Abstract
Introduction
Narcolepsy patients report lower health-related quality of life (HRQoL) than the general population, as measured by the Short Form-36 Health Survey (SF-36). This analysis evaluated whether changes in SF-36 correlated with physician-rated Clinical Global Impression of Change (CGI-C).
Methods
Data were from 209 of 228 narcolepsy patients participating in an 8-week clinical trial of sodium oxybate. Changes from baseline for SF-36 subscales (Physical Functioning, Role Physical, Bodily Pain, General Health, Vitality, Social Functioning, Role Emotional, and Mental Health) and the summary scores were evaluated for correlation with CGI-C overall and by treatment group. Correlations were calculated using the Pearson product-moment correlation coefficient (r).
Results
Correlations described an inverse relationship in scores, but a direct relationship in improvement; lower CGI-C scores (i.e., better) were associated with higher SF-36 subscale scores (i.e., improved HRQoL). Moderate and significant correlations were observed for Vitality (r = −0.464; P < 0.0001) and Role Physical (r = −0.310; P < 0.0001) subscales, but weak correlations were observed with other subscales including summary scores. Correlations were stronger at higher sodium oxybate doses for most SF-36 subscales.
Conclusion
Some aspects of HRQoL, measured by the SF-36, may be associated with narcolepsy. In particular, Vitality (indicative of energy and tiredness) and Role Physical (impact of physical function on daily roles) moderately correlated with overall change in status observed by clinicians. However, lack of strong correlations between SF-36 and CGI-C indicates differences in patient and clinician perspectives of disease, and suggest a need for broader assessment of the impact of narcolepsy and its treatment on patients.
Funding
Jazz Pharmaceuticals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Narcolepsy, a chronic neurologic disease with no known cure, is associated with an economic burden resulting from higher healthcare resource utilization and reduced productivity relative to those without narcolepsy, and a humanistic burden, since patients with narcolepsy report substantially impaired health-related quality of life (HRQoL) [1,2,3,4]. Studies that have used the 36-item Short Form Health Status survey (SF-36) [5], which is a generic patient-reported measure of HRQoL, have consistently shown lower scores on most SF-36 subscales in narcolepsy patients not only compared with the general population, but also relative to patients with other chronic conditions including obstructive sleep apnea, Parkinson’s disease, and epilepsy [6,7,8,9,10,11,12,13,14,15]. In particular, of the eight SF-36 subscales, the greatest differences between narcolepsy patients and the general population have been for the subscales of Vitality and Role Physical; the eight subscales of the SF-36 are Physical Function (PF; ability to perform physical activity), Role Physical (RP; assesses the impact of physical function on daily roles including work and daily activities), Bodily Pain (BP; presence of pain and its impact on limiting activities), General Health (GH; overall health status), Vitality (VT; energy and tiredness), Social Function (SF; ability to perform social activities), Role Emotional (RE; impact of emotional problems on participation in life activities such as work and other daily activities), and Mental Health (MH; general mood, i.e., anxiety, depression).
Clinical change in disease status is often assessed using a generic global measure, the Clinical Global Impression of Change (CGI-C) [16], which is a frequently used summary scale from the clinician’s perspective. The CGI-C asks the clinician to rate on a scale from 1 (=very much improved to 7 (=very much worse) how much the patient has improved from their perspective since baseline or an earlier assessment. An analysis of SF-36 data from a clinical trial of sodium oxybate (SXB) suggested dose-dependent effects of SXB on the SF-36, with significant improvements for the change from baseline relative to placebo with the 9 g/night dose on the SF-36 domains of VT, GH, PF, and SF, as well as the Physical Component Summary (PCS) [17]. Similarly, analysis of data from this clinical trial showed that the proportion of patients in each SXB treatment group who were rated as Much Improved/Very Much Improved by the investigator was significantly greater than placebo [18]. However, it is not clear if the clinician’s assessment using this scale accurately reflects what the patient may be experiencing with regard to the broader impact of narcolepsy on health status or HRQoL.
Patient-reported outcomes are becoming increasingly important to researchers and regulatory agencies, including the United States Food and Drug Administration [19]. This importance suggests not only that there is a need for evaluating changes in disease status from both the clinical and patient perspectives, but also a need to understand the similarities and differences in how clinicians and patients perceive and interpret the impact of changes in disease. Furthermore, it is useful to identify whether any of the SF-36 domains reflect narcolepsy-specific issues or correlate with the clinician’s perspective of a patient’s improvement or worsening. Therefore, the purpose of this analysis was to provide initial assessment of the degree of correlation between these outcomes using data from the previously mentioned clinical trial of SXB that included both the SF-36 and the CGI-C [17, 18, 20]; SXB is approved for the treatment of both excessive daytime sleepiness and cataplexy in patients with narcolepsy [21].
Methods
Study Design and Population
This post hoc analysis was based on data from an 8-week clinical trial of SXB for which the methodology and results have previously been published [18]. Patients were required to be ≥16 years of age and have a diagnosis of narcolepsy with cataplexy based on clinical history, an overnight polysomnogram, and a multiple sleep latency test. Randomization was to treatment with placebo or SXB 4.5 g, 6 g, or 9 g administered as two equally divided nightly doses. This article does not contain any new studies with human or animal subjects performed by any of the authors.
Outcomes
Both the CGI-C [16] and the SF-36 [5] were included as outcomes in the trial, with the former assessed at Week 8, and the latter as an exploratory efficacy endpoint at baseline, Week 4, and Week 8. The CGI-C was scored by the clinician from 1 = very much improved to 7 = very much worse. Scores on the SF-36 range from 0 to 100, with higher scores indicating better HRQoL. In addition to the eight subscales, the SF-36 derives two summary scales from positively weighting specific domains; the PCS, which is derived from weighting the domains of PF, RP, BP, and GH, and the Mental Component Summary (MCS), derived from weighting the domains of VT, SF, RE, and MH.
Statistical Analysis
This analysis was based on data that were available for 209 of the 228 patients in the intent-to-treat population of the study; 19 patients did not have both CGI-C and SF-36 results for inclusion in the correlation analysis. Missing Week 8 values on the SF-36 were imputed using the last observation (Week 4 values) carried forward. Regardless of treatment group, the change from baseline at Week 8 for all SF-36 subscales and the two summary scores were evaluated for correlation with the CGI-C at Week 8 using the Pearson product-moment coefficient (r), such that H 0: ρ = 0, where absolute values of r ≤ 0.30 represent a weak correlation, 0.30–0.50 are moderate, and ≥0.50 are strong [22]. Scatterplots and regression lines were developed to visualize the relationship between SF-36 and CGI-C. These scatterplot analyses estimated both the 95% confidence interval of the regression, as well as the 95% prediction limits, which indicate the predictive probability that future observations will fall within these boundaries. Additionally, Pearson correlation analysis was performed to determine the relationships between the CGI-C at Week 8 and changes from baseline in SF-36 subscales for each treatment group.
Results
Population Characteristics
Treatment groups were generally well-balanced for demographic characteristics, and the population was predominantly female (65.4%), white (86.0%), with a mean (SD) age of 40.5 (15.3) years. Baseline scores on the SF-36 were also well-balanced among the treatment groups (Table 1).
Correlation Between CGI-C and SF-36 Regardless of Treatment Group
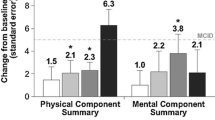
A general relationship was observed between scores on the CGI-C and SF-36, and this relationship was inverse as indicated by the negative correlation coefficients, such that CGI-C scores indicating greater improvement (i.e., lower scores) were associated with improved HRQoL with SF-36 domain scores (i.e., higher SF-36 scores) (Table 2). The correlations were moderate and significant for the subscales of VT (r = −0.464; P < 0.0001) and RP (r = −0.310; P < 0.0001). However, all other correlations were weak including those for PCS and MCS, although the correlations were significant except for the two subscales with the weakest correlations, BP and RE (Table 2).
Scatterplots of the relationship between CGI-C and the two SF-36 subscales that had moderate correlations, VT (Fig. 1a) and RP (Fig. 1b), show low R 2 values for the linear regression of both subscales, 0.215 and 0.096 for VT and RP, respectively, and wide 95% prediction limits, providing further visual representation that the correlations are not strong. For the other SF-36 subscales, the R 2 values of the linear regression ranged from 0.0079 (RE) to 0.0834 (SF) with similarly wide 95% prediction limits (data not shown).
Correlation Between CGI-C and SF-36 by Treatment Group
Correlations appeared to be dose dependent across SXB doses for PF, RP, VT, SF, and the PCS, with the SXB 9 g dose showing strong correlations for these subscales except SF for which the correlation was moderate (Table 3); the strongest correlation was for VT with SXB 9 g. Correlations were generally weak or moderate for the other subscales across all doses, consistent with overall results.
Discussion
Determining how global changes in disease and their impact are assessed and identifying concordance or discordance between clinician and patient perspectives can contribute to understanding what is considered an effective treatment. Correlation analyses such as those presented here help enhance this understanding by validating patient experiences as well as clinician observations.
While this analysis showed that most of the SF-36 subscales only weakly correlated with the CGI-C, it did identify two subscales with moderate correlations suggestive of changes in patient-reported domains that may be perceived by clinicians and likely enable them to determine patient improvement. These subscales, VT and RP, are also the subscales that are most frequently reported to have the lowest value (poorest outcome) among the SF-36 domains in patients with narcolepsy as well as the greatest difference in scores between narcolepsy patients and the general population [6,7,8]. In particular, VT can be interpreted as a measure of fatigue, which is frequently reported by patients with narcolepsy [26, 27]. In contrast, the low correlation coefficients and the lack of statistical significance in two other SF-36 subscales indicate domains where there either was not a good match between patient and clinician perspectives (RE) or did not adequately reflect disease-specific issues of patients with narcolepsy (BP). The correlation coefficients for PF, GH, SF, and MH as well as the PCS and MCS also suggest that despite showing statistical significance, these subscales may not be adequate as indicators of change in narcolepsy. Based on these results, it may be prudent to suggest that while the SF-36 can provide a general assessment of HRQoL in patients with narcolepsy, the use of other, more appropriate measures should be considered for evaluating global and disease-specific changes.
However, the low to moderate correlations also indicate lack of agreement, at least in part, between how clinicians and patients perceive improvements. This lack of agreement is consistent with the discordance between patient and clinician perspectives of global assessments of disease activity and treatment response that has also been reported in other diseases [23,24,25] suggesting that the variables contributing to a patient’s perception of disease may be different from that of the healthcare provider. Such a lack of agreement, especially with regard to the low correlations observed for mental health in the current analysis, further supports the need for narcolepsy assessment that incorporates a broader range of patient-reported outcomes that more closely align with patient perceptions of treatment effects.
Limitations
A limitation of this study is that it was a post hoc analysis, although data were available from most of the patients in the study. Additionally, the 8-week duration of the study may also be considered a limitation because it may have been too short to capture patient-reported changes in HRQoL; response onset, assessed as clinically meaningful improvements in EDS and cataplexy, were observed in most patients within 2 months, but a longer period was needed to achieve maximum response [28]. Finally, the study could also potentially be criticized for not evaluating factors that may confound the relationship. However, it should be noted that these correlations reflect within-subject assessments, since patient scores from both outcome measures were assessed within the same patient among those who had available data or who met the imputation guidelines.
Conclusion
Only two SF-36 subscales showed moderate correlation with overall change in status observed by clinicians, VT and RP, suggesting that improvement in these domains may be reflected by clinicians’ ratings. However, other SF-36 subscales as well as the summary scores expressed only a weak correlation with clinician ratings, indicating that some aspects of HRQoL measured by the SF-36 may assess symptoms of narcolepsy that are also recognized by physicians. However, there is also discordance between patient and physician perspectives of disease, further suggesting a need for a broader assessment of narcolepsy and treatment effects that emphasizes the patient perspective.
References
Dodel R, Peter H, Walbert T, Spottke A, Noelker C, Berger K, et al. The socioeconomic impact of narcolepsy. Sleep. 2004;27(6):1123–8.
Jennum P, Knudsen S, Kjellberg J. The economic consequences of narcolepsy. J Clin Sleep Med. 2009;5(3):240–5.
Black J, Reaven NL, Funk S, McGaughey K, Ohayon MM, Guilleminault C, et al. The burden of narcolepsy disease (BOND) study: healthcare utilization and cost findings. Sleep Med. 2014;15(5):522–9.
Flores NM, Villa KF, Black J, Chervin RD, Witt EA. The humanistic and economic burden of narcolepsy. J Clin Sleep Med. 2016;12(3):401–7.
Ware Jr JE, Kosinski M, Bjorner JB, Turner-Bowker D, Maruish ME. User’s manual for the SF-36v2™ health survey, 2nd edn. Lincoln: QualityMetric Incorporated; 2007.
Beusterien KM, Rogers AE, Walsleben JA, Emsellem HA, Reblando JA, Wang L, et al. Health-related quality of life effects of modafinil for treatment of narcolepsy. Sleep. 1999;22(6):757–65.
Dodel R, Peter H, Spottke A, Noelker C, Althaus A, Siebert U, et al. Health-related quality of life in patients with narcolepsy. Sleep Med. 2007;8(7–8):733–41.
Campbell AJ, Signal TL, O’Keeffe KM, Bakker JP. Narcolepsy in New Zealand: pathway to diagnosis and effect on quality of life. N Z Med J. 2011;124(1336):51–61.
David A, Constantino F, dos Santos JM, Paiva T. Health-related quality of life in Portuguese patients with narcolepsy. Sleep Med. 2012;13(3):273–7.
Ervik S, Abdelnoor M, Heier MS, Ramberg M, Strand G. Health-related quality of life in narcolepsy. Acta Neurol Scand. 2006;114(3):198–204.
Teixeira VG, Faccenda JF, Douglas NJ. Functional status in patients with narcolepsy. Sleep Med. 2004;5(5):477–83.
Daniels E, King MA, Smith IE, Shneerson JM. Health-related quality of life in narcolepsy. J Sleep Res. 2001;10(1):75–81.
Vignatelli L, D’Alessandro R, Mosconi P, Ferini-Strambi L, Guidolin L, De Vincentiis A, et al. Health-related quality of life in Italian patients with narcolepsy: the SF-36 health survey. Sleep Med. 2004;5(5):467–75.
Vignatelli L, Plazzi G, Peschechera F, Delaj L, D’Alessandro R. A 5-year prospective cohort study on health-related quality of life in patients with narcolepsy. Sleep Med. 2011;12(1):19–23.
Ozaki A, Inoue Y, Nakajima T, Hayashida K, Honda M, Komada Y, et al. Health-related quality of life among drug-naive patients with narcolepsy with cataplexy, narcolepsy without cataplexy, and idiopathic hypersomnia without long sleep time. J Clin Sleep Med. 2008;4(6):572–8.
Guy W. ECDEU assessment manual for psychopharmacology, revised. US Department of Health, Education, and Welfare publication (ADM 76-338). Rockville: National Institute of Mental Health; 1976.
Bogan R, Swick T, Mamdani M, Kovacevic-Ristanovic R, Lai C, Black J, et al. Evaluation of quality-of-life in patients with narcolepsy treated with sodium oxybate: use of the 36-item Short-Form Health Survey in a clinical trial. Neurol Ther. 2016;5(2):203–13.
Xyrem International Study Group. A double-blind, placebo-controlled study demonstrates sodium oxybate is effective for the treatment of excessive daytime sleepiness in narcolepsy. J Clin Sleep Med. 2005;1(4):391–7.
Food and Drug Administration. Guidance for Industry. Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. 2009. http://www.fda.gov/downloads/Drugs/Guidances/UCM193282.pdf. Accessed 8 Sept 2015.
Xyrem International Study Group. Further evidence supporting the use of sodium oxybate for the treatment of cataplexy: a double-blind, placebo-controlled study in 228 patients. Sleep Med. 2005;6(5):415–21.
Xyrem® [sodium oxybate] oral solution US prescribing information. Palo Alto: Jazz Pharmaceuticals, Inc; 2016.
Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale: Lawrence Erlbaum Associates; 1988.
Yalcin I, Viktrup L. Comparison of physician and patient assessments of incontinence severity and improvement. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(11):1291–5.
Tabolli S, Sampogna F, Pagliarello C, Paradisi A, Spagnoli A, Abeni D. Disease severity evaluation among dermatological out-patients: a comparison between the assessments of patients and physicians. J Eur Acad Dermatol Venereol. 2012;26(2):213–8.
Desthieux C, Hermet A, Granger B, Fautrel B, Gossec L. Patient-physician discordance in global assessment in rheumatoid arthritis: a systematic literature review with meta-analysis. Arthritis Care Res (Hoboken). 2016;68(12):1767–73.
Droogleever Fortuyn HA, Fronczek R, Smitshoek M, Overeem S, Lappenschaar M, Kalkman J, et al. Severe fatigue in narcolepsy with cataplexy. J Sleep Res. 2012;21(2):163–9.
U.S. Food and Drug Administration. The voice of the patient: narcolepsy. 2014. http://www.fda.gov/downloads/ForIndustry/UserFees/PrescriptionDrugUserFee/UCM402907.pdf. Accessed 7 July 2014.
Bogan RK, Roth T, Schwartz J, Miloslavsky M. Time to response with sodium oxybate for the treatment of excessive daytime sleepiness (EDS) and cataplexy in patients with narcolepsy. J Clin Sleep Med. 2015;11(4):427–32.
Acknowledgements
This study and publication charges were funded by Jazz Pharmaceuticals. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. Yejia Mao is acknowledged for statistical programming and graphical design. Under the direction of the authors, E. Jay Bienen, PhD, an employee of The Curry Rockefeller Group, LLC (CRG), provided medical writing assistance for this publication. Editorial assistance in formatting, proofreading, copy editing, and fact checking was also provided by CRG. Jazz Pharmaceuticals provided funding to CRG for support in writing and editing this manuscript. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
Dr. Bogan is a shareholder, a Board of Directors member, Chief Medical Officer, and an employee of SleepMed Inc; is a Board of Directors member for First Community Corporation, SC, and National Sleep Foundation; has received consultant fees from Aerial BioPharma, LLC, Jazz Pharmaceuticals, Inc., ApniCure, UCB, and Teva; has received industry-funded research support from Actelion, Boehringer Ingelheim, GSK, Jazz Pharmaceuticals, Inc., Vanda, Merck, Novo Nordisk, Pfizer, Schwarz, Sepracor, XenoPort, Eisai, Ventus, Philips, ResMed, ApniCure, Sensory Medical, J&J, Apnex, and Fisher Paykel; and has been a speakers’ bureau member for Teva, Jazz Pharmaceuticals, Inc., UCB, Vanda, Merck, and XenoPort. Dr Black is a part-time employee of Jazz Pharmaceuticals and shareholder of Jazz Pharmaceuticals plc. Dr. Swick is an employee of Neurology and Sleep Medicine Consultants; has received consultancy fees and/or honoraria from Jazz Pharmaceuticals, Vanda Pharmaceuticals, XenoPort Pharmaceuticals, UCB Pharma, Merck Pharma, Aerial BioPharma, LLC; has received research funding from Jazz Pharmaceuticals, Aerial BioPharma, LLC, GSK Pharmaceuticals, Otsuka Pharmaceuticals, Teva Pharmaceuticals, Vanda Pharmaceuticals; and is a speakers’ bureau member for Jazz Pharmaceuticals, XenoPort Pharmaceuticals, and UCB Pharma. Dr. Mamelak has received consultancy fees from Jazz Pharmaceuticals. Dr Kovacevic-Ristanovic has received honoraria as a speakers’ bureau member for Jazz Pharmaceuticals, Inc. Ms Villa is an employee of Jazz Pharmaceuticals who, in the course of this employment, has received stock options exercisable for, and other stock awards of, ordinary shares of Jazz Pharmaceuticals plc. Ms Mori is an employee of Jazz Pharmaceuticals who, in the course of this employment, has received stock options exercisable for, and other stock awards of, ordinary shares of Jazz Pharmaceuticals plc. Dr Montplaisir has received consultancy fees from Jazz Pharmaceuticals, Inc., Merck, UCB, and Valeant Pharmaceuticals; and has received research funding from GSK and Merck.
Compliance with Ethics Guidelines
This article does not contain any new studies with human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/DED8F0603144A186.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Bogan, R.K., Black, J., Swick, T. et al. Correlation of Changes in Patient-Reported Quality of Life With Physician-Rated Global Impression of Change in Patients With Narcolepsy Participating in a Clinical Trial of Sodium Oxybate: A Post Hoc Analysis. Neurol Ther 6, 237–245 (2017). https://doi.org/10.1007/s40120-017-0076-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-017-0076-6