Abstract
Introduction
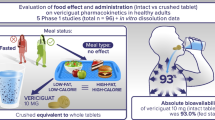
XEN496 is a novel, granular, immediate-release formulation of ezogabine intended for pediatric use. The objective of this study was to assess the effect of food on the pharmacokinetics (PK) of XEN496 and its N-acetyl metabolite (NAMR) in healthy volunteers.
Methods
Twenty-four adult subjects were enrolled in this phase 1, single center, open-label, randomized, single-dose, two-way crossover study. Subjects received 400 mg XEN496 as an oral suspension in both fed and fasted states separated by a 6-day washout period. Serial blood samples were collected up to 48 h post-administration. PK parameters evaluated included maximum observed plasma concentration (Cmax), time of maximum observed plasma concentration (Tmax), and area under the concentration–time curve (AUC(0–t) and AUCinf). Safety was assessed by laboratory evaluations, physical exam, and adverse event monitoring.
Results
For XEN496, median Tmax was 3 and 2 h in the fed and fasted states, respectively. AUC parameters in the fed and fasted states were equivalent, whereas food decreased Cmax of XEN496 by 32% compared to the fasted state. The ratio of geometric means [90% CI] for Cmax was 72% [64–82%]. For NAMR, food delayed Tmax by 1 h, while Cmax and AUC parameters were equivalent in the fed and fasted states. The safety profile of XEN496 in this study appeared comparable to that previously reported for ezogabine tablets.
Conclusion
The biopharmaceutical performance of XEN496 in this study was as expected for an immediate-release, granular dosage formulation, and generally comparable to that reported for ezogabine tablets. Future studies are needed to characterize the efficacy, safety, and PK of XEN496 in a pediatric population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Compounded or crushed ezogabine tablets had been used off-label to treat certain types of epilepsy in children, including KCNQ2-related developmental and epileptic encephalopathy; however, ezogabine tablets were withdrawn from the market in 2017. |
XEN496 is a novel, granular, immediate-release formulation of ezogabine that is being developed to treat infants and children with KCNQ2-related developmental and epileptic encephalopathy. |
What was learned from this study? |
Analysis of pharmacokinetic parameters following a single dose of 400 mg XEN496 in healthy adult volunteers under fed and fasted conditions showed that food reduced and delayed the peak plasma concentration of ezogabine, but did not affect the extent of systemic exposure compared to the fasted state. |
The biopharmaceutical performance of XEN496 in this study appeared comparable to that of ezogabine tablets, warranting further investigation of its pharmacokinetics, efficacy, and safety in a pediatric population. |
Introduction
Epilepsy is one of the most common childhood brain disorders. Worldwide, over 45 million people have been diagnosed with epilepsy, 4.7 million of whom are children between 1 and 4 years old [1]. In infants born to term, the incidence of seizures has been estimated at 0.5–3 per 1000 live births, but could be as high as 1–13% for pre-term births [2]. Without timely and adequate treatment, early onset epilepsy can lead to significant developmental, behavioral, and cognitive problems in children. Despite this burden, the development of anti-epileptic drugs for treatment of seizures in neonates and children has been slow [3].
Ezogabine, a KV7.2/KV7.3 potassium channel opener, was originally developed as adjunctive treatment for partial-onset epilepsy in adults [4,5,6]. Marketed as Potiga® tablets in the United States and as Trobalt® (retigabine) tablets in Europe, ezogabine was withdrawn from the market in 2017 for commercial reasons [7]. Compounded or crushed ezogabine had been used off-label to treat certain types of epilepsy in children, including KCNQ2-related developmental and epileptic encephalopathy (KCNQ2-DEE) [8, 9]. This condition is caused by loss-of-function missense mutations in KCNQ2 [10], leading to multiple, daily, treatment-resistant seizures often presenting within the first week of life [11].
XEN496 is a novel, granular, immediate-release formulation of ezogabine intended for pediatric use [12] that may have the potential to improve long-term outcomes in KCNQ2-DEE. XEN496 was shown to be bioequivalent to ezogabine tablets in a rat model, is chemically stable allowing for compatibility with feeding-bottle plastics, and in addition has a pleasant taste and mouth feel [12]. XEN496 granules may be dispersed in breast milk, infant formula, or soft foods prior to dosing, and are packaged in a way to allow for accurate body weight-based dosing [12].
The pharmacokinetics (PK) of the previously available commercial product have been extensively investigated [13,14,15]. Ezogabine is rapidly absorbed after both single and multiple oral doses [16]. While it was shown that a high-fat meal had no effect on the systemic exposure of ezogabine, the fed state did reduce variability observed in the rate of absorption compared to the fasted state [15].
This phase 1 trial characterized the PK profile of XEN496 and assessed the effect of food on PK parameters of XEN496 in healthy volunteers. Additionally, PK data obtained from this study were compared to historical PK data available from previously conducted studies with the tablet formulation of ezogabine. The tolerability and safety of XEN496 following single oral doses were also assessed.
Methods
Study Design and Participants
In this phase 1, single center, open-label, randomized, single-dose, two-way crossover study, approximately 24 subjects were planned to receive 400 mg XEN496 in the fed and fasted states. Healthy volunteer subjects were selected according to appropriate inclusion and exclusion criteria. Briefly, all subjects must have been healthy adult men or women aged between 18 and 55 years with no clinically relevant abnormalities as determined by medical history, physical examination (including ophthalmological), vital signs, 12-lead ECG, and clinical laboratory evaluation. Subjects had to have no current or recurrent disease that could have affected the action, absorption, or disposition of XEN496, or that could have affected clinical assessments or clinical laboratory evaluations. Subjects who were prone to orthostatic dysregulation, fainting, or blackouts, or who had a history of seizures or any seizure disorder were excluded from the study. Women who were pregnant or breastfeeding were excluded from the study, and all female subjects of childbearing potential agreed to use at least one form of highly effective contraception during the treatment period and for at least 90 days after the last dose of the study drug. Male subjects with partners who were pregnant, breastfeeding, or of childbearing potential agreed to use barrier contraception during the treatment period and for at least 90 days after the last dose of the study drug. The trial was carried out in accordance with the protocol (Pro00039280, approved by Advarra, Inc., an independent institutional review board registered with the US Department of Health and Human Services Office for Human Research Protections and US Food and Drug Administration under IRB#00000971), International Conference on Harmonisation (ICH) Good Clinical Practice (GCP), Declaration of Helsinki, and applicable regulatory requirements. All subjects provided written informed consent before completing any study-related procedures.
Drug Dosage and Subject Examination
As shown in Fig. 1, subjects were randomized equally into one of two treatment sequences, to be treated first in the fasted state and then the fed state, or to be treated first in the fed state and then the fasted state. During each treatment period, subjects entered the clinic the day before dosing and remained until discharged 48 h post-dose. Subjects received both a single dose of 400 mg XEN496 under fasted conditions and a single dose of 400 mg XEN496 under fed conditions separated by a 6-day washout period and returned for a follow-up visit 7 ± 3 days after completing the second treatment period.
Following an overnight fast of at least 10 h, subjects assigned the fed condition received a standardized high-fat, high-calorie meal 30 min before XEN496 administration. This meal included 240 mL whole milk, two large eggs, 4 oz of hash brown potatoes, two slices of toast, 9 g of butter, and two strips of bacon.
XEN496 was administered as a suspension, with the contents of one sachet, equaling 400 mg of the granular formulation, mixed thoroughly with 120 mL of water at ambient temperature in a glass. After administration, the glass was rinsed twice with approximately 60 mL of water at ambient temperature, both of which the subject was to drink. Subjects were observed to ensure they ingested the entire volume, including both rinses. Safety assessments and blood sampling for PK purposes were performed from pre-dose up to 48 h after study drug administration in each period.
PK and Safety Assessments
Blood samples were collected prior to each XEN496 administration and at 0.25, 0.5, 1, 1.5, 2, 3, 4, 8, 12, 24, and 48 h afterward. Twelve blood samples were collected during each treatment period in 4-mL lithium-heparin vacutainers, totaling 24 samples from each subject. Blood samples were processed to plasma and assayed for XEN496 and its primary human N-acetyl metabolite (NAMR) [17], using protein precipitation and validated high-performance liquid chromatography methods with tandem mass spectrometry detection (XNO-W9-336). The methods were validated based upon the US Food and Drug Administration Guidance for Industry, Bioanalytical Method Validation (May 2018) [18] and European Medicines Agency Guideline on Bioanalytical Method Validation (EMEA/CHMP/EWP/192217/2009 Rev.1 Corr. 2**) [19] over a calibration range of 1.00 ng/mL to 1000.00 ng/mL for both XEN496 and NAMR. The lower limit of quantification for both analytes was 1.00 ng/mL.
PK analyses were performed using non-compartmental methods. PK parameters that were assessed included maximum observed plasma concentration (Cmax), time of maximum observed plasma concentration (Tmax), area under the concentration–time curve from time zero to time of last observed quantifiable plasma concentration (AUC0–t), and area under the concentration–time curve extrapolated to infinity (AUC0–inf). The magnitude of the food effect on a single 400 mg dose of the granular formulation of ezogabine was described using Tmax, and the geometric mean ratio (GMR; fed/fasted) with associated 90% confidence interval (CI) for Cmax, AUC0–t, and AUC0–inf of XEN496 and NAMR in plasma. Cmax and Tmax were obtained directly from the raw concentration–time data. The terminal elimination rate constant (λz) was determined using log-linear regression of at least three concentration–time points visually judged to be in the terminal phase. The AUC0–t was determined using the linear trapezoidal rule for increasing concentrations and the logarithmic trapezoidal rule for decreasing concentrations. The AUC0–inf was calculated, where data permitted, as the sum of AUC0–t and Ct/λz, where Ct is the last quantifiable concentration. Additional parameters included the terminal elimination half-life (t1/2), estimated as Ln2/λz, the apparent volume of distribution during the terminal elimination phase (Vz/F), calculated as dose/λz × AUC0–inf, and the apparent total plasma clearance (CL/F), calculated as dose/AUC0–inf.
Safety was assessed by reported adverse events (AEs), clinical laboratory tests, vital sign measurements, ECG findings, American Urological Association Symptom Index (AUA-SI), and Columbia-Suicide Severity Rating Scale (C-SSRS) questionnaire responses, as well as findings from physical, ophthalmological, and neurological examinations.
Statistical Analysis
The natural logarithmic (ln)-transformation of Cmax, AUC0–t, and AUC0–inf, were used for all statistical inference. The 90% CIs for the exponential of the difference in least-squares (LS) means between fasted and fed status were calculated using the fasted state as the reference condition. Conditions were considered equivalent if the 90% CI of the GMR was within the conventional bioequivalence acceptance range of 80–125%.
The Tmax parameter distribution was compared between the fed and fasted treatments through a non-parametric approach (empirical distribution comparison and Wilcoxon’s rank sum test). Hodge–Lehman 95% CI was provided for the difference in the median Tmax between treatments.
PK parameter data from this study were compared to data obtained from the US Food and Drug Administration Center for Drug Evaluation and Research Clinical Pharmacology and Biopharmaceutics Review [20] of ezogabine tablets, specifically from VRX-RET-E22-104, a similarly designed open-label, randomized, single-dose, two-way crossover study, that assessed the PK of the retigabine 400 mg market image tablet formulation in 24 male subjects after a 10-h fast and 30 min after administration of a standard high-fat breakfast.
Results
Subject Disposition and Demographics
A total of 24 subjects were included in this study, with 12 subjects per treatment sequence (Fig. 2). After randomization, 23 subjects (96%) received XEN496 under fasted conditions and 22 subjects (92%) received XEN496 under fed conditions. All 24 subjects completed the safety assessment, while 21 subjects (87.5%) completed the PK assessment. Three subjects withdrew consent or were withdrawn from the study prior to dosing in the second treatment period for reasons other than safety or tolerability; these subjects were excluded from the PK analyses. Baseline subject demographic characteristics can be found in Table 1. The mean age of participants was 33.5 years (median 32, range 19–54); two-thirds were male, and all but two were White.
Pharmacokinetics
Absorption of XEN496 was relatively rapid under both fed and fasted conditions with median Tmax of 3 and 2 h, respectively. Thereafter, plasma concentrations declined in a mono-exponential manner, with mean t1/2 of 7.2 h in the fed and 8.8 h in the fasted state (Table 2). A similar disposition pattern and t1/2 were observed for NAMR. When compared to administration in the fasted state, administration of XEN496 under fed conditions slightly reduced and delayed the peak plasma concentration of XEN496 but did not affect the extent of its systemic exposure (Fig. 3). For both XEN496 and NAMR, median Tmax was delayed by 1 h under fed conditions. Overall, the interindividual variability (IIV) in PK parameters was low for both ezogabine and NAMR following administration of XEN496. The fed condition tended to reduce the IIV; for example, the %CV for Cmax, AUC0–t, and AUC0–inf ranged from 23–38% under the fasted state but was reduced to 18–25% under the fed state. The apparent volume of distribution during the terminal elimination phase (Vz/F), and the apparent total plasma clearance (CL/F) were similar under fed and fasted conditions, resulting in t1/2 of 7.17 ± 1.06 h and 8.83 ± 2.04 h, respectively.
Key plasma PK parameters of XEN496 and its primary metabolite NAMR following administration of 400 mg XEN496 under both fasted and fed conditions are summarized in Table 2. For XEN496, the AUC parameters in the fed and fasted states were equivalent while Cmax was close to equivalent (Table 3). In the case of XEN496, the GMR [90% CI] of fed/fasted states was 91% [84–99%] and 89% [82–96%] for AUC0–t and AUC0–inf, respectively, whereas the GMR [90% CI] for Cmax was 72% [64–82%], outside the reference range of 80–125%. For NAMR, AUC and Cmax were equivalent between fed and fasted states (Table 3). The GMR [90% CI] of NAMR was 112% [102–123%], 110% [105–116%], and 107% [102–113%] for Cmax, AUC0–t, and AUC0–inf, respectively.
The PK and statistical results of the XEN496 400 mg granular formulation were compared to historical data for the ezogabine 400 mg tablet formulation (Fig. 4), showing that food slightly affected the rate of absorption of both formulations. Food decreased Cmax following administration of the 400 mg granular formulation by 32% (Table 2), while a fed state was shown previously to increase Cmax by 38% following administration of the 400 mg tablet formulation (Table 4). Following administration of XEN496 or ezogabine tablet formulation, comparable systemic exposure, in terms of AUC0–inf, was observed under fed and fasted conditions (Table 5).
XEN496 (ezogabine granular formulation) comparison with historical data for ezogabine tablets [20]: Cmax and AUC0–inf. AUC0–inf area under the concentration–time curve extrapolated to infinity, Cmax maximum observed plasma concentration, NAMR N-acetyl metabolite. Data for orange bars for ezogabine tablets obtained from the US Food and Drug Administration Center for Drug Evaluation and Research; Application Number: 022345Orig1s000, Clinical Pharmacology and Biopharmaceutics Review(s) [20]
Safety
The treatment-emergent adverse events (TEAEs) experienced most commonly during the study were dizziness, reported by 12 subjects (52%) under fasted conditions and six subjects (27%) under fed conditions; oral hypoesthesia, reported by six subjects (26%) under fasted conditions and three subjects (14%) under fed conditions; and fatigue, reported by five subjects (22%) under fasted conditions and seven subjects (32%) under fed conditions, following administration of XEN496 (Table 6).
Most TEAEs were considered mild in intensity, with slightly higher incidences under fasted conditions compared to fed conditions. All of the TEAEs experienced by subjects resolved by the end of the study. Only two subjects (8%) experienced TEAEs that were considered severe; both TEAEs were considered possibly related to drug administration. One subject experienced syncope following a blood draw, about 1 h after receiving XEN496 during fasted conditions in period 1. Another subject experienced depressed mood 3 days after receiving XEN496 during fasted conditions in period 2.
During follow-up examination, one subject exhibited mild erythema in the left eye. The finding was considered clinically significant but unrelated to treatment and resolved approximately 23 h after onset. Overall, no clinically significant physical or neurological. examination findings were observed. Mean clinical laboratory, vital signs, and ECG values were generally within the reference range.
Discussion
In this open label, two-way crossover study, XEN496 performed as expected for an immediate-release granular dosage formulation. XEN496 has been developed using a modified quality by design approach to be suitable for administration to children, including newborns [12]. The granules containing ezogabine have good polymer compatibility and suitable particle size distribution leading to rapid dissolution, adequate stability, and ease of dosing on a body weight basis without requiring extemporaneous compounding [12]. In addition, XEN496 has a neutral taste profile, which could potentially enhance both patient and caregiver convenience and patient compliance.
The main finding of the current study is that administration of a single 400 mg dose of XEN496 in the fed state slightly reduced and delayed the peak plasma concentration of ezogabine, but did not affect the extent of systemic exposure compared to the fasted state. With respect to the major metabolite of XEN496, food also delayed the time to reach NAMR peak plasma concentration, but peak and total systemic exposure were not affected.
An absence of a food effect was not fully confirmed in this study, as the rate of absorption of ezogabine was delayed following single-dose administration of XEN496 in a fed compared to fasted state, and the fed/fasted GMR for ezogabine Cmax (72%) fell below the 80% reference range. The observed delay in Cmax in a fed condition for XEN496 may be related to the delay in the rate of absorption due to gastric emptying and thus sequential metabolism.
Comparison of the PK profile of ezogabine following administration of XEN496 granular formulation with that previously published for the tablet formulation showed that, unlike the tablet formulation, no distinct second peak was observed following administration of XEN496. Although some individual PK profiles showed a second peak after administration of XEN496, this was not a dominant feature of the average plasma concentration–time profile. The PK profile of ezogabine after administration of tablet formulation showed the first peak occurring at 0.67–1.5 h, with the second peak at 1.8–4.0 h, suggesting potential enterohepatic recirculation of the drug or other unknown mechanisms [17]. The lack of such a distinct second peak following administration of XEN496 suggests that mechanisms other than enterohepatic recirculation contribute, at least in part, to the formation of the second peak (e.g., differential absorption in different parts of the gastrointestinal tract). The upper part of the stomach has a pH of 4−6.5, with food residence time of 0.5–1 h, while the lower part has a pH of 1.5−4.0 with food residence time of 1 to 3 h [21]. The time of first and second peak of ezogabine following tablet formulation administration coincides with the residence time of ingested material in different parts of the stomach. In addition, the solubility of ezogabine is pH-dependent, with high solubility in acidic conditions (16 mg/mL at pH 1.6) and poor solubility in alkaline conditions (0.08 mg/mL at pH 5.0) [15], suggesting that a two-phase absorption profile is possible. Furthermore, these data suggest that XEN496 likely has a better dissolution profile, resulting in more uniform absorption of ezogabine and a smoother PK profile compared to that of the tablet formulation.
Comparison of the XEN496 granular formulation of ezogabine with the tablet formulation showed that, while total systemic exposure was comparable, food slightly affected the rate of absorption of both formulations but in opposite directions. Food decreased Cmax following administration of the 400 mg granular formulation by 32%, while a fed state increased Cmax by 38% following administration of the 400 mg tablet formulation. The reason for this observation is unknown but could be related to the larger surface area of the granule formulation and greater nonspecific binding to food particles. Alternatively, a tablet may have more time to dissolve with food and delayed gastric emptying compared to the fasted state, where Cmax was somewhat lower for the tablet than that observed for the granule formulation in the current study. Nonetheless, in the fed state, Cmax following administration of the 400 mg granular formulation was lower (29%) than that of tablet formulation, which may have implications for safety and tolerability. However, the modest differences observed in Cmax for these formulations under a fasted state may not be clinically meaningful, since pediatric patients typically are not dosed under fasted conditions. Moreover, comparable systemic exposure (i.e., AUC0–inf) following administration of XEN496 or ezogabine tablet formulation was observed under fed and fasted conditions, which is important since the AUC of ezogabine was a good predictor of efficacy [15]. Furthermore, lower Cmax under the fed state for XEN496 as compared to that of the tablet formulation may be beneficial in terms of managing Cmax-related central nervous system side effects.
Overall, the ratio of geometric means of XEN496 to ezogabine tablets for Cmax and AUC0–inf indicated that the AUC parameters for these formulations were equivalent while Cmax was relatively lower with XEN496 in the fed state. The Tmax of ezogabine after administration of a single 400 mg dose of XEN496 (2.0–3.0 h) or tablet formulation (1.8–2.5 h) was comparable regardless of food intake, as was t1/2 (7.2–8.8 h for XEN496 vs. 6.3 to 7.8 h for tablet formulation) [20]. Similarly, the PK parameters of NAMR were comparable after administration of a single 400 mg dose of XEN496 or tablet formulation. This further supports the comparability of PK parameters between the two ezogabine formulations at an equivalent dose of 400 mg [20].
XEN496 was safe and generally well tolerated in this phase 1 PK study in healthy volunteers. No deaths or serious AEs occurred in the study, and no subject was withdrawn for safety reasons. The safety profile of XEN496 appeared comparable to that of ezogabine tablets [6].
This study has several limitations. Firstly, because the tablet formulation of ezogabine is no longer commercially available, the tablet formulation was not administered to a control group in this study. Instead, data from this study were compared to historical data for the effect of food on PK parameters after administration of the tablet formulation of ezogabine. A second limitation is that the PK assessment was done in healthy adults, which may limit the generalizability of the results to the pediatric population for which XEN496 is being developed. However, previous research suggests that plasma levels of ezogabine in adult and pediatric subjects are comparable at milligrams-per-kilogram doses extrapolated from recommendations in adults. In a retrospective study of KCNQ2-DEE patients aged between 2 months and 6 years, administration of ezogabine was associated with improvement in patients’ seizures and developmental indicators. The study found that serum ezogabine levels in the infant patients were within the range seen in adult subjects when given the same weight-adjusted dose [22]. In addition, no new toxicities were observed in the infant patients. Overall, they tolerated the serum ezogabine levels well, with side effects that were dose-related, relatively minor, reversible, and consistent with those observed in adult patients (e.g., urinary retention, chromaturia, and somnolence) [22].
Conclusions
In conclusion, the biopharmaceutical performance of XEN496, a granular formulation of ezogabine suitable for pediatric use, appeared comparable to that of ezogabine tablets. Despite its off-label usage to treat KCNQ2-related seizures in children, ezogabine has not been widely studied in pediatric patients [23]. Future clinical studies will be required to establish the pharmacokinetics, efficacy, and safety of XEN496 in a pediatric population.
References
Olusanya BO, Wright SM, Nair MKC, Boo NY, Halpern R, Kuper H, et al. Global burden of childhood epilepsy, intellectual disability, and sensory impairments. Pediatrics. 2020;146(1):e20192623. https://doi.org/10.1542/peds.2019-2623.
Berg AT, Jallon P, Preux PM. The epidemiology of seizure disorders in infancy and childhood: definitions and classifications. Handb Clin Neurol. 2013;111:391–8. https://doi.org/10.1016/B978-0-444-52891-9.00043-9.
Pressler RM, Lagae L. Why we urgently need improved seizure and epilepsy therapies for children and neonates. Neuropharmacology. 2020;170: 107854. https://doi.org/10.1016/j.neuropharm.2019.107854.
Gunthorpe MJ, Large CH, Sankar R. The mechanism of action of retigabine (ezogabine), a first-in-class K+ channel opener for the treatment of epilepsy. Epilepsia. 2012;53(3):412–24. https://doi.org/10.1111/j.1528-1167.2011.03365.x.
Owen RT. Ezogabine: a novel antiepileptic as adjunctive therapy for partial onset seizures. Drugs Today (Barc). 2010;46(11):815–22. https://doi.org/10.1358/dot.2010.46.11.1556435.
Yamada M, Welty TE. Ezogabine: an evaluation of its efficacy and safety as adjunctive therapy for partial-onset seizures in adults. Ann Pharmacother. 2012;46(10):1358–67. https://doi.org/10.1345/aph.1R153.
Anticonvulsant Potiga Discontinued in June 2017. Neurology Live. https://www.neurologylive.com/view/anticonvulsant-potiga-discontinued-june-2017. Accessed 10 Nov 2021.
Nissenkorn A, Kornilov P, Peretz A, Blumkin L, Heimer G, Ben-Zeev B, et al. Personalized treatment with retigabine for pharmacoresistant epilepsy arising from a pathogenic variant in the KCNQ2 selectivity filter. Epileptic Disord. 2021;23(5):695–705. https://doi.org/10.1684/epd.2021.1315.
Walleigh DJ, Legido A, Valencia I. Ring chromosome 20: a pediatric potassium channelopathy responsive to treatment with ezogabine. Pediatr Neurol. 2013;49(5):368–9. https://doi.org/10.1016/j.pediatrneurol.2013.06.005.
Goto A, Ishii A, Shibata M, Ihara Y, Cooper EC, Hirose S. Characteristics of KCNQ2 variants causing either benign neonatal epilepsy or developmental and epileptic encephalopathy. Epilepsia. 2019;60(9):1870–80. https://doi.org/10.1111/epi.16314.
Kim HJ, Yang D, Kim SH, Won D, Kim HD, Lee JS, et al. Clinical characteristics of KCNQ2 encephalopathy. Brain Dev. 2021;43(2):244–50. https://doi.org/10.1016/j.braindev.2020.08.015.
Tandy MD, Cadieux JA, Namdari R. Using modified QbD to develop a novel pediatric formulation of ezogabine. PharmTech. https://www.pharmtech.com/view/using-modified-qbd-to-develop-a-novel-pediatric-formulation-of-ezogabine. Accessed 10 Nov 2021.
Crean CS, Tompson DJ. The effects of ethanol on the pharmacokinetics, pharmacodynamics, safety, and tolerability of ezogabine (retigabine). Clin Ther. 2013;35(1):87–93. https://doi.org/10.1016/j.clinthera.2012.12.003.
Ferron GM, Paul J, Fruncillo R, Richards L, Knebel N, Getsy J, et al. Multiple-dose, linear, dose-proportional pharmacokinetics of retigabine in healthy volunteers. J Clin Pharmacol. 2002;42(2):175–82. https://doi.org/10.1177/00912700222011210.
Tompson DJ, Crean CS. Clinical pharmacokinetics of retigabine/ezogabine. Curr Clin Pharmacol. 2013;8(4):319–31. https://doi.org/10.2174/15748847113089990053.
Patsalos PN, Berry DJ. Pharmacotherapy of the third-generation AEDs: lacosamide, retigabine and eslicarbazepine acetate. Expert Opin Pharmacother. 2012;13(5):699–715. https://doi.org/10.1517/14656566.2012.667803.
Orhan G, Wuttke TV, Nies AT, Schwab M, Lerche H. Retigabine/Ezogabine, a KCNQ/K(V)7 channel opener: pharmacological and clinical data. Expert Opin Pharmacother. 2012;13(12):1807–16. https://doi.org/10.1517/14656566.2012.706278.
Center for Drug Evaluation and Research. Bioanalytical Method Validation Guidance for Industry. US Food and Drug Administration. Published April 29, 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bioanalytical-method-validation-guidance-industry. Accessed 15 Dec 2021.
Bioanalytical method validation. European Medicines Agency. Published March 6, 2011. https://www.ema.europa.eu/en/bioanalytical-method-validation. Accessed 15 Dec 2021.
US Food and Drug Administration Center for Drug Evaluation and Research; Application Number: 022345Orig1s000, Clinical Pharmacology and Biopharmaceutics Review(s). 022345Orig1s000ClinPharmR.pdf. Accessed 2 Nov 2021.
Fallingborg J. Intraluminal pH of the human gastrointestinal tract. Dan Med Bull. 1999;46(3):183–96.
Millichap JJ, Park KL, Tsuchida T, Ben-Zeev B, Carmant L, Flamini R, et al. KCNQ2 encephalopathy: features, mutational hot spots, and ezogabine treatment of 11 patients. Neurol Genet. 2016;2(5): e96. https://doi.org/10.1212/NXG.0000000000000096.
Tompson DJ, Buraglio M, Andrews SM, Wheless JW. Adolescent clinical development of ezogabine/retigabine as adjunctive therapy for partial-onset seizures: pharmacokinetics and tolerability. J Pediatr Pharmacol Ther. 2016;21(5):404–12. https://doi.org/10.5863/1551-6776-21.5.404.
Acknowledgements
The authors wish to thank the volunteers who participated in this study.
Funding
This study was funded by Xenon Pharmaceuticals Inc., Burnaby, BC, Canada. The journal’s Rapid Service Fee was paid by Xenon Pharmaceuticals.
Medical Writing, Editorial, and Other Assistance
The authors thank Ernesto Aycardi, a former employee of Xenon Pharmaceuticals, for technical assistance. Medical writing and editorial services were provided by Ian Rochford and Mary Susan Prescott of Prescott Medical Communications Group, Inc. (Chicago, IL, USA) with financial support from Xenon Pharmaceuticals.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Rostam Namdari, Jay A. Cadieux, and Gregory N. Beatch were responsible for study conception and design, Constanza Luzon oversaw the conduct of the study, Rostam Namdari, Constanza Luzon, Jennifer Leung, and Gregory N. Beatch were responsible for data analysis and interpretation. Rostam Namdari, Constanza Luzon, Jay A. Cadieux, Jennifer Leung, and Gregory N. Beatch contributed to the writing and critical review of the manuscript, and all approved its submission.
Prior Presentation
The study was presented in part as an electronic poster at the 2020 American Academy of Neurology meeting, which was held virtually as of May 18, 2020 (Abstract #4918).
Disclosures
Rostam Namdari, Constanza Luzon, Jay A. Cadieux, Jennifer Leung, and Gregory N. Beatch are full-time employees of Xenon Pharmaceuticals.
Compliance with Ethics Guidelines
The trial was carried out in accordance with the protocol (Pro00039280, approved by Advarra, Inc., an independent institutional review board registered with the US Department of Health & Human Services Office for Human Research Protections and US Food and Drug Administration under IRB#00000971), International Conference on Harmonisation (ICH) Good Clinical Practice (GCP), Declaration of Helsinki, and applicable regulatory requirements. All participants provided written informed consent.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Namdari, R., Luzon, C., Cadieux, J.A. et al. Pharmacokinetics of XEN496, a Novel Pediatric Formulation of Ezogabine, Under Fed and Fasted Conditions: A Phase 1 Trial. Neurol Ther 11, 781–796 (2022). https://doi.org/10.1007/s40120-022-00343-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-022-00343-x