Abstract
Current guidelines exclusively recommend vitamin-K-antagonists (VKA) as anticoagulation for patients after mechanical aortic valve replacement due to the increased postoperative risk of valve thrombosis and thrombo-embolism. Strict and regular assessments are mandatory during VKA therapy to ensure a potent anticoagulatory effect within the desired range. From the patients’ perspective, VKA are associated with relevant interactions and side effects reducing the quality of life and contributing to a high number of patients not achieving the optimal therapeutic target. Direct oral anticoagulants (DOAC) have replaced VKA therapy in the past for several indications, e.g., atrial fibrillation. However, it is still unclear if DOACs could replace VKA therapy in patients after mechanical aortic valve replacement. While the PROACT-Xa study did not show a sufficient anticoagulatory effect of apixaban plus aspirin compared to VKA therapy in patients after mechanical aortic valve replacement, the direct thrombin inhibitor dabigatran and the oral factor Xa inhibitors apixaban and rivaroxaban showed promising results in comparable patient cohorts in smaller studies and case reports. Factor Xa inhibitors were able to prevent thrombosis and thrombo-embolic events in patients after mechanical aortic valve replacement. Therefore, factor Xa inhibitors or factor XI inhibitors could provide a potent alternative to VKA for patients after a mechanical aortic valve replacement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Life-long oral anticoagulation is mandatory after mechanical aortic valve replacement. |
Vitamin-K-Antagonist are the only approved oral anticoagulation after mechanical heart valve replacement. |
Dabigatran and Apixaban did not prevent thrombo-embolism in patients with a mechanical aortic valve. |
Rivaroxaban might still be an option after mechanical aortic valve replacement. |
Introduction
The artificial surface of a mechanical heart valve and the sewing ring can trigger a thrombus formation through an interplay of clotting processes including protein absorption, the adhesion of platelets, thrombin generation, and complement activation, finally leading to a coagulation cascade and activation of thrombin [1,2,3]. Activated thrombin, stimulated by factor Xa, is a potent platelet activator, which triggers fibrin polymerization, finally forming platelet aggregates [4, 5]. In addition to hemodynamic factors with regional turbulence and varying shear stress, the anatomic position of the prosthetic heart valve underlines the importance of hemodynamic factors as heart valve thrombosis has been reported 20 times more often in the slower-flow right-sided cardiac chamber or occurred two to three times more frequently in the mitral valve position than in the aortic valve [6, 7].
Following current AHA/ACC (American Heart Association/American College of Cardiology) and ESC/EACTS (European Society of Cardiology/European Association of Cardio-Thoracic Surgery) guidelines, vitamin K antagonists are the only approved anticoagulatory medication for patients with mechanical heart valves (VKA) [8, 9]. VKA therapy can effectively prevent thrombosis and thrombo-embolic events after a mechanical heart valve implantation but needs strict monitoring to maintain the narrow therapeutic window as the international normalized ration (INR) needs to be measured frequently to monitor the anticoagulatory effects of VKA treatment [10]. Patients after a mechanical aortic valve replacement without additional risk factors should maintain an INR range of 2–3 [11]. The effectiveness of a long-term VKA therapy documented that patients’ INR values vary significantly outside the recommended range due to non-compliance or other side conditions resulting either in a higher risk for either thrombo-embolic or bleeding events [12, 13]. Therefore, evidence has been gathered that the variability of the INR values is a strong predictor of morbidity and mortality after a mechanical valve replacement [14]. The multiple pharmaco-dynamic and –kinetic interactions of VKA with other drugs and food negatively impacts patients’ quality of life [15].
Direct oral anticoagulants (DOAC) including the factor II inhibitor dabigatran and the factor Xa inhibitors apixaban, edoxaban, and rivaroxaban have been approved for the prevention of stroke in patients with non-valvular atrial fibrillation (AF) showing at least the same effectiveness as VKA [16,17,18]. Until now, DOACs have not been approved for patients after a mechanical aortic valve replacement but could provide a reasonable alternative to VKA, consequently leading to a higher acceptance of the more durable mechanical aortic heart valve [8].
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Factor II inhibitor
Dabigatran directly inhibits thrombin (factor II) and is approved for the treatment of non-valvular AF, deep vein thrombosis, and thrombo-embolism and in patients with acute coronary syndrome [16, 19, 20].
Following the confirmation, that dabigatran is able to prevent thrombus formation in mechanical heart valves and positive findings in an animal model using a mechanical aortic bi-leaflet valve conduit a clinical study was initiated [21].
The clinical setting—RE-ALIGN study (randomized phase II study to evaluate the safety and pharmacokinetics of oral dabigatran-etexilate in patients after heart valve replacement)—compared warfarin in patients with mechanical heart valves with an anticoagulation with dabigatran. The primary aim of RE-ALIGN was to evaluate the dosing regimen for dabigatran in patients with a mechanical heart valve irrespective of valve position (aortic, mitral, or both) [22]. The study had to be stopped early after an interim analysis due to an elevated number of adverse events in the dabigatran group with thrombo-embolism and bleeding. Hence, RE-ALIGN could not detect any benefits of an anticoagulation with dabigatran compared to dabigatran.
This result might have been related to several aspects including the excretion of dabigatran leading to a wide range of anticoagulatory effects [23]. Taking into account the reversible inhibition of factor II by dabigatran, VKAs interact with several factors of the coagulation cascade such as factors II, VII, IX, X and proteins C and S in a noncompetitive manner and may therefore be more potent than dabigatran for anticoagulation in patients with mechanical heart valves. Furthermore, the possibility that the thrombin generation induced by the surface of the mechanical heart valve or the sewing ring overwhelms the inhibitory effects of dabigatran has to be considered as another potential reason [24]. Furthermore, the time frame in which a new anticoagulation regimen might be tested has to be considered since the vast majority of thrombo-embolic events occur within 90 days after surgery [25]. As also discussed in the editorial of the article, the basis on which the aimed dabigatran dosage through target plasma levels of 50 ng/ml or higher was examined could be problematic by itself: The desired dabigatran level was translated from the RELY-trial (randomized evaluation of long-term anticoagulation therapy) for the prevention of strokes in patients with atrial fibrillation and dabigatran anticoagulation. Hence, the different indication in patients with mechanical heart valves and its thrombogenic surface, differences in the blood flow and shear stress, as well as other patients’ characteristics may lead to an alternate thrombus formation and cause of thrombo-embolism compared to the patient collective in the RE-LY study [25]. Another potential source of error in the early anticoagulation with dabigatran in patients with a mechanical heart valve could be the altered bioavailability of the drug in the early postoperative phase affecting its plasma levels because of gut dysfunctions and malabsorption [25]. However, anticoagulation with VKAs face similar problems and require a good anticoagulation control due to drug–drug interactions and fluid shifts with more possible over- or under-dosing and INR lapses [26].
Factor Xa Inhibitor
Apixaban and rivaroxaban act as direct factor Xa inhibitors on free and clot bound factor Xa in prothrombinase complexes [27, 28]. The factor Xa inhibitors have been approved for several indications, e.g., for the treatment of non-valvular AF and deep vein thrombosis.
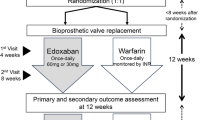
The possible effectiveness of rivaroxaban as an anticoagulant for mechanical heart valves was first examined in an in vitro study and animal models using a bi-leaflet mechanical valve conduit, which bypassed the ligated descending aorta, showing a significantly lower thrombus formation and platelet deposition without increasing the risk of severe events [29, 30]. In a case series, rivaroxaban was investigated in patients after an isolated mechanical mitral valve replacement and within 90 days of the follow-up, none of the patients showed severe adverse events such as thrombus formation, thrombo-embolic or bleeding events [31]. Subsequently, the authors initiated an open-label pilot study, the “RIWA study – rivaroxaban versus warfarin in patients with mechanical heart valves”, to systematically compare rivaroxaban with INR-adjusted warfarin in patients with a mechanical heart valve to prevent them from thrombo-embolic events and any type of stroke. Patients were eligible for enrollment if mechanical heart valve replacement, aortic or mitral, was conducted at least 3 months previously. With enrollment, and after 90 days of follow-up (23 patients in the rivaroxaban group and 21 in the warfarin group), patients underwent a transesophageal echocardiography and computed tomography of the head. The primary composite endpoint with stroke, transient ischemic attack, silent brain infarct, and systemic embolization occurred in one patient (transient ischemic attack) under DOAC with rivaroxaban and three patients with VKA (ischemic stroke n = 2, silent brain infarct n = 1), reaching no statistically significant difference. No case of disabling stroke was observed. One patient in the VKA group died due to a myocardial infarction, no case of major bleeding or non-major bleeding was reported. The assessed echocardiography parameters showed no significant differences with regard to pressure gradients and peak velocity when compared for the distinct prostheses and groups. In this study, rivaroxaban was comparable to VKA concerning thromboembolic and bleeding events in patients with mechanical heart valves [32]. Hence, the authors conclude that rivaroxaban might be a safe alternative to VKA with respect to thromboembolic and bleeding events in patients with mechanical heart valves [33]. Moreover, another study group published their results for rivaroxaban in ten patients with a low-risk profile and mechanical aortic heart valves. Within the follow-up for 6 months, no case of thrombo-embolic, valve thrombosis, bleeding, or death occurred [34].
Apixaban was similarly tested in an animal model with a mechanical heart valve, as the mean thrombus weight was lowest in the intravenous apixaban group. In contrast to the warfarin group, no adverse events for bleeding were observed, and the authors concluded that apixaban might be a promising alternative to VKA for the prevention of thrombus formation in patients with a mechanical heart valve [35].
Subsequently, the PROACT Xa study—a randomized, multicenter, open-label, clinical trial to evaluate the efficacy and safety of apixaban versus warfarin in patients with a mechanical On-X aortic heart valve—investigating apixaban and warfarin in patients after the implantation of an On-X heart valve was initiated [36]. Within this study, patients with a mechanical On-X aortic valve were randomized at least 3 months after surgery at 64 sites in the USA. The rationale for the On-X mechanical aortic valve was a propagated lower INR as investigated in earlier studies. Patients in the PROACT-Xa study were randomized 1:1 either to receive apixaban (5 mg bid) or warfarin (VKA) with a targeted INR of 2.0–3.0. The aim of the study was to demonstrate a non-inferiority of apixaban oral anticoagulation compared to warfarin for the combined primary endpoint including valve thrombosis and valve related thromboembolism. The primary safety endpoint of the study was defined as major bleeding events.
Between May 2020 and September 2022, 863 patients of the initially planned 990 patients were randomized as the study was stopped prematurely due to a higher rate of thromboembolic events in the apixaban group. In conclusion, the PROACT-Xa study was not able to prove a non-inferiority for apixaban in patients with an On-X mechanical aortic valve. Aortic valve thrombosis occurred in three patients in the apixaban group as none was documented in the VKA cohort. Furthermore, valve-related thromboembolisms occurred more often in the apixaban group with 17 documented cases versus six events in the VKA group. Major bleeding events were recorded in 17 cases in the apixaban group and 21 cases in the VKA group [37]. Regarding the primary endpoint, the PROACT-Xa study failed to demonstrate a non-inferiority for apixaban compared to VKA in the prevention of valve thrombosis and thromboembolism in patients with an On-X mechanical aortic valve as the study was stopped prematurely regarding a higher number of thromboembolic events in the DOAC group. The difference in bleeding events showed no statistical significance [37].
The PROACT-Xa only randomized patients with an On-X-mechanical aortic valve replacement at least 3 months prior, who primarily received VKA after surgery. The investigators of the PROACT-Xa study addressed concerns raised after the failed RE-ALIGN study, which yielded at the hypercoagulatory state in the first 3 months after surgery and mechanical valve replacement [7]. Furthermore, the investigators only included patients with a mechanical valve in aortic position and used the On-X valve, which is meant to show favorable hemodynamic parameters and flow characteristics and can be anticoagulated less strict (INR 1.5–2.0 instead of 2.0–3.0) compared to other mechanical aortic valves [37]. The higher rate of thromboembolic events in the apixaban group cannot be assigned to a certain cause. In addition, all participants received aspirin orally in addition to the oral anticoagulation. Details of included studies are displayed in Table 1.
Comment
DOACs are contraindicated in patients with a mechanical heart valve in the current guidelines of the American College of Cardiology/American Heart Association (ACC/AHA) as well as in the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) [8, 9]. This statement is based on the outcomes of the RE-ALIGN study (dabigatran versus warfarin in patients with mechanical heart valves) [23]. Following the outcomes of the Re-ALIGN study, no further examinations with DOACs in patients with a mechanical aortic valve replacement were conducted. Because of the unfavorable side effects of VKA and to avoid a life-long anticoagulation with VKA, patients often decide against a mechanical valve while preferring a biological prosthesis. The decision for a biological prosthesis is often associated with a premature valve deterioration and the risk of re-operation. The guideline recommendations for an oral anticoagulation with VKA in patients after a mechanical aortic valve replacement is based on historical studies [10, 38].
With DOACs, new promising drugs might be available for these patients with the chance for higher patient comfort and safety due to fewer drug–drug and drug–food interactions, no need for INR measurements and fewer fluctuations of an effective drug level. However, the benefit of this additional convenience needs to be assessed according to the associated risk difference as the possible opportunity to find a new anticoagulation other than VKA for patients with mechanical heart valve should not be abandoned [39]. Pharmacodynamic effects may also explain the negative findings of the RE-ALIGN phase II dose validation study, which had to be stopped early because of a higher incidence of thrombo-embolism and bleeding in the dabigatran group [23]. Taking into account the reversible inhibition of factor II by dabigatran, VKAs interact with several factors of the coagulation cascade such as factors II, VII, IX, X, and proteins C and S in a noncompetitive manner and may therefore be more potent than dabigatran for anticoagulation in patients with mechanical heart valves. The possibility that the thrombin generation induced by the surface of the mechanical heart valve or the sewing ring overwhelms the inhibitory effects of dabigatran has to be considered as another potential reason [24].
Of note, the design of the RE-ALIGN study including the target plasma level needs to be critically discussed: the desired dabigatran level was translated from the RELY-trial (randomized evaluation of long-term anticoagulation therapy) for prevention from stroke in patients with atrial fibrillation and dabigatran anticoagulation. Hence, the different indication in patients with mechanical heart valves and its thrombogenic surface may be associated with higher thrombus formation. Another potential explanation of the results could be the altered bioavailability of the drug in the early postoperative phase affecting its plasma levels because of gut dysfunctions and malabsorption [25].
The possible opportunity to find a new anticoagulation other than VKA for patients with mechanical heart valve should not be abandoned because of the negative findings from only one trial and factor Xa inhibitors such as apixaban and rivaroxaban could be promising. One reason why the usage of factor Xa inhibitors might be more promising than dabigatran is the different approach in the coagulation cascade taking into consideration that activated factor Xa triggers the generation of thrombin by factor 1.000 [40]. Thereby, this upstream inhibition of the coagulation cascade by rivaroxaban or apixaban might be more potent in preventing thrombo-embolic events in patients with mechanical heart valves [41].
In the effort of finding an alternative anticoagulation, the study design of PROACT-Xa was modified and differed significantly from the RE-ALIGN study. With apixaban, a DOAC with a favorable pharmacodynamics and pharmacokinetic was chosen [36]. With the inclusion of patients at least 3 months after the mechanical aortic valve replacement, the critical postoperative phase was bypassed. Nevertheless, patients needed to be switched from VKA to apixaban, which includes a critical phase with the interruption of the anticoagulation and the risk valve thrombosis formation and thromboembolic events. The On-X mechanical aortic valve is suggested to have a lower thrombogenicity and can be anticoagulated with a lower INR, but within the PROACT-Xa study the investigators used the initial INR (2.0–3.0) plus aspirin 81 mg daily [37, 42]. Furthermore, the basis of the power analysis of the primary endpoint was based on preliminary studies for the On-X mechanical valve, which alongside with the objective performance criterion of the Food and Drug Administration bases on heterogenic data [36, 42].
Due to the lack of comparable cardiovascular studies, the design and the determination of the non-inferiority margin is not trivial. Therefore, the PROACT-Xa study is exemplary and may serve partially as an example for further studies to identify an alternative oral anticoagulation for patients after a mechanical aortic valve replacement.
The use of the factor Xa inhibitor rivaroxaban could be promising. Animal studies and case series with patients using a mechanical aortic valve and rivaroxaban anticoagulation showed promising outcomes for the prevention of mechanical heart valve thrombosis [30,31,32]. Taking these issues and the pitfalls from the RE-ALIGN trial into account, several important issues to possibly avoid severe event rates in patients with mechanical heart valves and DOAC can be addressed. In detail, the authors discuss key points with regard to hemodynamics and thrombogenicity. Specifically, a mechanical valve with low thrombogenicity in aortic position should be used, and only patients with preserved ejection fraction, a low bleeding risk, and no state of hypercoagulability should be considered for DOAC evaluation [7, 39].
Conclusions
A life-long anticoagulation with VKAs negatively affects the quality of a patient’s life because of the necessity of permanent control of INR ranges and several drug–drug and drug–food interactions. VKAs are the only approved anticoagulation for patients with a mechanical heart valve. The outcome of “The proof of concept-trial – The RIWA study” by Duraes et al. showed promising data for rivaroxaban in patients with mechanical heart valves, also including mechanical mitral valves and patients with AF – hence, patients at a higher risk of thrombo-embolic events. Currently, it is not possible to make a final judgement about factor Xa inhibitors and their impact on the prevention of thrombo-embolic events in patients with mechanical heart valves. Therefore, precise prospective and randomized clinical studies are needed to evaluate the effectiveness of the factor Xa inhibitor rivaroxaban.
References
Dangas GD, Weitz JI, Giustino G, Makkar R, Mehran R. Prosthetic heart valve thrombosis. J Am Coll Cardiol. 2016;68(24):2670–89.
Wiegner R, Chakraborty S, Huber-Lang M. Complement-coagulation crosstalk on cellular and artificial surfaces. Immunobiology. 2016;221(10):1073–9.
Jaffer IH, Stafford AR, Fredenburgh JC, Whitlock RP, Chan NC, Weitz JI. Dabigatran is less effective than warfarin at attenuating mechanical heart valve-induced thrombin generation. J Am Heart Assoc. 2015;4(8): e002322.
Stavrou E, Schmaier AH. Factor XII: what does it contribute to our understanding of the physiology and pathophysiology of hemostasis & thrombosis. Thromb Res. 2010;125(3):210–5.
Mackman N, Tilley RE, Key NS. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler Thromb Vasc Biol. 2007;27(8):1687–93.
Roudaut R, Serri K, Lafitte S. Thrombosis of prosthetic heart valves: diagnosis and therapeutic considerations. Heart. 2007;93(1):137–42.
Aimo A, Giugliano RP, De Caterina R. Non-vitamin K antagonist oral anticoagulants for mechanical heart valves: is the door still open? Circulation. 2018;138(13):1356–65.
Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. 2021;143(5):e35–71.
Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2021. https://doi.org/10.1093/ejcts/ezac209.
Cannegieter SC, Rosendaal FR, Briet E. Thromboembolic and bleeding complications in patients with mechanical heart valve prostheses. Circulation. 1994;89(2):635–41.
Seiler C. Management and follow up of prosthetic heart valves. Heart. 2004;90(7):818–24.
Orensky IA, Holdford DA. Predictors of noncompliance with warfarin therapy in an outpatient anticoagulation clinic. Pharmacotherapy. 2005;25(12):1801–8.
Kimmel SE, Chen Z, Price M, Parker CS, Metlay JP, Christie JD, et al. The influence of patient adherence on anticoagulation control with warfarin: results from the international normalized ratio adherence and genetics (IN-RANGE) study. Arch Intern Med. 2007;167(3):229–35.
Butchart EG, Payne N, Li HH, Buchan K, Mandana K, Grunkemeier GL. Better anticoagulation control improves survival after valve replacement. J Thorac Cardiovasc Surg. 2002;123(4):715–23.
Almeida Gde Q, Noblat Lde A, Passos LC, do Nascimento HF. Quality of life analysis of patients in chronic use of oral anticoagulant: an observational study. Health Qual Life Outcomes. 2011;9:91.
Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–51.
Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–91.
Proietti M, Romanazzi I, Romiti GF, Farcomeni A, Lip GYH. Real-world use of apixaban for stroke prevention in atrial fibrillation: a systematic review and meta-analysis. Stroke. 2018;49(1):98–106.
Eikelboom JE, Weitz JI. Dabigatran etexilate for prevention of venous thromboembolism. Thromb Haemost. 2009;101(1):2–4.
Eriksson BI, Dahl OE, Buller HR, Hettiarachchi R, Rosencher N, Bravo ML, et al. A new oral direct thrombin inhibitor, dabigatran etexilate, compared with enoxaparin for prevention of thromboembolic events following total hip or knee replacement: the BISTRO II randomized trial. J Thromb Haemost. 2005;3(1):103–11.
McKellar SH, Abel S, Camp CL, Suri RM, Ereth MH, Schaff HV. Effectiveness of dabigatran etexilate for thromboprophylaxis of mechanical heart valves. J Thorac Cardiovasc Surg. 2011;141(6):1410–6.
Van de Werf F, Brueckmann M, Connolly SJ, Friedman J, Granger CB, Hartter S, et al. A comparison of dabigatran etexilate with warfarin in patients with mechanical heart valves: THE Randomized, phase II study to evaluate the safety and pharmacokinetics of oral dabigatran etexilate in patients after heart valve replacement (RE-ALIGN). Am Heart J. 2012;163(6):931-7.e1.
Eikelboom JW, Connolly SJ, Brueckmann M, Granger CB, Kappetein AP, Mack MJ, et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369(13):1206–14.
Sharma S, Singh S. Dabigatran in patients with mechanical heart valves. N Engl J Med. 2014;370(4):381–2.
Hylek EM. Dabigatran and mechanical heart valves–not as easy as we hoped. N Engl J Med. 2013;369(13):1264–6.
Eikelboom JW, Brueckmann M, van de Werf F. Dabigatran versus warfarin in patients with mechanical heart valves: reply. J Thromb Haemost. 2014;12(3):426.
Mueck W, Stampfuss J, Kubitza D, Becka M. Clinical pharmacokinetic and pharmacodynamic profile of rivaroxaban. Clin Pharmacokinet. 2014;53(1):1–16.
Byon W, Garonzik S, Boyd RA, Frost CE. Apixaban: a clinical pharmacokinetic and pharmacodynamic review. Clin Pharmacokinet. 2019;58(10):1265–79.
Kaeberich A, Reindl I, Raaz U, Maegdefessel L, Vogt A, Linde T, et al. Comparison of unfractionated heparin, low-molecular-weight heparin, low-dose and high-dose rivaroxaban in preventing thrombus formation on mechanical heart valves: results of an in vitro study. J Thromb Thrombolysis. 2011;32(4):417–25.
Greiten LE, McKellar SH, Rysavy J, Schaff HV. Effectiveness of rivaroxaban for thromboprophylaxis of prosthetic heart valves in a porcine heterotopic valve model. Eur J Cardiothorac Surg. 2014;45(5):914–9.
Duraes AR, Bitar YSL, Lima MLG, Santos CC, Schonhofen IS, Filho JAL, et al. Usefulness and safety of rivaroxaban in patients following isolated mitral valve replacement with a mechanical prosthesis. Am J Cardiol. 2018;122(6):1047–50.
Duraes AR, de Souza Lima Bitar Y, Filho JAL, Schonhofen IS, Camara EJN, Roever L, et al. Rivaroxaban versus warfarin in patients with mechanical heart valve: rationale and design of the RIWA study. Drugs RD. 2018;18(4):303–8.
Duraes AR, de Souza Lima Bitar Y, Schonhofen IS, Travassos KSO, Pereira LV, Filho JAL, et al. Rivaroxaban versus warfarin in patients with mechanical heart valves open-label proof-of-concept trial-the RIWA study. Am J Cardiovasc Drugs. 2020. https://doi.org/10.1007/s40256-020-00449-3.
Roost E, Weber A, Alberio L, Englberger L, Reineke D, Keller D, et al. Rivaroxaban in patients with mechanical heart valves: a pilot study. Thromb Res. 2020;186:1–6.
Lester PA, Coleman DM, Diaz JA, Jackson TO, Hawley AE, Mathues AR, et al. Apixaban versus warfarin for mechanical heart valve thromboprophylaxis in a swine aortic heterotopic valve model. Arterioscler Thromb Vasc Biol. 2017;37(5):942–8.
Jawitz OK, Wang TY, Lopes RD, Chavez A, Boyer B, Kim H, et al. Rationale and design of PROACT Xa: a randomized, multicenter, open-label, clinical trial to evaluate the efficacy and safety of apixaban versus warfarin in patients with a mechanical On-X aortic heart valve. Am Heart J. 2020;227:91–9.
Wang T, Svensson L, Wen J, Vekstein A, Gerdisch M, Rao V, et al. Apixaban or warfarin in patients with an On-X mechanical aortic valve. NEJM Evid. 2023;2(7):2300067.
Mok CK, Boey J, Wang R, Chan TK, Cheung KL, Lee PK, et al. Warfarin versus dipyridamole-aspirin and pentoxifylline-aspirin for the prevention of prosthetic heart valve thromboembolism: a prospective randomized clinical trial. Circulation. 1985;72(5):1059–63.
Gerfer S, Grandoch M, Wahlers T, Kuhn E. Factor Xa Inhibitors for patients after mechanical heart valve replacement? Thorac Cardiovasc Surg. 2023. https://doi.org/10.1055/s-0041-1736242.
Mann KG, Brummel K, Butenas S. What is all that thrombin for? J Thromb Haemost. 2003;1(7):1504–14.
Chan NC, Weitz JI, Eikelboom JW. Anticoagulation for mechanical heart valves: will oral factor Xa inhibitors be effective? Arterioscler Thromb Vasc Biol. 2017;37(5):743–5.
Torella M, Torella D, Chiodini P, Franciulli M, Romano G, De Santo L, et al. LOWERing the INtensity of oral anticoaGulant Therapy in patients with bileaflet mechanical aortic valve replacement: results from the “LOWERING-IT” Trial. Am Heart J. 2010;160(1):171–8.
Funding
No funding or sponsorship was received for this study or publication of this article.
Author information
Authors and Affiliations
Contributions
Concept and design: Stephen Gerfer. Statistical analysis: Stephen Gerfer. Drafting the manuscript: Stephen Gerfer, Thorsten Wahlers, Elmar Kuhn.
Corresponding author
Ethics declarations
Conflict of Interest
We hereby certify that there is no conflict of interest for Stephen Gerfer, Thorsten Wahlers, and Elmar Kuhn.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Gerfer, S., Wahlers, T. & Kuhn, E. Is There an Alternative Oral Anticoagulation to Vitamin-K-Antagonists for Patients with Mechanical Aortic Valve Replacement? – A Literature Review. Cardiol Ther (2024). https://doi.org/10.1007/s40119-024-00371-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40119-024-00371-8