neurol Ther 11, 1583–1594 (2022). https://doi.org/10.1007/s40120-022-00390-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-022-00390-4