Abstract
Introduction
To evaluate the value of plasma exchange (PE) for patients with three subtypes of demyelinating optic neuritis (ON): aquaporin-4 (AQP4) antibody-positive ON (AQP4-ON), myelin oligodendrocyte glycoprotein (MOG) antibody-positive ON (MOG-ON), and AQP4 and MOG double-antibody-seronegative ON (D-ON).
Methods
A single-center prospective study compared the logarithm of the minimum angle of resolution (logMAR) best-corrected visual acuity (BCVA) at most severe onset, 1 day before intravenous high-dose methylprednisolone (IVMP) treatment, 1 day before PE treatment, after five-cycles of PE therapy, and at 1-, 3-, and 6-month follow-up visits. The proportions of eyes in each visual outcome category were also compared. Logistic regression and a receiver operating characteristic curve were used to analyze predicted factors for VA improvement.
Results
A total of 124 ON attacks of 122 patients were included. No significant differences were found in BCVA (P = 0.659) before and after PE therapy for 22 D-ON attacks, but VA improved in two of six MOG-ON patients. In 95 AQP4-ON patients suffering 96 attacks, the mean logMAR BCVA markedly improved and was steadily maintained after five-cycles of PE treatments (adjusted P < 0.001), with VA exhibiting a significantly increasing trend (adjusted P = 0.001) after PE treatment. The combination of the number of previous ON episodes and the time window to PE treatment showed accuracy of 74.7% for predicting an improvement in BCVA score ≥ 2 levels. In addition, a combination of logMAR VA before PE and the time window to PE treatment resulted in 83.4% accuracy in predicting whether VA would regain 1.0 logMAR.
Conclusion
PE therapy effectively improves visual outcomes for AQP4-ON patients, but offers limited value for D-ON patients. Early initiation greatly increases likelihood of achieving VA improvement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This study aimed to evaluate the value of plasma exchange (PE) for patients with three subtypes of demyelinating optic neuritis (ON), including aquaporin-4 (AQP4) antibody-positive ON (AQP4-ON), myelin oligodendrocyte glycoprotein (MOG) antibody-positive ON (MOG-ON), and AQP4 and MOG double-antibody-seronegative ON (D-ON). |
PE treatment leads to visual functional improvement in AQP4-ON patients. |
Early initiation of PE treatment increases the likelihood of improvement in visual acuity in AQP4-ON patients. |
PE treatment may have limited value for D-ON patients. |
Introduction
Demyelinating optic neuritis (ON), which can cause acute or subacute vision loss, occurs worldwide, but incidence varies among races [1]. The discovery of two biomarkers, aquaporin-4 (AQP4) and myelin oligodendrocyte glycoprotein (MOG) antibodies, has allowed recognition of the cause of atypical ON, which is characterized by severe visual loss (worse than 6/60 or 20/200), no recovery within 3 weeks of onset, and progression of visual loss for more than 2 weeks [1]. The AQP4 antibody is pathogenic and highly specific for neuromyelitis optica spectrum disorder (NMOSD) [2], whilst the MOG antibody is a biomarker of MOG antibody-associated disorder (MOG-AD) [3]. AQP4 antibody-positive ON (AQP4-ON), which is dominant in the Asian population, is associated with more severe episodes and poor visual recovery [4]. In contrast, MOG antibody-related ON (MOG-ON) exhibits no distinct ethnic predilection, although associated with recurrent ON [5, 6]. With no biomarker currently recognized, double-antibody-negative ON (D-ON), exhibits a distinct clinical presentation, and idiopathic ON [7, 8] or multiple sclerosis (MS)-related ON [9] without a recognized biomarker may also be included as “D-ON.” Demyelinating ON tends to relapse, leading to cumulative blindness and disability, so timely and effective treatment is essential to save vision. Intravenous high-dose methylprednisolone (IVMP) is the standard first-line treatment for acute ON, while plasma exchange (PE) is performed as a rescue method in patients with ON refractory to IVMP treatment. PE has been reported to be useful in treating steroid-resistant ON [10,11,12,13], severe ON due to NMOSD [14,15,16], and recurrent ON at risk for poor recovery [17].
The seventh edition of the clinical treatment guidelines of the American Society for Apheresis (2016) identified PE as the recommended grade IB and class II indications for the treatment of acute NMOSD [18]. Several studies and subsequent meta-analyses have revealed that PE can lower disease activity and improve functional scoring (Expanded Disability Status Scale, EDSS) in NMOSD [15, 19,20,21,22,23,24,25,26,27,28]. However, confounding factors and different disease entities can lead to several contradictions. One concern is whether different antibody subtypes are related to prognosis. While some studies have concluded that a positive AQP4 antibody result is associated with time to complete improvement [29, 30], others have suggested that AQP4-Ab-seronegative patients are associated with a superior response to PE therapy [17, 20]. There is limited evidence about the efficacy of PE for MOG-ON or D-ON patients. Benefit-related factors, including the correlation between the benefit of PE and the timing of PE initiation are also somewhat controversial. Most studies concur that early initiation may increase the benefit to NMOSD patients [17, 20, 21, 25, 29, 30], while some studies reported no strong correlation between the interval from relapse to PE treatment and 3-month functional scoring [14], and another study concluded that the time window from the onset of attack to PE initiation was not significantly associated with treatment outcome in children with acute inflammatory demyelinating central nervous system (CNS) syndromes [24]. Due to the scarcity of plasma resources, determining the optimal timing of treatment is valuable for clinical guidance. Our previous study showed that PE treatment effectively improved the visual outcomes of a small group of patients experiencing their first attack of severe acute isolated ON [28]. Clinically, many ON patients suffer a long disease duration, experiencing episodes of relapse. However, research on the therapeutic effect of PE for these patients is scarce. This study aimed to evaluate the efficacy of PE in improving visual outcomes in ON patients with different serum antibodies after treatment with IVMP and to determine the factors associated with visual improvement.
Methods
Study Design and Participants
A single-center cohort study, which included all consecutive patients presenting at the Department of Ophthalmology, Chinese People’s Liberation Army General Hospital was conducted between January 2015 and October 2021. The study protocol was approved by the Ethics Committee for Human Research of the hospital (approval number S2017-093-01) and was in accordance with the tenets of the Helsinki Declaration and the ICH-GCP guidelines. Written informed consent was obtained from all study subjects. Consent to publish the patient-level data presented in Table S2 was also obtained.
The diagnosis of ON was based on the criteria described by the Optic Neuritis Treatment Trial [31, 32]. The inclusion and exclusion criteria followed our previous study [30], but with no restriction on the times of attacks, initial visual acuity (VA), the disease duration, or the presentation of a related demyelinating disease of the CNS, such as transverse myelitis (TM), area postrema syndrome, acute disseminated encephalomyelitis, or MS.
The recruited patients were administered IVMP at 1 g daily for 3–5 days (total dose, 5-10 g; treatment duration, 3–14 days), followed by orally administered prednisone (starting at 1 mg/kg/day) without any other treatment. According to the patients’ VA tested 1 day after the last course of IVMP treatment, PE was recommended to patients who were “unresponsive to initial IVMP therapy.” In those patients elevated to PE after IVMP treatment, five cycles of PE were administered after the end of IVMP treatment, combined with oral prednisone (starting at 1 mg/kg/day). No other rescue therapy was added during PE treatment. Additional immunosuppressants, including rituximab, azathioprine, and mycophenolate mofetil, were prescribed to the patients after completion of PE treatment.
“Unresponsive to initial IVMP therapy” was defined as no obvious visual improvement of more than three lines of Snellen VA [30] or inability to read the highest letter on the Snellen chart at 10 feet at the end of IVMP treatment.
Ophthalmic Examinations
Patients underwent a comprehensive ophthalmic examination. Best-corrected visual acuity (BCVA) was measured at each visit, including the worst acuity at onset, 1 day before IVMP therapy, 1 day after IVMP therapy, 1 day before PE therapy, and after the fifth cycle of PE therapy. BCVA follow-up (1, 3, and 6 months after PE treatment) was conducted via telephone or outpatient follow-up visits. The Snellen VA of 20/400 was recorded when the subjects were just able to read the highest line of the Snellen chart at 10 feet. If the vision was worse than 20/400, the VA was recorded as counting fingers (CF) acuity, and converted to Snellen VA. The conversion of CF acuity to Snellen acuity is as follows: VA of 20/800 (CF at 10 feet), VA of 20/1000 (CF at 8 feet) and VA of 20/2000 (CF at 4 feet). For subjects who were unable to detect CF at a distance of > 3 feet, the VA was assessed and categorized into the following, in descending order: CF, perception of hand motion (HM), light perception (LP), or no light perception (NLP). The Snellen scale was converted into the logarithm of the minimum angle of resolution (logMAR) scale for statistical analysis.
Spectral-domain optical coherence tomography (OCT) (Zeiss Stratus OCT system; Carl Zeiss Meditec, Inc., Dublin, CA, USA) and flash visual evoked potential (F-VEP) (Roland RETI-scan 32 system; Roland Consult GmbH, Brandenburg, Germany) examinations were performed during hospitalization. The thickness of the peripheral retinal nerve fiber layer (pRNFL) and macular inner limiting membrane retinal pigment epithelium (mILM-RPE), N2-P2 peak-to-peak amplitude, and P2 peak latency of the F-VEP were recorded.
Detection of AQP4 and MOG Autoantibodies
Serum samples from patients hospitalized after 2016 were tested for the presence of AQP4 and MOG autoantibodies using a cell-based assay (Euroimmun, Lübeck, Germany) before the first PE treatment. Samples from patients hospitalized before 2016 were only tested for the presence of AQP4 autoantibodies. Only seven patients with AQP4 autoantibody positivity before 2016 were recruited. Recruited subjects were divided into three subtypes of ON depending on their detected serum antibodies—AQP4-ON, MOG-ON, and D-ON.
Therapeutic Plasma Exchange
Double-filtration plasmapheresis (Plasauto EZ, Asahi Kasei Medical, Tokyo, Japan) was performed on all recruited patients, who received a total of five cycles of treatment, administered every other day over 10 days. During a treatment session, during which approximately 2000–3500 mL of plasma was removed, each patient received a 1.0–1.5 plasma volume exchange with 10% albumin and plasma replacement fluid.
Outcome Assessment
The visual outcome score was categorized into 10 levels: Snellen VA of 20/20, scotoma, but with VA better than 20/30, 20/30 ≥ VA > 20/60, 20/60 ≥ VA > 20/200, 20/200 ≥ VA > 20/800, 20/800 ≥ VA > 20/2000, CF, HM, LP, and NLP. Changes in VA were defined at each time point as the primary outcome.
Statistical Analysis
To avoid selection bias, only one eye was randomly selected using a random number table if the patients’ eyes were simultaneously bilaterally involved. Statistical analyses were conducted using SPSS version 26.0 (IBM Corp., Armonk, NY, USA) or GraphPad Prism 8.0.2 (GraphPad Software, San Diego, CA, USA). Continuous data are presented as the mean ± standard deviation. The Mann–Whitney U test was used to compare the differences for continuous data and the chi-square test, corrected chi-square test, or Fisher’s exact test to compare categorical variables. The differences in VA value (logMAR) and the proportions of eyes in each VA category at the seven time points were evaluated using the Friedman test, together with the post hoc Wilcoxon signed-rank test. Statistical significance in the Wilcoxon signed-rank test was adjusted by Bonferroni correction. P < 0.05 was considered statistically significant.
Backward stepwise multiple logistic regression analyses were performed to identify critical factors associated with visual improvement after the fifth cycle of PE treatment in the AQP4-ON group. These factors included sex, age at receiving PE treatment, disease duration, disease phenotype, history of autoimmune disease, time window from onset to IVMP treatment, time window from onset to PE treatment, logMAR VA before PE, number of previous ON episodes, and bilateral eye involvement. Body mass index (BMI) [30], thickness of pRNFL (excluding one AQP4-ON patient with optic disc edema) [33], and mILM-RPE [34] were also included as factors for analysis, because they were identified to be associated with visual outcomes in previous studies. According to the significance in clinical practice and based on the statistical estimation for maintaining balance between compared groups, VA improvement scores ≥ 2 levels and VA < 1.0 logMAR were selected as the outcome indicator for visual improvement: the ratio of VA improvement scores ≥ 2 to VA improvement scores < 2 was equal to 1:2; the ratio of VA < 1.0 logMAR to VA ≥ 1.0 logMAR was equal to 1:3. An independent variable with P < 0.05 was considered a critical factor. Establishing a regression equation of the combined predictor and receiver–operating characteristic (ROC) curve was used to analyze the highest sensitivity and specificity by using the Youden index for the single and combined predictor to achieve VA score improvement ≥ 2 levels and regain less than 1.0 logMAR vision after the fifth cycle of PE therapy.
Results
Demographic and Clinical Characteristics of Optic Neuritis Patients and Comparisons Between Different Antibody Subtypes
A flowchart of patient inclusion is shown in Fig. 1. During the mean 30.21 ± 17.29 months follow-up visits, three AQP4-ON patients and four D-ON patients were lost to follow-up. A total of 122 consecutive patients receiving PE to treat 124 episodes were ultimately eligible for analyses (Table 1), consisting of 95 (77.5%) patients in the AQP4-ON group, 6 (4.8%) in the MOG-ON group, and 21 (17.7%) in the D-ON group. During the 28.11 ± 16.05 months follow-up time, none of the subjects in the D-ON group had developed MS or other forms of inflammatory CNS disorders, except for one patient who was diagnosed as NMOSD. Among the 21 patients (22 attacks), only one patient had better onset vision (20/50), but it was a recurrent attack. All the other patients had poor onset VA worse than 20/200. All the D-ON patients were “unresponsive to initial IVMP therapy.” There was no MS or idiopathic ON patients in our cohort, and only one patient with NMOSD.
Comparison of the AQP4-ON group and D-ON group revealed no significant differences (all P > 0.05) in gender, age of onset, age of PE initiation, attack phenotype, frequencies of ocular pain, history of area postrema syndrome, systemic disease including hypertension, diabetes mellitus and autoimmune diseases, malignant tumor, and time window from onset to IVMP or PE initiation. Owing to a longer disease duration and a higher proportion of ON history (both P < 0.001), the AQP4-ON group had a significantly thinner average thickness of the pRNFL (P = 0.001) and mILM-RPE (P = 0.042), whereas the D-ON group had a significantly higher frequency of bilateral involvement (P < 0.001), a lower proportion of TM history (P = 0.035), and higher BMI (P = 0.007). No statistical difference in F-VEP amplitude and latency was found between the AQP4-ON and D-ON groups.
In terms of the six MOG-ON patients (three male) receiving PE treatment (Table S2), the age of onset varied from 15 to 54 years, and age of PE initiation was > 18 years. Two patients experienced bilateral involvement. The number of ON attacks in this group ranged from 3 to 12. None experienced other CNS attacks before or were combined with systemic disease. There was a difference in gender balance compared with AQP4-ON, with significantly fewer female MOG-ON patients (P = 0.021), but no statistically significant difference was observed for other clinical characteristics. In contrast, a longer disease duration (P = 0.033), lower BMI (P = 0.045), and a thinner average pRNFL (P = 0.018) were observed in MOG-ON patients compared with the D-ON group.
In the total group, the mean time windows from onset to IVMP initiation, onset to PE initiation, and IVMP initiation to PE initiation were 10.85 ± 13.83 days, 38.31 ± 27.31 days and 27.47 ± 24.45 days, respectively (Table 1).
Changes in logMAR Visual Acuity at Different Time Points Before and After Plasma Exchange Treatment in ON Patients with Different Serum Antibodies
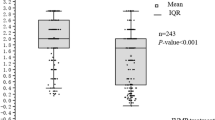
In the D-ON group, there was no statistical difference in mean logMAR VA of the studied eyes at seven points (Friedman P = 0.659, Fig. 2a, Table S1).
Visual acuity (logMAR) of the studied eyes over the study period. a The visual acuity (logMAR) of the studied eyes in the D-ON group. The mean VA (logMAR) values of the patients at the onset, 1 day before IVMP therapy, 1 day before PE therapy, after five cycles of PE therapy, and at the 1-, 3-, and 6-month follow-up visits were 3.95 ± 1.23, 3.40 ± 1.47, 2.75 ± 1.45, 2.59 ± 1.57, 2.47 ± 1.69, 2.95 ± 1.77, and 2.39 ± 1.96, respectively. The VA was not statistically different among different time points (Friedman P = 0.659). b The visual acuity (logMAR) of the MOG-ON eyes. The red, green, black, purple, blue, and brown lines represent patients 1, 2, 3, 4, 5, and 6, respectively. c The visual acuity (logMAR) of the studied eyes in the AQP4-ON group. The mean VA (logMAR) values of the patients were 3.33 ± 1.51, 3.16 ± 1.63, 2.22 ± 1.21, 1.61 ± 1.14, 1.87 ± 1.46, 1.94 ± 1.49, and 1.85 ± 1.52, respectively. The VA improved after PE therapy (Friedman P < 0.001). The data are presented as the mean ± standard deviation. The horizontal axis represents the time points at the onset, 1 day before IVMP therapy, 1 day before PE therapy, after five cycles of PE therapy, and at the 1-, 3-, and 6-month follow-up visits, respectively. *Adjusted P < 0.05, **adjusted P < 0.01, and ***adjusted P ≤ 0.001, compared with the VA at 1 day before PE therapy; ### adjusted P < 0.001, compared with the VA at 1 day before IVMP therapy; ns no statistical differences. IVMP intravenous methylprednisolone; PE plasma exchange; VA visual acuity; D-ON double-antibody-negative optic neuritis; MOG myelin oligodendrocyte glycoprotein; AQP4 aquaporin-4; ON optic neuritis
In the MOG-ON group, two patients improved significantly following treatment, with one improving from NLP to a final score of 0.1 logMAR; two patients (patient 2 and patient 3) had unchanged VA and another (patient 6) remained CF after PE treatment, and patient 5 had no more than one level improvement (Fig. 2b, Table S2).
The mean logMAR VA improved markedly after PE treatment in the AQP4-ON group. The VA at the worst onset, 1 day before IVMP treatment, and 1 day before PE therapy (Table S1) ranged from 3.33 ± 1.51 to 2.22 ± 1.21 and did not change significantly after IVMP treatment (adjusted P = 0.525, Fig. 2c). PE therapy significantly improved VA (P = 0.001), but after completing the five-cycle PE therapy there was no further significant change (all adjusted P > 0.999, Table S1, Fig. 2c). A significant improvement was found between the VA 1 day before IVMP treatment and four time points after PE treatment (after five cycles of PE therapy and at 1-, 3-, and 6-month follow-up visits) (Fig. 2c, Table S1).
Before commencement of PE therapy, there was a wide range in VA in the AQP4-ON group. The proportions in VA categories did not differ between the onset and before IVMP treatment (Table S3), but a significant difference existed after IVMP treatment (adjusted P = 0.035, Fig. 3, Table S3), with a relatively large proportion improving from NLP (45.8%) before IVMP treatment to HM (20.8%) and CF (25%) after IVMP treatment (Fig. 3, Table S3). A significantly increasing trend in VA was observed after PE treatment (adjusted P < 0.001, Fig. 3, Table S3) resulting in about half of the patients demonstrating an improvement in VA from HM and CF to CF and 20/200 ≥ VA > 20/800. Notably, the VA of two subjects improved to 20/20 and of three to scotoma but with VA better than 20/30 (Table S3). Some follow-up VA data were lost due to patient failure to undergo examination on time. However, there was no significant difference in the proportion of VA classification 1 day after PE treatment and at the 1-, 3-, and 6-month follow-up visits (adjusted P > 0.999, Fig. 3). Finally, 6.3% of patients recorded an improvement in VA to 20/20. A similar proportion improved to scotoma but with VA better than 20/30, and 10% recovered to 20/30 ≥ VA > 20/60 (Table S3).
Visual outcome categorization of AQP4-ON patients during the plasma exchange treatment period. The proportions of patients in each visual outcome category 1 day before IVMP treatment were not significantly different from those at the onset (adjusted P > 0.999). A significant trend in visual acuity improvement was observed from five-cycle PE treatment to the 6-month follow-up visit as compared with the visual outcome before the IVMP therapy (Friedman P < 0.001). The proportions of patients in each visual outcome category shifted significantly towards an improved distribution after PE therapy as compared with those 1 day before PE therapy (P = 0.001), and 6-month follow-up visits as compared with those at 1 day before PE therapy (P = 0.043). The proportions in each visual outcome category at the 1-, 3-, and 6-month follow-up visits were the same as those after the five-cycle PE therapy (adjusted P > 0.999). The data are presented as cumulative percentages. *Adjusted P < 0.05 and ***adjusted P ≤ 0.001, compared with the VA 1 day before PE treatment; #adjusted P < 0.05 and ###adjusted P < 0.001, compared with the VA 1 day before IVMP treatment. CF ability to count fingers; HM ability to perceive hand motion; LP light perception; NLP no light perception; IVMP intravenous methylprednisolone; PE plasma exchange; VA visual acuity; AQP4 aquaporin-4; ON optic neuritis
Visual Outcome Improvement-Associated Factors in the AQP4-ON Group
Two independent variables were identified to be associated with a visual improvement ≥ 2 levels after the fifth cycle of PE treatment in AQP4-ON patients: the number of previous ON episodes (P = 0.048) and time window from onset to PE treatment (P = 0.003) (Table S4). According to the regression coefficient, a predictive regression equation was established: combined factor (Y) = (−1) × time window from onset to PE treatment (days) − 11.11 × the number of previous ON episodes.
Similarly, three independent variables were determined to be statistically significant in multivariate analysis of VA < 1.0 logMAR after the fifth cycle of PE treatment in the AQP4-ON group: the logMAR VA before PE treatment (P = 0.001), the time window from onset to PE treatment (P = 0.009), and bilateral eye involvement (P = 0.047). The predictive factor (Y) = (−1) × time window from onset to PE treatment − 22.73 × the logMAR VA before PE treatment was calculated (Table S5).
Predictive Factors for Visual Acuity Improvement ≥ 2 Levels After Plasma Exchange Treatment in the AQP4-ON Group
A ROC analysis was performed with the combined factor as the predictor and success in achieving an improvement of ≥ 2 levels after PE treatment as the dependent variable. The area under the curve (AUC) was determined to be 0.747 (Fig. 4a), the Youden optimal criterion of the combined factor > −49.78, and sensitivity and specificity were 80.6% and 64.6%, respectively. Both the individual AUCs of the time window from onset to PE treatment and the number of previous ON episodes as individual predictors of success in improving two levels after PE treatment were lower (0.691 and 0.564, respectively).
A receiver operating characteristic (ROC) curve of the predictors after PE treatment in the AQP4-ON group. a The predictors of VA improvement ≥ 2 levels. The combined factors include the number of previous ON episodes and time window from onset to PE treatment. b The predictors of success in regaining less than 1.0 logMAR (20/20) vision. The combined factors include the logMAR VA before PE and the time window from onset to PE treatment. PE plasma exchange; VA visual acuity; AQP4 aquaporin-4; ON optic neuritis
Predictive Factors for Regaining Less than 1.0 logMAR Vision (~ Snellen 20/200) After Plasma Exchange Treatment in the AQP4-ON Group
A similar analysis was conducted with success to regain less than 1.0 logMAR as the dependent variable. A combination of logMAR VA before PE treatment and time window from onset to PE treatment reached the optimal AUC of 0.834 (Fig. 4b) with a Youden optimal criterion of > −83.42, sensitivity of 87%, and specificity of 64.4%. The addition of bilateral eye involvement led to a reduced AUC (0.808) relative to that with the combined factor (0.834).
Visual Improvement Scores at Different Time Windows from Onset to Plasma Exchange Treatment in the AQP4-ON Group
The visual improvement scores at four time windows (9–20 days, 21–30 days, 31–60 days, 61–157 days) for PE initiation were analyzed. Delay in the treatment reduced the likelihood of achieving visual improvement (Table S6, Fig. 5). Specifically, no patients achieved a visual improvement score > 3 when the treatment time window exceeded 60 days.
See Tables S1–S6 in the electronic supplementary material.
Safety Assessment of PE Treatment
Among the 124 attacks which received PE treatment, allergic reaction occurred on seven occasions, but the symptoms mainly involved a transient rash. No other serious adverse events occurred in the recruited subjects. All recruited subjects completed the full course of five-cycle PE treatment without any interruption. In the group of excluded patients, four patients did not complete the five-cycle PE treatment due to personal economic problems and deficient plasma. Another patient was excluded because the PE therapy was completed in other hospital. There were no adverse events reported by any of these patients.
Discussion
This paper reports the findings of a relatively large cohort study of PE rescue treatment for ON attacks unresponsive to IVMP, which revealed that PE treatment effectively improved VA in AQP4-ON patients. This study also identified the time window from onset to PE initiation as the key factor for VA prognosis. The concept “Time is Vision” should be emphasized in the treatment of acute ON.
A previous study reported that patients with D-ON often displayed early development of severe visual impairment, with about 75% of patients experiencing very poor outcomes after the first ON attack. Recovery of VA in the D-ON group was as poor as that of the AQP4-ON group [7, 8]. There is currently a paucity of data on D-ON. Mild D-ON patients may have excellent functional central acuity recovery spontaneously or recover after IVMP treatment, rendering PE therapy unnecessary. In this cohort study, 18 of 22 D-ON patients experienced their first attack with a mean PE initiation of 22.78 ± 11.37 days (range 7–49 days). However, VA improvement was limited even after five-cycle PE therapy (Friedman P = 0.659, Fig. 2a), indicating that PE therapy may be of limited benefit for patients with severe D-ON.
As a large proportion of MOG-ON patients achieved relatively better prognosis after IVMP, only a small cohort of MOG-ON patients were recruited in the current study; therefore, only descriptive data could be reported for this condition (Table S2, Fig. 2b). For two patients (patient 2 and patient 4), the treatment was implemented after their first ON attack. Both had bilateral eye involvement and were of similar age (47 years and 54 years), and their VA before PE treatment was NLP. However, patient 4 recovered to 20/25, whilst patient 2 remained NLP. Other than the small age gap, the major difference was the time window from onset to PE initiation (19 and 75 days, respectively). Of the remaining four patients, patient 1 had experienced 12 ON attacks over the past 7 years, and received timely PE treatment within 11 days of onset. The worst VA before treatment was 20/50, after IVMP but before PE was 20/30, after PE was 20/25, and finally achieving 20/20 Snellen VA. For the other three patients who had different ages of PE initiation, experiencing between three and six ON episodes over the past 3–20 years, although PE initiation was relatively timely (within 20 days for patient 3 and patient 5), no obvious VA improvement occurred. Patient 4 improved dramatically from NLP to 20/25 after receiving PE treatment. However, it was still difficult to ensure that the improvement after PE in these patients was due to the additional PE treatment. This issue will need to be addressed in further studies.
In contrast, the mean logMAR VA measurements in the AQP4-ON group improved markedly after five cycles of PE treatment (Friedman P < 0.001, Fig. 2c), and the VA of 95 eyes was steadily maintained, despite a longer disease duration (3.52 ± 4.25 years) and a higher proportion of past ON history (70.8%). The proportions of patients in each visual outcome category shifted significantly toward an improved distribution after PE therapy (adjusted P = 0.001, Fig. 3, Table S3). These results indicate that PE therapy effectively improves visual outcomes in AQP4-ON patients.
It was observed that VA improvement leveled off, showing no difference in VA or proportion of patients in each VA category at four time points after PE treatment. A multivariate analysis was conducted to determine the factors associated with achieving VA score improvement ≥ 2 levels, which produced a combined predictive factor equation: the combined factor (Y) = (−1) × time window from onset to PE treatment (days) − 11.11 × the number of previous ON episodes. The derived ROC curve demonstrated that a combination of the number of previous ON episodes and the time window of PE initiation could more efficiently predict the occurrence of VA score improvement ≥ 2 levels after PE treatment and the cutoff point that optimizes the balance between sensitivity and specificity was −49.78 (sensitivity 80.6% and specificity of 64.6%) (Fig. 4a). This indicates that the time window is 49.8 days for patients with their first attack of ON, 38.6 days for patients with one previous ON episode, 27.5 days for patients with two, 16.5 days for patients with three, and 5.3 days for patients with four. It also reveals a low probability of achieving a score improvement of two or more levels after PE therapy for patients with five or more episodes.
The same analysis showed that the logMAR VA before PE treatment combined with the time window from onset to PE treatment could predict the likelihood of regaining less than 1.0 logMAR vision with the cutoff point −83.42 (87% sensitive and 64.4% specific) (Fig. 4b) and the predictive factor (Y) = (−1) × time window from onset to PE treatment − 22.73 × the logMAR VA before PE treatment. That is, the treatment time window was 22 days for patients with HM VA before PE treatment and 51.6 days for patients with CF VA before PE treatment. The histogram directly demonstrated that patients who received early initiation of PE treatment had a greater opportunity to achieve increased improvement in VA. When the treatment time window exceeded 60 days, there was a low probability of achieving a visual improvement score > 3 (Fig. 5, Table S6).
In summary, the smaller number of attacks, the better VA before treatment, and the shorter treatment time window are indicators of the likely success of the PE therapy. Early diagnosis of ON, together with timely commencement of PE to prevent permanent visual disability should be given higher priority [35]. Our results emphasize that “Time is Tissue,” a core principle in neuroimmunology, and the precept that “Time is Vision” should be considered in either IVMP or PE initiation therapy [36], viewing AQP4-ON as an emergency. Because less serious adverse events occurred in our cohort, we would recommend the treatment approach for demyelinating ON as follows: after patients are diagnosed with demyelinating ON, initiation of IVMP treatment is recommended as soon as possible, followed by oral prednisone. When AQP4-ON can be confirmed and is “unresponsive to initial IVMP therapy,” we recommend escalating to five-cycle PE treatment immediately for AQP4-ON patients. The recommended time window is within 49.8 days for patients with first attack of ON, 38.6 days for patients with one previous ON episode, 27.5 days for patients with two episodes, 16.5 days for patients with three episodes, and 5.3 days for patients with four episodes. The treatment time window for PE should be within 22 days for patients with VA of HM and 51.6 days for VA of CF.
Previous studies have shown that progressive axonal loss may follow acute inflammatory demyelination, and ongoing subclinical inflammation may continue to develop over several years after an attack, as evidenced by a reduction in the pRNFL and decrease in the macular ganglion cell layer and inner plexiform layer (GCIPL) in longitudinal OCT studies [37, 38] and a mean decrease in the optic nerve area over a 1-year follow-up period in serial magnetic resonance imaging (MRI) studies [39, 40]. Further episodes of ON, leading to additional demyelination and failure of remyelination of the initial lesion, may contribute to conduction block, and over time, to secondary axonal degeneration [41]. In addition, the loss of critical macular axons may affect the ability of the visual system to compensate. Therefore, timely control of inflammation and prevention of recurrence are crucial. The rationale behind the improvement of prognosis by PE is as follows: The principle of PE is to separate the patient’s blood into plasma and blood cells via a blood cell separator to remove harmful and pathogenic components in the plasma and replace these with an equal amount of fluid and then transfuse the blood cells and PE fluid back into the patient [42]. PE can improve disease activity by reducing circulating antibodies, abnormal plasma proteins or cytokines, and other pathogenic macromolecules [42]. Early initiation of PE treatment can alleviate complement activation and subsequent inflammatory tissue destruction in AQP4-ON patients by clearance of AQP4-IgG, a critical driver in the evolving NMOSD lesion, which may decrease the synthesis and secretion of complement components, cytokines, and chemokines. Timely removal of complement protein may block the crosstalk between astrocytes and microglia [43, 44], rapidly blocking ganglion cell loss and preserving visual function [45]. In the future, we intend to evaluate the relationship between the pathogenic AQP4 antibody and prognosis, as well as to establish a model of PE efficacy and determine the concentration of the pathogenic AQP4 antibody.
This study does have several limitations. Firstly, only six patients with MOG-ON were included in this study of PE therapy, allowing only a descriptive analysis about the changes in logMAR VA with PE treatment in MOG-ON. The number of patients with D-ON was also relatively small, so a larger study is still needed to support the conclusions of this study for D-ON. Secondly, as this was a one-arm study, the effect of IVMP treatment is difficult to exclude. No statistically significant differences existed in mean logMAR VA before and after IVMP treatment, but a difference existed after IVMP treatment in each VA category. Further research should include equivalent-baseline ON patients who only received treatment for acute IVMP, in order to separate the effect of the IVMP therapy. Thirdly, the AQP4 antibody titer was not quantified longitudinally because of difficulty in conducting follow-ups related to changes in antibody titer changes. Thus, the correlation between therapeutic effect and the change in antibody titer should be investigated to improve the clinical estimation of the value of the therapy. Moreover, because some of the attacks were recurrent, and the patients received the initiation IVMP for their first attack outside the study site, the information on the initiation of IVMP, such as time or dosage, was missing. Furthermore, due to the paucity of plasma resources, not all the patients could receive PE treatment at the expected time after onset. There is currently no evidence-based data about the exact initiation window for PE therapy. These factors lead to inconsistency in the time window for therapeutic PE. However, based on the large time window of PE initiation to onset, which ranged from 9–157 days (some patients’ PE initiation > 60 days), in this observational study, the time window of PE initiation which benefited prognosis could be determined. Lastly, optic disc swelling causes difficulties in assessing the degree of severity. One (0.1%) AQP4-ON patient with optic disc edema was excluded from the analyses. Unfortunately, neither GCIPL nor pRNFL was collected at follow-up in the current study.
Conclusion
The most salient points of this study are the importance of antibody detection and serotyping of acute ON, to guide the use of PE treatment. PE therapy effectively improves visual outcomes for AQP4-ON patients, but provides limited value for D-ON patients. This study has provided a statistically rational treatment time window for AQP4-ON patients dependent on the previous history of their disease. In summary, the earlier the treatment commences, the greater the benefits.
References
Toosy AT, Mason DF, Miller DH. Optic neuritis. Lancet Neurol. 2014;13(1):83–99.
Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364(9451):2106–12.
Waters P, Woodhall M, O’Connor KC, et al. MOG cell-based assay detects non-MS patients with inflammatory neurologic disease. Neurol Neuroimmunol Neuroinflamm. 2015;2(3):e89.
Chan KH, Lee R, Lee JC, et al. Central nervous system inflammatory demyelinating disorders among Hong Kong Chinese. J Neuroimmunol. 2013;262(1–2):100–5.
Ramanathan S, Mohammad S, Tantsis E, et al. Clinical course, therapeutic responses and outcomes in relapsing MOG antibody-associated demyelination. J Neurol Neurosurg Psychiatry. 2018;89(2):127–37.
Jurynczyk M, Messina S, Woodhall MR, et al. Clinical presentation and prognosis in MOG-antibody disease: a UK study. Brain. 2017;140(12):3128–38.
Deschamps R, Gueguen A, Lecler A, et al. Acute idiopathic optic neuritis: not always benign. Eur J Neurol. 2018;25(11):1378–83.
Akaishi T, Nakashima I. Visual prognosis in seronegative idiopathic optic neuritis finally elucidated: as bad as that in anti-AQP4 antibody-positive optic neuritis. Eur J Neurol. 2018;25(11):1305–6.
Ishikawa H, Kezuka T, Shikishima K, et al. Epidemiologic and clinical characteristics of optic neuritis in Japan. Ophthalmology. 2019;126(10):1385–98.
Skorupka N, Miclea A, Jalowiec KA, et al. Visual Outcomes of Plasma Exchange Treatment of Steroid-Refractory Optic Neuritis: A Retrospective Monocentric Analysis. Transfus Med Hemother. 2019;46(6):417–22.
Mori S, Kurimoto T, Ueda K, Nakamura M. Short-term effect of additional apheresis on visual acuity changes in patients with steroid-resistant optic neuritis in neuromyelitis optica spectrum disorders. Jpn J Ophthalmol. 2018;62(4):525–30.
Mason MC, Marotta DA, Kesserwani H. Steroid-resistant double-seronegative optic neuritis responds favorably to plasma exchange. Cureus. 2021;13(5):e15260.
Roesner S, Appel R, Gbadamosi J, Martin R, Heesen C. Treatment of steroid-unresponsive optic neuritis with plasma exchange. Acta Neurol Scand. 2012;126(2):103–8.
Li R, Wang J, Li C, et al. Rescue immunoadsorption treatment for neuromyelitis optica spectrum disorder attacks unresponsive to intravenous methylprednisolone. J Neuroimmunol. 2021;356:577604.
Zhang L, Zhuang Y, Liu X, et al. The efficacy of therapeutic apheresis in patients with refractory neuromyelitis optica spectrum disorders: a single-center retrospective study. Ann Palliat Med. 2021;10(3):3105–14.
Deschamps R, Gueguen A, Parquet N, et al. Plasma exchange response in 34 patients with severe optic neuritis. J Neurol. 2016;263(5):883–7.
Jiao Y, Cui L, Zhang W, et al. plasma exchange for neuromyelitis optica spectrum disorders in Chinese patients and factors predictive of short-term outcome. Clin Ther. 2018;40(4):603–12.
Schwartz J, Padmanabhan A, Aqui N, et al. Guidelines on the use of therapeutic apheresis in clinical practice-evidence-based approach from the writing committee of the American Society for Apheresis: the seventh special issue. J Clin Apher. 2016;31(3):149–62.
Huang X, Wu J, Xiao Y, Zhang Y. Timing of plasma exchange for neuromyelitis optica spectrum disorders: a meta-analysis. Mult Scler Relat Disord. 2021;48:102709.
Yu HH, Qin C, Zhang SQ, et al. Efficacy of plasma exchange in acute attacks of neuromyelitis optica spectrum disorders: a systematic review and meta-analysis. J Neuroimmunol. 2020;350:577449.
Songthammawat T, Srisupa-Olan T, Siritho S, et al. A pilot study comparing treatments for severe attacks of neuromyelitis optica spectrum disorders: Intravenous methylprednisolone (IVMP) with add-on plasma exchange (PLEX) versus simultaneous ivmp and PLEX. Mult Scler Relat Disord. 2020;38:101506.
Siritho S, Nopsopon T, Pongpirul K. Therapeutic plasma exchange vs conventional treatment with intravenous high dose steroid for neuromyelitis optica spectrum disorders (NMOSD): a systematic review and meta-analysis. J Neurol. 2021;268(12):4549–62.
Kleiter I, Gahlen A, Borisow N, et al. Apheresis therapies for NMOSD attacks: a retrospective study of 207 therapeutic interventions. Neurol Neuroimmunol Neuroinflamm. 2018;5(6):e504.
Savransky A, Rubstein A, Rios MH, et al. Prognostic indicators of improvement with therapeutic plasma exchange in pediatric demyelination. Neurology. 2019;93(22):e2065–73.
Bonnan M, Valentino R, Debeugny S, et al. Short delay to initiate plasma exchange is the strongest predictor of outcome in severe attacks of NMO spectrum disorders. J Neurol Neurosurg Psychiatry. 2018;89(4):346–51.
Llufriu S, Castillo J, Blanco Y, et al. Plasma exchange for acute attacks of CNS demyelination: Predictors of improvement at 6 months. Neurology. 2009;73(12):949–53.
Abboud H, Petrak A, Mealy M, Sasidharan S, Siddique L, Levy M. Treatment of acute relapses in neuromyelitis optica: Steroids alone versus steroids plus plasma exchange. Mult Scler. 2016;22(2):185–92.
Bonnan M, Valentino R, Olindo S, Mehdaoui H, Smadja D, Cabre P. Plasma exchange in severe spinal attacks associated with neuromyelitis optica spectrum disorder. Mult Scler. 2009;15(4):487–92.
Restrepo-Aristizabal C, Giraldo LM, Giraldo YM, et al. PLEX: the best first-line treatment in nmosd attacks experience at a single center in Colombia. Heliyon. 2021;7(4):e06811.
Tan S, Ng TK, Xu Q, et al. Vision improvement in severe acute isolated optic neuritis after plasma exchange treatment in Chinese population: a prospective case series study. Ther Adv Neurol Disord. 2020;13:1756286420947977.
Gotkine M. Neuromyelitis optica and the optic neuritis treatment trial. Arch Neurol. 2008;65(11):1545–6.
Beck RW, Cleary PA, Anderson MM, et al. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. The Optic Neuritis Study Group. N Engl J Med. 1992;326(9):581–8.
Zhao X, Qiu W, Zhang Y, et al. a prospective case-control study comparing optical coherence tomography characteristics in neuromyelitis optica spectrum disorder-optic neuritis and idiopathic optic neuritis. BMC Ophthalmol. 2018;18:247.
Sotirchos ES, Filippatou A, Fitzgerald KC, et al. Aquaporin-4 IgG seropositivity is associated with worse visual outcomes after optic neuritis than MOG-IgG seropositivity and multiple sclerosis, independent of macular ganglion cell layer thinning. Mult Scler. 2020;26(11):1360–71.
Villoslada P, Martinez-Lapiscina EH. Time is vision: the importance of the early discovery and diagnosis of optic neuritis. Mult Scler. 2017;23(14):1806–7.
Stiebel-Kalish H, Hellmann MA, Mimouni M, et al. Does time equal vision in the acute treatment of a cohort of AQP4 and MOG optic neuritis? Neurol Neuroimmunol Neuroinflamm. 2019;6(4):e572.
Gabilondo I, Martinez-Lapiscina EH, Fraga-Pumar E, et al. Dynamics of retinal injury after acute optic neuritis. Ann Neurol. 2015;77(3):517–28.
Klistorner A, Arvind H, Garrick R, Graham SL, Paine M, Yiannikas C. Interrelationship of optical coherence tomography and multifocal visual-evoked potentials after optic neuritis. Invest Ophthalmol Vis Sci. 2010;51(5):2770–7.
Hickman SJ, Brierley CM, Brex PA, et al. Continuing optic nerve atrophy following optic neuritis: a serial MRI study. Mult Scler. 2002;8(4):339–42.
Hickman SJ, Toosy AT, Jones SJ, et al. A serial MRI study following optic nerve mean area in acute optic neuritis. Brain. 2004;127(Pt 11):2498–505.
McGavern DB, Murray PD, Rivera-Quinones C, Schmelzer JD, Low PA, Rodriguez M. Axonal loss results in spinal cord atrophy, electrophysiological abnormalities and neurological deficits following demyelination in a chronic inflammatory model of multiple sclerosis. Brain. 2000;123(Pt 3):519–31.
Okafor C, Ward DM, Mokrzycki MH, Weinstein R, Clark P, Balogun RA. Introduction and overview of therapeutic apheresis. J Clin Apher. 2010;25(5):240–9.
Chen T, Bosco DB, Ying Y, Tian DS, Wu LJ. The emerging role of microglia in neuromyelitis optica. Front Immunol. 2021;12:616301.
Chen T, Lennon VA, Liu YU, et al. Astrocyte-microglia interaction drives evolving neuromyelitis optica lesion. J Clin Invest. 2020;130(8):4025–38.
Soelberg K, Specovius S, Zimmermann HG, et al. Optical coherence tomography in acute optic neuritis: a population-based study. Acta Neurol Scand. 2018;138(6):566–73.
Acknowledgements
We are grateful to all participants in this study. We would like to thanks all optometrists and nurses at the Department of Ophthalmology in the Chinese People's Liberation Army General Hospital for their assistance in this project.
Funding
This work was supported by the National Key Research and Development Program (project code: 2018YFE0113900 to S.W.) and the National Natural Science Foundation of China (project code: 81870662 to S.W. and project code: 81800822 to. S.T.) including the study and the journal's Rapid Service Fee.
Editorial Assistance
Editorial assistance in the preparation of this article was provided by Dr Maureen Boost, a part-time staff member, from School of Optometry, The Hong Kong Polytechnic University. The salary of Dr Maureen Boost on editorial assistance was paid by the School of Optometry, The Hong Kong Polytechnic University.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and give their approval for this version to be published.
Author Contributions
Conceptualization: Junxia Fu, Shaoying Tan; Methodology: Junxia Fu, Yongping Wang, Shaoying Tan; Formal analysis and investigation: Junxia Fu, Yongping Wang, Hongen Li, Huanfen Zhou, Honglu Song, Mingming Sun, Shaoying Tan; Writing - original draft preparation: Junxia Fu, Shaoying Tan; Writing - review and editing: Junxia Fu, Shaoying Tan, Shihui Wei; Funding acquisition: Shaoying Tan and Shihui Wei; Clinical data collection and curation: Junxia Fu, Huanfen Zhou, Quangang Xu, Shihui Wei; Supervision: Shaoying Tan and Shihui Wei.
Disclosures
The authors (Junxia Fu, Yongping Wang, Hongen Li, Huanfen Zhou, Honglu Song, Mingming Sun, Quangang Xu, Shaoying Tan, Shihui Wei) declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Compliance with Ethics Guidelines
The study protocol was approved by the Ethics Committee for Human Research of the hospital (approval number S2017-093-01) and was in accordance with the tenets of the Helsinki Declaration and the ICH-GCP guidelines. Written informed consent was obtained from all study subjects. The consents to publish the patient-level data presented in Table S2 were also obtained.
Data Availability
As far as permitted, according to data protection requirements and consent provided by the participants, original data are available from the corresponding author on request from any qualified investigator within 5 years of publication.
Open Access
This article is licensed under a Creative Commons Attribution-Non Commercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
Author information
Authors and Affiliations
Corresponding authors
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Fu, J., Wang, Y., Li, H. et al. Efficacy of Plasma Exchange Treatment for Demyelinating Optic Neuritis Associated with Various Serum Antibodies: A Prospective Cohort Study. Neurol Ther 11, 797–813 (2022). https://doi.org/10.1007/s40120-022-00344-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-022-00344-w