Abstract
Introduction
Limited information is available on people’s experiences of living with Alzheimer’s disease (AD) at earlier stages. This study assessed awareness of diagnosis among people with early-stage AD and its impact on different person-centered outcome measures.
Methods
We conducted an observational, cross-sectional study in 21 memory clinics in Spain. Persons aged 50–90 years, diagnosed with prodromal or mild AD (NIA/AA criteria), a Mini Mental State Examination (MMSE) score ≥ 22, and a Clinical Dementia Rating-Global score (CDR-GS) of 0.5 or 1.0 were recruited. The Representations and Adjustment to Dementia Index (RADIX) was used to assess participants’ beliefs about their condition and its consequences.
Results
A total of 149 persons with early-stage AD were studied. Mean (SD) age was 72.3 (7.0) years and 50.3% were female. Mean duration of AD was 1.4 (1.8) years. Mean MMSE score was 24.6 (2.1) and 87.2% had a CDR-GS score of 0.5. Most participants (n = 84, 57.5%) used a descriptive term related to specific AD symptoms (e.g., memory difficulties) when asked what they called their condition. Participants aware of their diagnosis using the term AD (n = 66, 45.2%) were younger, had more depressive symptoms, and poorer life satisfaction and quality of life compared to those without awareness of their specific diagnosis. Practical and emotional consequences RADIX scores showed a significant negative correlation with Quality of Life in Alzheimer’s Disease score (rho = − 0.389 and − 0.413, respectively; p < 0.0001). Years of education was the only predictor of awareness of AD diagnosis [OR = 1.04 (95% CI 1.00–1.08); p = 0.029].
Conclusions
Awareness of diagnosis was a common phenomenon in persons with early-stage AD negatively impacting their quality of life. Understanding illness representations in earlier stages may facilitate implementing optimized care that supports improved quality of life and well-being.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
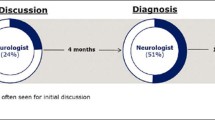
Awareness of the condition and its diagnosis among persons with early-stage Alzheimer’s disease has been little explored. |
Awareness of Alzheimer’s disease diagnosis was a common phenomenon in persons in early stages negatively impacting their quality of life. |
The identification of persons with awareness of their diagnosis, especially in the early stages of the disease, should be a goal to implement individualized strategies to preserve quality of life. |
Introduction
Alzheimer’s disease (AD) is the most common type of dementia and its prevalence continues to increase mainly because of population growth and ageing [1]. Impaired awareness of cognitive and functional deficits or anosognosia is a common phenomenon in persons with AD with a prevalence ranging from 21% to 81% [2, 3]. Low awareness predicts progression to dementia in both subjects with normal cognition and persons with mild cognitive impairment by an average of 3 years [4, 5].
The impact of anosognosia on AD persons and their caregivers has been extensively studied, but awareness of the condition and its diagnosis has only recently begun to be explored [2, 3, 6,7,8,9,10,11,12]. Quinn et al. developed the Representations and Adjustment to Dementia Index (RADIX), a specific instrument to assess people’s beliefs about dementia and their impact on subsequent adjustment [13]. In a sample of 917 persons with mild-to-moderate dementia in the UK, those with awareness of their condition reported a higher rate of depression and lower indices of well-being and psychological resources [12].
AD care should focus on person-centered outcomes [14, 15]. Maintaining autonomy and quality of life based on persons’ preferences and beliefs is essential, especially in the early stage of the disease when decision-making capacity is less impaired [14,15,16]. The aim of this study was to assess awareness of diagnosis among persons with early-stage AD and its impact on different psychological and behavioral dimensions.
Methods
This is an observational, cross-sectional study conducted at 21 hospital-based memory clinics. Key eligibility criteria included age between 50 and 90 years old, a diagnosis of prodromal or mild AD (National Institute on Aging/Alzheimer’s Association criteria), a Mini Mental State Examination (MMSE) score ≥ 22, and a Clinical Dementia Rating-Global score (CDR-GS) of 0.5 or 1.0 [17, 18]. Participants were consecutively recruited in the context of their regular follow-up visits between February and June 2021. This study was approved by the research review board of Hospital de la Santa Creu i Sant Pau, Barcelona, Spain (reference number EC/20/396/6186 [OBS]), and performed in accordance with the 1964 Helsinki Declaration and its later amendments. According to Spanish legislation (Orden SAS/3470/2009), non-interventional studies require approval by a single ethics committee and this approval applies to all participating centers. All participants provided written informed consent.
Outcome Measures
The RADIX is a brief, self-report instrument validated in people with mild to moderate dementia to assess their understanding of the condition and its consequences in five components: identity, cause, disease course over time (timeline), possibilities for controlling the disease, and practical and emotional consequences [13]. A nine-question screening checklist is included to screen out those who did not identify any difficulties or changes. Identity and cause are open questions to capture the perspective of the person with dementia. A list of options of possible causes is offered to the respondent including ageing, changes within the brain, disease or physical condition, hereditary condition, lifestyle events, or don’t know. Questions on timeline and control are single items rated on a four-point scale. For practical and emotional consequences, responses to the questions are rated on a four-point scale (from strongly disagree to strongly agree) and can be summed to give an overall score and then divided by 4 and 5 to give the mean score, respectively. Higher scores indicate greater negative consequences. This questionnaire was developed in English and is available at https://medicine.exeter.ac.uk/reach/publications/. In this study, a Spanish version was used after previous forward/backward translations.
The Quality of Life in Alzheimer’s Disease (QoL-AD), Functional Activities Questionnaire (FAQ), Beck Depression Inventory-Fast Screen (BDI-FS), Beck Hopelessness Scale (BHS), Satisfaction With Life Scale (SWLS), and General Self-Efficacy Scale (GSES) were used to gather information on quality of life, functioning, mood, feelings of hopelessness, life satisfaction, and self-efficacy, respectively [19,20,21,22,23,24]. Cognition was assessed using the Alzheimer Disease Assessment Scale-Cognitive (ADAS-Cog13) [25]. The QoL-AD is a 13-item self-report questionnaire designed to assess quality of life of persons with a diagnosis of AD [19]. Each item is rated using a four-point scale ranging from 1 (poor) to 4 (excellent). Higher scores indicate better quality of life. The FAQ is a 10-item, informant-based instrument to measure activities of daily living in elderly people [20]. Each item is rated using a four-point scale ranging from 0 (normal) to 3 (dependent). A cutoff ≥ 6 indicates functional impairment. The BDI-FS is self-report seven-item questionnaire assessed on a four-point scale (no symptoms to severe symptoms) [21]. Total score ranges from 0 to 21 with a cutoff ≥ 4 indicating the presence of depressive symptoms. The BHS is a 20-item self-report inventory to measure three major components of hopelessness: feelings about the future, loss of motivation, and future expectations [22]. Each optimistic response is scored as 0 and each pessimistic response is scored as 1. Total score ranges from 0 to 20 with higher values indicating higher levels of hopelessness. A cutoff ≥ 9 indicates moderate-to-severe hopelessness and identifies a high-risk group for potential suicide [26]. Participants indicated how much they agree or disagree with each of the five items of the SWLS using a seven-point scale that ranges from 1 (strongly disagree) to 7 (strongly agree) [23]. A cutoff score ≥ 25 indicates that respondents are satisfied or extremely satisfied with their life. The GSES is a 10-item self-report instrument to assess whether a person believes he or she can perform novel or difficult tasks or cope with adversity in different domains of functioning [24]. Each item is rated using a four-point scale ranging from 1 (not at all true) to 4 (exactly true). Higher scores indicate higher levels of an optimistic self-belief.
Definitions
Awareness of diagnosis was defined when participants used a diagnostic term or descriptive terms with specific symptoms to refer to the diagnosis of their illness (RADIX diagnosis item) [13]. Awareness of AD diagnosis was defined when participants specifically used the word Alzheimer to refer to the diagnosis of their condition. Participants who were able to give the diagnosis using the term AD and considered that their condition was going to worsen over time were classified as high awareness group (RADIX diagnosis and timeline items) [12, 13].
Statistical Analysis
Logistic regression was used to explore predictors of AD awareness using sociodemographic, clinical, cognitive, and psychological/behavioral characteristics. Variables with a statistical relationship to awareness and a p value of less than 0.2 were candidates for the model. Stepwise automatic variable selection method was applied in model building. Correlations between RADIX components and quality of life were also analyzed.
Results
A total of 149 persons with early-stage AD were studied. Mean (SD) age was 72.3 (7.0) years and 50.3% were female. Mean duration of AD was 1.4 (1.8) years. Mean MMSE score was 24.6 (2.1) and 87.2% had a CDR-GS score of 0.5. Sociodemographic and clinical characteristics are shown in Table 1.
Only three persons (2%) did not complete any of the RADIX screening items. Most participants (n = 84, 57.5%) used a descriptive term related to specific AD symptoms (e.g., memory difficulties) when asked what they called their condition. Don’t know (22.7%), ageing (21.4%), brain changes (20.7%), and hereditary condition (15.6%) were the most common reported causes. Illness representations according to RADIX responses are shown in Table 2.
In total 116 (79.4%) participants were aware of their condition (Table 2) and 66 (45.2%) used the term AD when asked if they knew their specific diagnosis (Table 3). These persons were younger, had more depressive symptoms, and poorer life satisfaction and quality of life compared to those without awareness of their specific diagnosis (Table 3).
Nineteen (13%) participants were able to give the AD diagnosis and considered that their condition was going to worsen over time. A logistic regression approach was used to explore predictors of awareness among these participants. Years of education was the only predictor of high awareness of AD diagnosis [OR = 1.04 (95% CI 1.00–1.08); p = 0.02] (Table 4).
Practical and emotional consequences RADIX scores showed a significant negative correlation with QoL-AD score (rho = − 0.389 and − 0.413, respectively; p < 0.0001).
Discussion
The impact of impaired awareness on persons with AD and their caregivers has been extensively studied [2, 6,7,8, 27, 28]. Anosognosia was associated with poor autonomy, greater frequency and severity of behavioral symptoms, lower treatment adherence, greater caregiver burden, and discrepancy in perception of quality of life between people with AD and their caregivers [2, 27, 28].
Different studies have shown that persons with AD are still able to describe their problems, experiences, and preferences at different stages of the disease [10, 14, 15]. In a qualitative and quantitative study with mild and moderate AD persons, Trindade et al. found that awareness of condition was more preserved than awareness of specific functional deficits [10]. Mayelle et al. using the Awareness of Self and Disease Assessment scale found that awareness in AD is heterogeneous and can fluctuate over time with three different patterns (deficit, stability, or improvement) [11]. However, little is known about the impact of having awareness of diagnosis, especially at earlier stages of the disease.
In our study, awareness of diagnosis was a common phenomenon with 45% of persons being able to use the term AD in a population with a mean disease duration of 1.4 years. Awareness was associated with the presence of depressive symptoms, poor life satisfaction, and quality of life.
Similar findings were found in a recent systematic review in persons with mild to moderate AD by Azocar et al. [28]. Most studies showed a negative association between impaired awareness and depression and anxiety (Pearson correlations ranging from 0.3 to 0.7). The IDEAL (Improving the experience of Dementia and Enhancing Active Life) study investigated illness representations using the RADIX questionnaire in a population with mild-to-moderate dementia in the UK [12]. The participants (11.2%) categorized by high awareness of their dementia diagnosis and knowledge of its causes and prognosis reported a higher rate of depressive symptoms and lower satisfaction with life, optimism, self-efficacy and self-esteem [12]. Younger age and depressed mood were stronger predictors of this awareness group than the level of cognitive or functional impairment [12]. In our study, the only predictor of awareness of AD was years of education.
Persons’ beliefs and expectations about an illness determine their emotional reactions and coping responses [29]. Persons prefer to be informed about their AD diagnosis, which has been associated with a better quality of life [15, 30]. However, having a better awareness of AD diagnosis correlates with more depressive symptoms and poorer quality of life [12]. In our study, a negative hope for the future, one of the most important factors for suicide, was also highly prevalent [26]. Therefore, identifying and understanding persons’ representations of AD from the onset of the disease may be essential for early implementation of individualized interventions to enable well-being and maintenance of independence where possible.
Our study has some limitations that deserve mention. First, the aim of this study was to assess awareness of condition and its associated factors using a comprehensive battery of self-report measurements. Traditionally, self-report measures have been considered insensitive in this disease [31]. The discrepancy between persons’ and caregivers’ scores on quality of life and other symptom dimensions begins even with very mild cognitive impairment. Second, although the RADIX is a useful and validated tool to understand dementia representations, the use of open questions and the listing of options for possible causes in this questionnaire can be challenging for respondents and the reliability of their subjective assessments [12, 13]. Third, given the cross-sectional design of the study, we have no information about participants’ potential changes in their levels of awareness over time [9, 11]. Finally, this study did not collect whether the participants had received any information on the disease and its long-term prognosis. Therefore, we cannot rule out that a greater awareness of the diagnosis may reflect a better provision of information.
Conclusions
Awareness of diagnosis was a common phenomenon in persons with early-stage AD and negatively impacted their quality of life and well-being. Awareness of diagnosis could be a signal to identify early in order to implement specific strategies to prevent depression and preserve quality of life. The approach to such actions must be based on a holistic view of the person and their environment and must be treated in a multidisciplinary way.
References
GBD 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022;7:105–25.
Cacciamani F, Houot M, Gagliardi G, et al. Awareness of cognitive decline in patients with Alzheimer’s disease: a systematic review and meta-analysis. Front Aging Neurosci. 2021;13: 697234.
Alexander CM, Martyr A, Savage SA, Morris RG, Clare L. Measuring awareness in people with dementia: results of a systematic scoping review. J Geriatr Psychiatry Neurol. 2021;34:335–48.
Munro CE, Donovan NJ, Amariglio RE, et al. The impact of awareness of and concern about memory performance on the prediction of progression from mild cognitive impairment to Alzheimer disease dementia. Am J Geriatr Psychiatry. 2018;26:896–904.
Hanseeuw BJ, Scott MR, Sikkes SAM, et al. Evolution of anosognosia in Alzheimer’s disease and its relationship to amyloid. Ann Neurol. 2020;87:267–80.
Mak E, Chin R, Ng LT, Yeo D, Hameed S. Clinical associations of anosognosia in mild cognitive impairment and Alzheimer’s disease. Int J Geriatr Psychiatry. 2015;30:1207–14.
Yoon B, Shim YS, Hong YJ, et al. Anosognosia and its relation to psychiatric symptoms in early-onset Alzheimer disease. J Geriatr Psychiatry Neurol. 2017;30:170–7.
Conde-Sala JL, Turró-Garriga O, Piñán-Hernández S, et al. Effects of anosognosia and neuropsychiatric symptoms on the quality of life of patients with Alzheimer’s disease: a 24-month follow-up study. Int J Geriatr Psychiatry. 2016;31:109–19.
Jacus JP, Mayelle A, Voltzenlogel V, et al. Modelling awareness in Alzheimer’s disease. J Alzheimers Dis. 2020;76:89–95.
Trindade PGE, Santos RL, Johannessen A, Neto JPS, Dourado MCN. Awareness of functional status: people with Alzheimer’s disease abilities to self-report impairment in activities of daily living. J Alzheimers Dis Rep. 2020;4:405–15.
Mayelle A, Hazebrouck C, El Haj M, et al. Awareness for people with Alzheimer’s disease: profiles and weekly trajectories. Front Aging Neurosci. 2022;13: 781426.
Alexander CM, Martyr A, Gamble LD, et al. Does awareness of condition help people with mild-to-moderate dementia to live well? Findings from the IDEAL programme. BMC Geriatr. 2021;21:511.
Quinn C, Morris RG, Clare L. Beliefs about dementia: development and validation of the representations and adjustment to dementia index (RADIX). Am J Geriatr Psychiatry. 2018;26:680–9.
DiBenedetti DB, Slota C, Wronski SL, et al. Assessing what matters most to patients with or at risk for Alzheimer’s and care partners: a qualitative study evaluating symptoms, impacts, and outcomes. Alzheimers Res Ther. 2020;12:90.
Wehrmann H, Michalowsky B, Lepper S, Mohr W, Raedke A, Hoffmann W. Priorities and preferences of people living with dementia or cognitive impairment - a systematic review. Patient Prefer Adherence. 2021;15:2793–807.
Lamont RA, Nelis SM, Quinn C, et al. Psychological predictors of “living well” with dementia: findings from the IDEAL study. Aging Ment Health. 2020;24:956–64.
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98.
Berg L. Clinical dementia rating (CDR). Psychopharmacol Bull. 1988;24:637–9.
Logsdon RG, Gibbons LE, McCurry SM, Teri L. Assessing quality of life in older adults with cognitive impairment. Psychosom Med. 2002;64:510–9.
Marshall GA, Zoller AS, Lorius N, et al. Functional activities questionnaire items that best discriminate and predict progression from clinically normal to mild cognitive impairment. Curr Alzheimer Res. 2015;12:493–502.
Elben S, Dimenshteyn K, Trenado C, et al. Screen fast, screen faster: a pilot study to screen for depressive symptoms using the beck depression inventory fast screen in parkinson’s disease with mild cognitive impairment. Front Neurol. 2021;12: 640137.
Dyce JA. Factor structure of the Beck hopelessness scale. J Clin Psychol. 1996;52:555–8.
Pavot W, Diener E, Colvin CR, Sandvik E. Further validation of the Satisfaction with Life Scale: evidence for the cross-method convergence of well-being measures. J Pers Assess. 1991;57:149–61.
Schwarzer R, Jerusalem M. Generalized Self-Efficacy scale. In: Weinman J, Wright S, Johnston M, editors. Measures in health psychology: A user’s portfolio. Causal and control beliefs. Windsor: NFER-NELSON; 1995. p. 35–37.
Mohs RC, Knopman D, Petersen RC, et al. Development of cognitive instruments for use in clinical trials of antidementia drugs: additions to the Alzheimer’s Disease Assessment Scale that broaden its scope. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(Suppl 2):S13–21.
McMillan D, Gilbody S, Beresford E, Neilly L. Can we predict suicide and non-fatal self-harm with the beck hopelessness scale? A meta-analysis. Psychol Med. 2007;37:769–78.
Conde-Sala JL, Reñé-Ramírez R, Turró-Garriga O, et al. Clinical differences in patients with Alzheimer’s disease according to the presence or absence of anosognosia: implications for perceived quality of life. J Alzheimers Dis. 2013;33:1105–16.
Azocar I, Livingston G, Huntley J. The association between impaired awareness and depression, anxiety, and apathy in mild to moderate Alzheimer’s disease: a systematic review. Front Psychiatry. 2021;12: 633081.
Diefenbach MA, Leventhal H. The common-sense model of illness representation: theoretical and practical considerations. J Soc Distress Homeless. 1996;5:11–38.
Mate KE, Pond CD, Magin PJ, Goode SM, McElduff P, Stocks NP. Diagnosis and disclosure of a memory problem is associated with quality of life in community based older Australians with dementia. Int Psychogeriatr. 2012;24:1962–71.
Hongisto K, Väätäinen S, Martikainen J, et al. Self-rated and caregiver-rated quality of life in Alzheimer disease with a focus on evolving patient ability to respond to questionnaires: 5-year prospective ALSOVA cohort study. Am J Geriatr Psychiatry. 2015;23:1280–9.
Acknowledgements
Funding
This study was funded by the Medical Department of Roche Farma Spain (ML42346). The sponsor also funded the journal’s Rapid Service fee.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors made a significant contribution to the work reported, whether in study conception, design, execution, acquisition of data, analysis and interpretation, or in all these areas; all took part in drafting, revising or critically reviewing the article; all gave final approval of the version to be published; all have agreed on the journal to which the article has been submitted; and all agree to be accountable for all aspects of the work.
Disclosures
This study was funded by the Medical Department of Roche Farma Spain (ML42346). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Alberto Villarejo-Galindo discloses honoraria from a consulting/advisory role with KRKA, Kern Pharma, Exeltis, Esteve, Roche, AbbVie, Schwabe, Neuraxpharm, Nutricia, and Alter. Antonio del Olmo-Rodríguez discloses honoraria from a consulting/advisory role with Alter, Biocross, Biogen, KRKA, Esteve, Schwabe, Nutricia, and Lilly. Emilio Franco-Macías discloses honoraria from a consulting/advisory role with Kern Pharma, Esteve, Roche, and Neuraxpharm. Mercè Boada discloses honoraria from a consulting/advisory role with Grifols, Araclon Biotech, Roche, Lilly, Merck, Biogen, Zambon, Novo-Nordisk, Bioiberica, Biogen, Eisai, Servier, and Schwabe Pharma; funding sources with Life Molecular Imaging, Bioiberica, and Schwabe; and grants from CIBERNED, EU/EFPIA, Instituto de Salud Carlos III (ISCIII), Fundación La Caixa, and Grífols. Rafael Arroyo discloses being an advisory board participant and receiving speaking fees from Almirall, Bayer, Biogen, Merck, Novartis, Roche, Sanofi, and Teva. Albert Lleó discloses honoraria from a consulting/advisory role with Grifols, Fujirebio-Europe, Novartis, Roche, Otsuka, Nutricia, Zambón, Biogen, Lilly, and KRKA. Co-author of a patent on markers of synaptopathy in neurodegenerative diseases (EP18382175.0, PCT/EP2019/056535). Elena García-Arcelay and Jorge Maurino are employees of Roche Farma Spain. Gerard Piñol-Ripoll, Félix Viñuela, Almudena Ibañez de la Peña, Mario Riverol, Albert Puig-Pijoan, Pedro Abizanda-Soler, Miquel Baquero-Toledo, Inmaculada Feria-Vilar, Mircea Balasa, Ángel Berbel, Eloy Rodríguez-Rodríguez, Alba Vieira-Campos, Guillermo García-Ribas, and Silvia Rodrigo-Herrero declare no competing interests.
Compliance with Ethics Guidelines
This study was approved by the research review board of Hospital de la Santa Creu i Sant Pau, Barcelona, Spain (reference number EC/20/396/6186 [OBS]), and performed in accordance with the 1964 Helsinki Declaration and its later amendments. According to Spanish legislation (Orden SAS/3470/2009), non-interventional studies require approval by a single ethics committee and this approval applies to all participating centers. Participants provided written informed consent.
Data Availability
Qualified researchers may request access to individual patient-level data through the corresponding author. The datasets generated during the analysis of the study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Villarejo-Galende, A., García-Arcelay, E., Piñol-Ripoll, G. et al. Awareness of Diagnosis in Persons with Early-Stage Alzheimer’s Disease: An Observational Study in Spain. Neurol Ther 11, 1183–1192 (2022). https://doi.org/10.1007/s40120-022-00367-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-022-00367-3