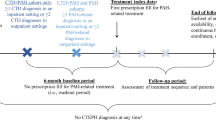
Abstract
Introduction
Data on real-world clinical practice and outcomes of patients with pulmonary arterial hypertension associated with connective tissue disease (CTD-PAH) are scarce. The OPUS/OrPHeUS studies enrolled patients newly initiating macitentan, including those with CTD-PAH. This analysis describes patient characteristics, treatment patterns, outcomes, and safety profiles of patients with CTD-PAH newly initiating macitentan in the US using the OPUS/OrPHeUS combined dataset.
Methods
OPUS was a prospective, US, multicenter, long-term, observational drug registry (April 2014–June 2020). OrPHeUS was a retrospective, US, multicenter medical chart review (October 2013–March 2017). The characteristics, treatment patterns, safety, and outcomes during macitentan treatment of patients with CTD-PAH and its subgroups systemic sclerosis (SSc-PAH), systemic lupus erythematosus (SLE-PAH), and mixed CTD (MCTD-PAH) were descriptively compared to patients with idiopathic/heritable PAH (I/HPAH).
Results
The combined OPUS/OrPHeUS population included 2498 patients with I/HPAH and 1192 patients with CTD-PAH (708 SSc-PAH; 159 SLE-PAH; 124 MCTD-PAH, and 201 other CTD-PAH etiologies). At macitentan initiation for patients with I/HPAH and CTD-PAH, respectively: 61.2 and 69.3% were in World Health Organization functional class (WHO FC) III/IV; median 6-min walk distance was 289 and 279 m; and 58.1 and 65.2% received macitentan as combination therapy. During follow-up, for patients with I/HPAH and CTD-PAH, respectively: median duration of macitentan exposure observed was 14.0 and 15.8 months; 79.0 and 83.0% experienced an adverse event; Kaplan–Meier estimates (95% confidence limits [CL]) of patients free from all-cause hospitalization at 1 year were 60.3% (58.1, 62.4) and 59.3% (56.1, 62.3); and Kaplan–Meier estimates (95% CL) of survival at 1 year were 90.5% (89.1, 91.7) and 90.6% (88.6, 92.3).
Conclusions
Macitentan was used in clinical practice in patients with CTD-PAH and its subgroups, including as combination therapy. The safety and tolerability profile of macitentan in patients with CTD-PAH was comparable to that of patients with I/HPAH.
Trial Registration
OPsumit® Users Registry (OPUS): NCT02126943; Opsumit® Historical Users cohort (OrPHeUS): NCT03197688; www.clinicaltrials.gov
Graphical abstract available for this article.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Patients with connective tissue disease associated with pulmonary arterial hypertension (CTD-PAH) form the largest PAH etiological subgroup after idiopathic/heritable PAH (I/HPAH). |
However, patient characteristics, treatment patterns, safety profile, and outcomes of patients with CTD-PAH (including patients with systemic sclerosis [SSc], systemic lupus erythematosus [SLE], and mixed connective tissue disease [MCTD]-PAH) newly initiating macitentan are not well understood. |
The OPUS/OrPHeUS studies provide detailed insight into real-world clinical practice and management of patients with CTD-PAH and its subgroups, with differing disease severities and comorbidity burdens. |
What was learned from the study? |
Macitentan was used as part of combination therapy in most patients with CTD-PAH; however, contrary to guideline recommendations, a considerable proportion remained on monotherapy therapy at follow-up. |
Outcomes were similar between patients with CTD-PAH and I/HPAH and there were no unexpected safety findings, supporting the safety and tolerability of macitentan, including as a combination therapy in patients with CTD-PAH. |
Digital Features
This article is published with digital features, including a graphical abstract, to facilitate understanding of the article. To view digital features for this article, go to https://doi.org/10.6084/m9.figshare.25196996.
Introduction
Pulmonary arterial hypertension (PAH) is a progressive and debilitating disease characterized by elevated pressure in arteries in the lungs, and can manifest as a complication of connective tissue diseases (CTD) [1,2,3]. The most common causes of CTD associated PAH (CTD-PAH) are systemic sclerosis (SSc), systemic lupus erythematosus (SLE), and mixed connective tissue disease (MCTD), while less common causes are rheumatoid arthritis, dermatomyositis, and Sjögren’s syndrome [1]. The CTD-PAH patient population is the largest PAH etiological subgroup after patients with idiopathic PAH (IPAH) [2, 3], accounting for approximately 15–30% of the PAH population [4,5,6]. Data from REVEAL show that within the CTD-PAH population, 62, 17, and 8% of patients had SSc-PAH, SLE-PAH, and MCTD-PAH, respectively [7], with 1- and 5-year survival estimates of 80 and 44% for patients with CTD-PAH versus 88 and 64% for patients with IPAH [8]. The long-term trials SERAPHIN, GRIPHON, and AMBITION have shown that patients with CTD-PAH respond to PAH-specific therapies, including combination therapy [1, 9, 10]. In the pivotal randomized controlled trial (RCT) SERAPHIN, patients with PAH including those with CTD-PAH (which accounted for 30.5% of the enrolled patients) were treated with the oral endothelin receptor antagonist (ERA) macitentan 10 mg as part of a monotherapy or combination therapy regimen, which resulted in a significant reduction (by 45%; p < 0.001) in the risk of a composite mortality/morbidity events [10]. Results from REPAIR also demonstrate the beneficial effects of macitentan on right ventricular function and structure in patients with PAH, including those with CTD-PAH [11]. Furthermore, a recent meta-analysis that included RCTs evaluated the addition of a PAH-specific therapy to a patient’s current care. In this analysis, patients were stratified according to whether they were receiving no PAH-specific treatment, monotherapy, or dual combination therapy; additional PAH-specific therapy resulted in a 36% reduction in the risk of morbidity/mortality events compared to controls, in patients with CTD-PAH, and in the overall PAH population [12].
In addition to progress in therapeutic development, early detection of PAH through systematic screening and timely treatment of patients with CTD-PAH is advocated due to potential survival benefits, especially in the SSc-PAH subpopulation, which have been shown to have worse survival compared to other CTD-PAH patient subgroups [1, 13, 14]. However, despite these recent advances, there is still a lack of data on real-world clinical practices, on clinical outcomes, and the use of PAH-specific therapies in patients with CTD-PAH, including with macitentan.
Opsumit® (macitentan) is an oral ERA administered once daily for the treatment of PAH to reduce the risks of disease progression and hospitalization for patients with PAH [15, 16]. The prospective Opsumit® Users (OPUS) registry was set up in 2014 to enable further evaluation of the hepatic safety profile of macitentan. OPUS was designed to characterize the safety, clinical characteristics, and outcomes of patients newly treated with macitentan in routine clinical practice [17]. The Opsumit® Historical Users cohort study (OrPHeUS) was initiated to supplement the OPUS registry data. Data from both studies provide a unique insight into contemporary real-world clinical practice for the management of a broad range of patients with PAH, including those with CTD-PAH, and report real-life clinical outcomes of these patients, complementing the findings of RCTs and other registries. This article describes the baseline characteristics, treatment patterns, safety profile, and outcomes in terms of hospitalizations and survival in patients with CTD-PAH newly treated with macitentan in the combined OPUS/OrPHeUS population. The overall CTD-PAH population and the CTD-PAH patient subgroups are descriptively compared with the idiopathic/heritable PAH (I/HPAH) population.
Methods
OPUS and OrPHeUS Study Design
As previously described, OPUS was a prospective, multicenter, long-term, US, observational drug registry (NCT02126943) that ran between April 2014 and June 2020. OrPHeUS was a retrospective, multicenter, US, medical chart review (NCT03197688) that captured individual patient data from October 2013 to March 2017 [17]. Both studies enrolled patients newly initiating macitentan. Data collection in OrPHeUS was designed to be similar to that of OPUS. Patients were excluded from OPUS if they were enrolled in an ongoing clinical trial, and from OrPHeUS if they were enrolled in a clinical trial involving macitentan; patients enrolled in OPUS were not allowed to participate in OrPHeUS. OPUS and OrPHeUS were initiated as a post-marketing requirement to evaluate the potential for hepatic risks with macitentan. The FDA announced in September 2019 that the post-marketing requirement had been fulfilled.
Monitoring and Ethical Approval
OPUS and OrPHeUS were executed in accordance with Good Pharmacoepidemiology Practices [18] and the 2008 Declaration of Helsinki ethical principles. Ethical approval was received from independent ethics committees/institutional review boards of participating centers (Supplementary Material I). The protocols were reviewed by the US FDA with written informed consent obtained from all patients in OPUS, including for publication of anonymized patient data (informed consent was not required in OrPHeUS as an Institutional Review Board [IRB] waiver was obtained). IRB approvals were provided by the Western IRB and Quorum (now Advarra) (OPUS registry; Western IRB approval number 2014‐0816, Quorum Review File number 29120/Advarra Pro00035124) and WCG‐IRB (OrPHeUS study; IRB numbers 2017‐8051 and 2017‐2348).
Observations and Assessments
Data collection (on demographics, baseline characteristics, treatment patterns, safety, hospitalizations, and deaths) during macitentan treatment in OPUS and OrPHeUS has been previously described [17]. Information was collected per routine clinical practice and no assessments were mandated. The observation period was from the date of macitentan initiation (which may not have coincided with the date of diagnosis) to study end, or until the first of death, loss to follow-up, withdrawal of consent, or macitentan discontinuation plus 30 days. For both OPUS and OrPHeUS, follow-up data were defined as at least one observation after macitentan initiation. In OPUS, adverse events (AEs) were recorded. In OrPHeUS, hepatic adverse events (HAEs) and HAEs of special interest (HAESIs) were identified from the clinical data collected; however, due to the retrospective design, no other AE reporting was conducted. For OPUS, the Independent Liver Safety Data Review Board (ILSDRB) reviewed and assessed all reported HAESIs. The ILSDRB additionally reviewed all HAESIs identified in OrPHeUS that met the biochemical criteria of a potential Hy’s law case, using available information from the electronic case report form (Supplementary Material II). As edema and anemia are common (occurring in ≥ 1 in 10 patients) side effects associated with ERA and macitentan use [15, 16], the AEs of special interest (AESIs) of edema and anemia/hemoglobin decrease were also investigated in OPUS.
Statistical and Other Analyses
Statistical and other analyses in OPUS and OrPHeUS have been previously described [17]. All analysis groups described here were derived from the OPUS/OrPHeUS PAH population, and included patients with follow-up data who had PAH entered as the only reason for macitentan prescription; patients with multiple pulmonary hypertension (PH) diagnoses or PAH etiologies were excluded. The reasons for macitentan prescription were investigator-assessed and were used to classify patients with CTD-PAH etiology into the CTD-PAH subgroups SSc-PAH, SLE-PAH, and MCTD-PAH; patients with other forms of CTD-PAH were included in the overall CTD-PAH group only. An I/HPAH patient group is included for reference, as it is the most well-characterized form of PAH. Patient and treatment characteristics were found to be similar in the OPUS and OrPHeUS datasets following heterogeneity analyses, and it was deemed appropriate to combine both into one dataset [17].
For this analysis from the combined OPUS/OrPHeUS population, the CTD-PAH group and CTD-PAH subgroups SSc-PAH, SLE-PAH, MCTD-PAH are presented, and descriptively compared with the I/HPAH group. Analyses of clinical characteristics and treatment patterns were descriptive. All analyses were conducted until the end of the observation period. Event rates (for HAEs, HAESIs, AEs [OPUS only], discontinuation of macitentan, hospitalization, and death) were calculated using time to the first event. Patients were included in each analysis until the first occurrence of the specified event, or until the end of observation period, whichever occurred first. Patients experiencing non-fatal events who did not discontinue macitentan treatment were able to continue in the study (i.e., they were not censored from other event analyses). All Poisson models included log (exposure time) as an offset to account for varying length of patients’ time on treatment. Confidence limits (CL [95%]) for rates per person-year were estimated using an unadjusted Poisson model. Treatment escalation, hospitalizations and deaths are presented using Kaplan–Meier (KM) estimates; curves were truncated at the time point when < 10% of patients in any of the cohorts were at risk, in accordance with Pocock’s stopping rule [19]. Imputations for missing values were applied for incomplete or missing dates; no other data imputations were made (see Supplementary Material III for more details). Fixed imputations were used based on other information (e.g., 1st or 15th of the month if the day of the month was missing). Rules were added to avoid conflicts (e.g., between start and stop dates). If the patient died or was lost to follow-up and no discontinuation date was provided, the discontinuation date was the date of death (for deaths) or date of last information (for loss to follow-up), respectively.
Results
Study Population and Characteristics
As previously described, 155 sites contributed patients to the combined OPUS and OrPHeUS database [17]. Of these, 150 sites enrolled patients with I/HPAH and 121 enrolled patients with CTD-PAH. The combined OPUS/OrPHeUS population consisted of 5654 patients of whom 81.9% (N = 4626) had a diagnosis that included PAH (WHO Group 1) and had follow-up data [17]. Out of these, there were 4459 patients with PAH as the only reason for macitentan prescription: 2498 (56.0%) patients had I/HPAH and 1192 (26.7%) patients had CTD-PAH, including 708 patients with SSc-PAH, 159 with SLE-PAH, and 124 with MCTD-PAH. There were 201 patients with CTD-PAH subgroup etiologies other than SSc-PAH, SLE-PAH, or MCTD-PAH. To note, as the current analysis excluded patients with multiple PH diagnoses or PAH etiologies, the CTD-PAH and I/HPAH groups herein comprise 47 and 100 patients less than the populations reported in McLaughlin et al., 2022 [17].
Patient characteristics at macitentan initiation are shown in Table 1. The majority of patients in all groups were White (range 52.6–79.7%). Compared to patients with I/HPAH, patients with CTD-PAH were more likely to be female (73.1 vs. 86.2%), in World Health Organization functional class (WHO FC) III/IV (61.2 vs. 69.3%) and were less likely to be obese (34.1 vs. 20.6%) and diabetic (27.9 vs. 12.8%). In all groups at baseline, data were frequently missing for 6-min walk distance (6MWD) and brain natriuretic peptide/N-terminal pro-brain natriuretic peptide (BNP/NT-proBNP) risk category. Compared to the other CTD-PAH subgroups, patients with SSc-PAH tended to be older (median age 64 years) and had the longest time from diagnosis to macitentan initiation (7.9 months). They had more impaired functional status (median 6MWD 274 m, 68.9% in WHO FC III/IV) and the highest proportion of patients with hypertension and edema. Patients with SLE-PAH were the youngest (median age 49 years), mostly female (95.6%), comprised a larger proportion of Black or African American (32.7%) and Hispanic-Latino (20.1%) patients, had less severe disease (median 6MWD 344 m, 57.3% in WHO FC III/IV) and the highest proportion of obesity and anemia compared to the other CTD-PAH subgroups. Patients with MCTD-PAH had the shortest time from diagnosis (4.6 months), with proportionally more patients in WHO FC III/IV (82.4%), and higher proportion of patients with diabetes, and renal insufficiency compared to the other CTD-PAH subgroups. At macitentan initiation, approximately half of patients across all groups had been diagnosed less than 6 months before enrollment (incident patients). Other relevant medical history at macitentan initiation is described in Table S1.
Treatment Patterns
Prior to macitentan initiation, 61.4% of patients with I/HPAH and 68.4% of patients with CTD-PAH had received at least one previous PAH-specific therapy (Table 2). At macitentan initiation, compared to patients with I/HPAH a higher proportion of patients with CTD-PAH received macitentan as part of combination therapy (58.1 vs. 65.2%, respectively; Fig. 1), most commonly combining macitentan with a phosphodiesterase 5 inhibitor (PDE5i; Table 2). This trend was similar at 6- and 12-month follow-up. Across the CTD-PAH subgroups, patients with MCTD-PAH had the highest rate of combination therapy at all three timepoints, while patients with SLE-PAH had the lowest (Fig. 1). At 1 and 2 years, patients with CTD-PAH were more likely to escalate from monotherapy to combination therapy, and from double to triple therapy, compared to patients with I/HPAH, with the MCTD-PAH patient group being the most likely to escalate at 2 years, compared to all other groups (Table 2; Fig. 2). Across all CTD-PAH groups, patients were more likely to escalate from monotherapy to double combination therapy, compared to double to triple therapy, with the exception of the SLE-PAH group at 1 year. Patterns of treatment changes over time are also shown in more detail in Figs. S1 and S2.
Treatment regimen at macitentan initiation, 6 months and 12 months after macitentan initiation. Percentages may not add to 100% due to rounding. Double therapy includes macitentan in combination with one other class of PAH therapy; triple therapy includes macitentan in combination with two other classes of PAH therapy. Classes of PAH therapy include PDE5i, prostanoids (oral, inhaled, or intravenous/subcutaneous), sGCs, and investigational drug (≥ 3 PAH therapies only; OrPHeUS only). aIncludes two patients receiving > 3 classes of PAH therapy; bIncludes one patient receiving > 3 classes of PAH therapy; cIncludes three patients receiving > 3 classes of PAH therapy. CTD-PAH PAH associated with connective tissue disease, I/HPAH idiopathic/heritable PAH, MCTD-PAH PAH associated with mixed connective tissue disease, PAH pulmonary arterial hypertension, PDE5i phosphodiesterase-5 inhibitor, sGCs soluble guanylate cyclase stimulator, SLE-PAH PAH associated with systemic lupus erythematosus, SSc-PAH PAH associated with systemic sclerosis
Kaplan–Meier estimates of percentage escalating from A monotherapy to combination therapy and B double to triple therapy. CTD-PAH PAH associated with connective tissue disease, I/HPAH idiopathic/heritable PAH, MCTD-PAH PAH associated with mixed connective tissue disease, PAH pulmonary arterial hypertension, SLE-PAH PAH associated with systemic lupus erythematosus, SSc-PAH PAH associated with systemic sclerosis
Safety and Tolerability
The safety and tolerability profile of macitentan was similar between patients with I/HPAH and CTD-PAH. During follow-up, the median duration of macitentan exposure observed was 14.0 and 15.8 months for the I/HPAH and CTD-PAH groups, respectively. A comparable proportion of patients with I/HPAH and CTD-PAH discontinued macitentan due to an AE (17.4 and 16.3%, respectively), with the lowest discontinuations observed in the SLE-PAH and MCTD-PAH groups (12.6 and 12.9%, respectively). AEs were recorded only in OPUS; 79.0 and 83.0% of patients with I/HPAH and CTD-PAH, respectively, experienced an AE. The most common AEs are described in Table 3. The proportion of patients experiencing an AESI of edema was similar between those with I/HPAH (27.7%) and with CTD-PAH (30.3%); this was slightly higher for patients with SSc-PAH (34.2%) and lower for those with SLE-PAH (23.1%). The proportion of patients experiencing an AESI of anemia/hemoglobin decrease was also similar for patients with I/HPAH and CTD-PAH (9.8 and 13.0%, respectively), with the highest levels for patients with SSc-PAH (15.6%) and lowest for patients with MCTD-PAH (5.0%). In the combined OPUS/OrPHeUS dataset, 7.8 and 7.7% of patients with I/HPAH and CTD-PAH, respectively, experienced an HAE. Similar to the I/HPAH group, the proportion and incidence rates of HAEs, HAESIs, and liver abnormalities were low in the CTD-PAH group (Table 4).
Hospitalization and Survival
At 1 year, KM estimates showed that 60.3% (95% CL 58.1, 62.4) of patients with I/HPAH, and 59.3% (95% CL 56.1, 62.3) of patients with CTD-PAH were free from all-cause hospitalization. Similar KM estimates were observed for the SSc-PAH and SLE-PAH groups, with more hospitalizations in the MCTD-PAH group at 1 year. At 30 months, patients with SLE-PAH had the highest free from all-cause hospitalization KM estimate (95% CL), at 49.5% (39.4, 58.8), and patients with MCTD-PAH had the lowest, at 35.9% (24.8, 47.2) (Fig. 3; Table 5).
Kaplan–Meier estimates of time from macitentan initiation to first all-cause hospitalization. The y-axis has been truncated at 40%. CTD-PAH PAH associated with connective tissue disease, I/HPAH idiopathic/heritable PAH, MCTD-PAH PAH associated with mixed connective tissue disease, PAH pulmonary arterial hypertension, SLE-PAH PAH associated with systemic lupus erythematosus, SSc-PAH PAH associated with systemic sclerosis
The KM estimates (95% CL) of survival at 1 year for patients with I/HPAH and CTD-PAH were 90.5% (89.1, 91.7) and 90.6% (88.6, 92.3), respectively. Similar estimates were observed for patients with SSc-PAH (89.8% [86.9, 92.0]), SLE-PAH (92.5% [86.4, 95.9]), and MCTD-PAH (93.8% [86.7, 97.2]). At 3 years, the KM survival estimates (95% CL) for patients with I/HPAH and CTD-PAH were 75.7% (73.1, 78.2) and 74.3% (70.5, 77.7), respectively, with the highest survival in patients with SLE-PAH (84.7% [74.7, 91.0]) (Fig. 4; Table 5).
Kaplan–Meier estimate of survival from macitentan initiation. The y-axis has been truncated at 70%. CTD-PAH PAH associated with connective tissue disease, I/HPAH idiopathic/heritable PAH, MCTD-PAH PAH associated with mixed connective tissue disease, PAH pulmonary arterial hypertension, SLE-PAH PAH associated with systemic lupus erythematosus, SSc-PAH PAH associated with systemic sclerosis
Discussion
The combined OPUS/OrPHeUS dataset is the largest new-users database for macitentan in the US, and includes a large proportion of patients with CTD-PAH. Data collection spanned the years 2013–2020, providing contemporary, real-world data on the management and outcomes of patients with CTD-PAH, albeit limited to those receiving macitentan. Here, we show that macitentan is used in newly diagnosed and prevalent patients with CTD-PAH, including in those with SSc-PAH, SLE-PAH, and MCTD-PAH, as part of a combination therapy regimen in the majority of patients, and tolerability and safety are comparable to the I/HPAH patient population and consistent with previous safety reports [10, 20].
In OPUS/OrPHeUS, patients with CTD-PAH comprised approximately a quarter of the PAH follow-up cohort. This is similar to previous reports where patients with CTD-PAH represented 24–34% of patients with PAH [5, 7]. In our analyses, the median ages for the overall CTD-PAH group and I/HPAH group were similar (62 vs. 64 years), while the median ages differed between the CTD subgroups: patients with SLE-PAH and MCTD-PAH were younger compared to patients with SSc-PAH (49, 57, and 64 years, respectively). This similarity in age between the overall CTD-PAH and I/HPAH groups in OPUS/OrPHeUS contrasts with earlier registries where patients with CTD-PAH were older than patients with IPAH (mean age 57 for patients with CTD-PAH versus 50 years for IPAH)[7], and older than other patients with PAH (mean age 56 for patients with CTD-PAH versus 51 years for PAH) [12]. This may reflect the changing demographics of patients with PAH, however, as OPUS and OrPHeUS were macitentan drug registries, there was the potential for bias in patient selection and therefore the study population may not be directly comparable with disease registries. In OPUS/OrPHeUS, patients with CTD-PAH were less likely to be obese and diabetic than patients with I/HPAH and the proportion of Black or African-American patients in the SLE-PAH group and the proportion of White patients in the SSc-PAH group were consistent with previous reports [21]. Additionally, the demographics and characteristics of patients with SSc-PAH and SLE-PAH were similar to previous reports from disease registries [22, 23], despite OPUS/OrPHeUS being a drug registry.
The current 2022 European Society of Cardiology/European Respiratory Society (ESC/ERS) PH treatment guidelines, as well as those effective at the time of study conduct, recommend that patients with CTD-PAH be treated according to the same algorithm as patients with IPAH [24,25,26,27,28,29]. Compared to patients with I/HPAH, a higher proportion of patients with CTD-PAH initiated macitentan as part of combination therapy and were more likely to escalate therapy up to 2 years after macitentan initiation. Overall, the MCTD-PAH group had the highest proportion of patients who escalated treatment up to 2 years after macitentan initiation. The MCTD-PAH group had the worst clinical presentation at macitentan initiation among the other subgroups; with a high proportion of patients with a low baseline 6MWD (a quarter of patients with a 6MWD < 152 m), and the highest proportion of patients in WHO FC III/IV at macitentan initiation (82.4%). Additionally, the MCTD-PAH group had a high proportion of patients with hospitalizations (50.8%). These factors could have contributed to the urgency to escalate therapy in these patients.
Exposure to macitentan was similar in patients with I/HPAH and CTD-PAH, with comparable proportions of patients discontinuing treatment due to an AE/HAE. AE profiles in OPUS were similar across the groups, and comparable to observations in previous RCTs assessing PAH therapies [1, 9], including ERAs. The incidence of HAEs, HAESIs, and liver abnormalities were low, and in line with the known safety profile of macitentan [10, 20]. Overall, these data show that the administration of macitentan, including as part of a combination therapy regimen, is well tolerated in newly diagnosed and prevalent patients with CTD-PAH and subgroups with varying characteristics.
We found the overall rates of first hospitalization and survival were similar between patients with I/HPAH and CTD-PAH in OPUS/OrPHeUS, in contrast to the REVEAL registry that enrolled patients from 2006 to 2009, and the recent COMPERA PAH-disease registry [8, 30]. Several recent studies have shown that survival has improved in patients with CTD-PAH in the last 10 years, which may be related to improved screening of patients with CTD for PAH, leading to earlier detection and initiation of initial combination treatment [12, 22, 30,31,32]. In REVEAL, only 39.5% of patients with CTD-PAH were on combination therapy at enrollment [7], whereas 65.2% were on combination therapy in OPUS/OrPHeUS. The differences in outcomes might also be due to the type of registry (disease versus drug), where patients may enroll in a disease registry at different times along their PAH journey; the time from diagnosis to enrollment for patients with CTD-PAH in REVEAL was mean (standard deviation) 27 (30) months [7], whereas in OPUS/OrPHeUS median (Q1, Q3) time from diagnosis to enrollment for patients with CTD-PAH was 6 (1, 35) months. The outcomes of patients with CTD-PAH in our study indicate progress has been made for early diagnosis and improved treatment options, however, outcomes could be further enhanced with increased use of initial combination therapy, as per the 2015 and recent 2022 ESC/ERS guidelines [26,27,28,29].
The OPUS and OrPHeUS studies provide valuable insights into the real-world management of patients with PAH newly treated with macitentan, including in patients with CTD-PAH, although their observational nature is associated with limitations. Firstly, as OPUS and OrPHeUS were drug registries, there is the possibility of bias with respect to the type of patients enrolled, and results may not be directly comparable with disease registries. In both studies, follow-up data were collected according to routine clinical practice without protocol-mandated rules or assessments. As such, data on patient baseline and disease characteristics are incomplete, particularly for WHO FC and 6MWD, which may indicate that for a large proportion of patients accurate risk assessment was not performed as recommended in the 2015 ESC/ERS guidelines, relevant at the time of the study [17, 26, 27]. Many parameters reported here (e.g., CTD-PAH diagnosis and deaths) were investigator-assessed and were not adjudicated. The sample sizes of the CTD-PAH subgroups are small (with corresponding wide 95% confidence limits), and the results from the time-to-event analysis should be interpreted with caution due to the small number of events. It should also be noted that the comparisons between the populations are descriptive. Finally, there are differences in the data between both studies [17], with decreased robustness in the data from OrPHeUS due to the retrospective nature of a medical chart review.
Conclusions
The OPUS/OrPHeUS dataset shows that macitentan was used in a heterogenous CTD-PAH population that included patients with SSc-PAH, SLE-PAH, and MCTD-PAH. The majority of patients received macitentan as part of combination therapy, and escalation from monotherapy to combination therapy was more likely in patients with CTD-PAH compared to I/HPAH. Outcomes were similar, and a considerable proportion of patients received monotherapy, contrary to the 2015 and 2022 ESC/ERS guidelines. Safety and tolerability of macitentan in patients with CTD-PAH were comparable to I/HPAH, including when administered as part of combination therapy.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Gaine S, Chin K, Coghlan G, Channick R, Di Scala L, Galie N, et al. Selexipag for the treatment of connective tissue disease-associated pulmonary arterial hypertension. Eur Respir J. 2017;50:1602493. https://doi.org/10.1183/13993003.02493-2016.
Kato M, Atsumi T. Pulmonary arterial hypertension associated with connective tissue diseases: a review focusing on distinctive clinical aspects. Eur J Clin Invest. 2018. https://doi.org/10.1111/eci.12876.
Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Resp Crit Care. 2006;173:1023–30. https://doi.org/10.1164/rccm.200510-1668OC.
Rhee RL, Gabler NB, Sangani S, Praestgaard A, Merkel PA, Kawut SM. Comparison of treatment response in idiopathic and connective tissue disease-associated pulmonary arterial hypertension. Am J Resp Crit Care. 2015;192:1111–7. https://doi.org/10.1164/rccm.201507-1456OC.
Farber HW, Miller DP, Poms AD, Badesch DB, Frost AE, Muros-Le Rouzic E, et al. Five-year outcomes of patients enrolled in the REVEAL Registry. Chest. 2015;148:1043–54. https://doi.org/10.1378/chest.15-0300.
Humbert M, Sitbon O, Yaïci A, Montani D, O’Callaghan DS, Jais X, et al. Survival in incident and prevalent cohorts of patients with pulmonary arterial hypertension. Eur Respir J. 2010;36:549–55. https://doi.org/10.1183/09031936.00057010.
Chung L, Liu J, Parsons L, Hassoun PM, McGoon M, Badesch DB, et al. Characterization of connective tissue disease-associated pulmonary arterial hypertension from REVEAL: identifying systemic sclerosis as a unique phenotype. Chest. 2010;138:1383–94.
Benza RL, Miller DP, Barst RJ, Badesch DB, Frost AE, McGoon MD. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest. 2012;142:448–56. https://doi.org/10.1378/chest.11-1460.
Coghlan JG, Galie N, Barbera JA, Frost AE, Ghofrani HA, Hoeper MM, et al. Initial combination therapy with ambrisentan and tadalafil in connective tissue disease-associated pulmonary arterial hypertension (CTD-PAH): subgroup analysis from the AMBITION trial. Ann Rheum Dis. 2017;76:1219–27. https://doi.org/10.1136/annrheumdis-2016-210236.
Pulido T, Adzerikho I, Channick R, Delcroix M, Galiè N, Ghofrani A, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med. 2013;369:809–18. https://doi.org/10.1056/NEJMoa1213917.
Vonk Noordegraaf A, Channick R, Cottreel E, Kiely DG, Marcus JT, Martin N, et al. The REPAIR study: effects of macitentan on RV structure and function in pulmonary arterial hypertension. JACC Cardiovasc Imaging. 2022;15:240–53. https://doi.org/10.1016/j.jcmg.2021.07.027.
Khanna D, Zhao C, Saggar R, Mathai SC, Chung L, Coghlan JG, et al. Long-term outcomes in patients with connective tissue disease-associated pulmonary arterial hypertension in the modern treatment era: meta-analyses of randomized, controlled trials and observational registries. Arthritis Rheumatol. 2021;73:837–47. https://doi.org/10.1002/art.41669.
Humbert M, Yaici A, de Groote P, Montani D, Sitbon O, Launay D, et al. Screening for pulmonary arterial hypertension in patients with systemic sclerosis: clinical characteristics at diagnosis and long-term survival. Arthritis Rheumatol. 2011;63:3522–30. https://doi.org/10.1002/art.30541.
Khanna D, Gladue H, Channick R, Chung L, Distler O, Furst DE, et al. Recommendations for screening and detection of connective-tissue disease associated pulmonary arterial hypertension. Arthritis Rheumatol. 2013;65:3194–201. https://doi.org/10.1002/art.38172.
Opsumit® (macitentan). Full prescribing information. Actelion Pharmaceuticals US, Inc. June 2023.
Opsumit® (macitentan). Summary of product characteristics. Janssen Pharmaceuticals Ltd. December 2022.
McLaughlin VV, Channick R, Kim NH, Frantz RP, McConnell J, Melendres-Groves L, et al. Safety of macitentan for the treatment of pulmonary hypertension: real-world experience from the OPsumit® USers Registry (OPUS) and OPsumit® Historical USers cohort (OrPHeUS). Pulm Circ. 2022;12: e12150. https://doi.org/10.1002/pul2.12150.
International Society for Pharmacoepidemiology. Guidelines for good pharmacoepidemiology practices (GPP). Pharmacoepidemiol Drug Saf. 2008;17:200–8. https://doi.org/10.1002/pds.3891.
Pocock SJ, Clayton TC, Altman DG. Survival plots of time-to-event outcomes in clinical trials: good practice and pitfalls. Lancet. 2002;359:1686–9. https://doi.org/10.1016/S0140-6736(02)08594-X.
Dingemanse J, Sidharta PN, Maddrey WC, Rubin LJ, Mickail H. Efficacy, safety and clinical pharmacology of macitentan in comparison to other endothelin receptor antagonists in the treatment of pulmonary arterial hypertension. Expert Opin Drug Saf. 2014;13:391–405. https://doi.org/10.1517/14740338.2014.859674.
Al-Naamani N, Paulus JK, Roberts KE, Pauciulo MW, Lutz K, Nichols WC, Kawut SM. Racial and ethnic differences in pulmonary arterial hypertension. Pulm Circ. 2017;7:793–6. https://doi.org/10.1177/2045893217732213.
Hachulla E, Launay D, Boucly A, Mouthon L, de Groote P, Cottin V, et al. Survival improved in patients aged ≤ 70 years with systemic sclerosis-associated pulmonary arterial hypertension during the period 2006 to 2017 in France. Chest. 2020;157:945–54. https://doi.org/10.1016/j.chest.2019.10.045.
Weatherald J, Boucly A, Launay D, Cottin V, Prévot G, Bourlier D, et al. Haemodynamics and serial risk assessment in systemic sclerosis associated pulmonary arterial hypertension. Eur Respir J. 2018;52:18000678. https://doi.org/10.1183/13993003.00678-2018.
Galiè N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Respir J. 2009;34:1219–63. https://doi.org/10.1183/09031936.00139009.
Galiè N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J. 2009;30:2493–537. https://doi.org/10.1093/eurheartj/ehp297.
Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 2015;46:903–75. https://doi.org/10.1183/13993003.01032-2015.
Galiè N, Humbert M, Vachiéry JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37:67–119. https://doi.org/10.1093/eurheartj/ehv317.
Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43:3618–731. https://doi.org/10.1093/eurheartj/ehac237.
Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2023;61:2200879. https://doi.org/10.1183/13993003.00879-2022.
Hoeper MM, Pausch C, Grünig E, Staehler G, Huscher D, Pittrow D, et al. Temporal trends in pulmonary arterial hypertension: results from the COMPERA registry. Eur Respir J. 2022;59:2102024. https://doi.org/10.1183/13993003.02024-2021.
Pan J, Lei L, Zhao C. Comparison between the efficacy of combination therapy and monotherapy in connective tissue disease associated pulmonary arterial hypertension: a systematic review and meta-analysis. Clin Exp Rheumatol. 2018;36:1095–102.
Hassan HJ, Naranjo M, Ayoub N, Housten T, Hsu S, Balasubramanian A, et al. Improved survival for patients with systemic sclerosis-associated pulmonary arterial hypertension: the Johns Hopkins Registry. Am J Respir Crit Care Med. 2022. https://doi.org/10.1164/rccm.202204-0731OC.
Acknowledgements
We thank the participants of these studies.
Medical Writing, Editorial, and Other Assistance
Medical writing assistance was provided by Anna Chapman-Barnes, PhD, and Sola Lawal, PhD, of eluSCIdate Ltd (Meggen, Switzerland). The graphical abstract support was provided Shaun Hall, Victoria Atess, and Lisa Berridge of Ashfield MedComms, an Inizio Company (Macclesfield, UK). Medical writing support and the development of the graphical abstract were funded by Actelion Pharmaceuticals Ltd, a Janssen Pharmaceutical Company of Johnson and Johnson. Clinical evaluation of hepatic safety was performed by the ILSDRB: Willis Maddrey MD (chairperson), Paul Watkins MD, and James Freston, MD.
Funding
The OPUS Registry and the OrPHeUS medical chart review were sponsored by Actelion Pharmaceuticals Ltd, a Janssen Pharmaceutical Company of Johnson & Johnson, who also funded the journal’s Rapid Service Fee.
Author information
Authors and Affiliations
Contributions
All authors made a substantial contribution to the manuscript. Richard Channick, Kelly M Chin, Vallerie V McLaughlin, Nick H Kim, Stefano Turricchia, and Rose Ong contributed to the conception and design of the study in collaboration with the funders. Richard Channick, Kelly M Chin, Vallerie V McLaughlin, Matthew R Lammi, Roham T Zamanian, and Nick H Kim were involved in the collection of the data. Lada Mitchell was responsible for the statistical analyses. All authors were involved in interpretation of the data, development of the manuscript, and approval of the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Richard Channick served as a scientific committee member for Janssen Pharmaceutical Companies of Johnson & Johnson; served on an advisory board for Janssen Pharmaceutical Companies of Johnson & Johnson and Bayer; received research grants / support from Janssen Pharmaceutical Companies of Johnson & Johnson and United therapeutics; received speaker fees from Janssen Pharmaceutical Companies of Johnson & Johnson, and Bayer; received consultancy fees from Janssen Pharmaceutical Companies of Johnson & Johnson, Bayer and Third pole. Kelly M Chin has served as a scientific committee member for Janssen Pharmaceutical Companies of Johnson & Johnson; has received research grants / support from Janssen Pharmaceutical Companies of Johnson & Johnson, Altavant, Acceleron, United Therapeutics, Pfizer, Merck, Gossamer Bio; has received support for travel to meetings from Janssen Pharmaceutical Companies of Johnson & Johnson; and has received consultancy fees from Janssen Pharmaceutical Companies of Johnson & Johnson, Altavant, Acceleron, United Therapeutics, Gossamer Bio and Merck. Vallerie V McLaughlin served as a scientific committee member from Janssen Pharmaceutical Companies of Johnson & Johnson; received research grants from Aerovate, Altavant, Gossamer Bio, Janssen Pharmaceutical Companies of Johnson & Johnson, Merck, and SoniVie; and received consultant fees from Aerami, Aerovate, Altavant, Bayer, Caremark, Corvista, Gossamer Bio, Janssen Pharmaceutical Companies of Johnson & Johnson, L.L.C, Merck and United Therapeutics. Matthew R Lammi has received research grants / support from Janssen Pharmaceutical Companies of Johnson & Johnson, Gilead, Bayer, United Therapeutics, Altavant and Acceleron. Roham T Zamanian is a patent holder (FK-506 in PAH); has served as a scientific medical advisor for Morphogenic-IX; has received consulting fees from Vivus, Pfizer, and Selten; and has received grants / support from Janssen Pharmaceutical Companies of Johnson & Johnson and United Therapeutics. Stefano Turricchia and Lada Mitchell are employees of Janssen Pharmaceutical Companies of Johnson & Johnson. Rose Ong is an employee of Janssen Pharmaceutical Companies of Johnson & Johnson; has received support for travel to meetings from Janssen Pharmaceutical Companies of Johnson & Johnson; holds stock or stock options with Janssen Pharmaceutical Companies of Johnson & Johnson; and spouse is an employee of Roche. Nick H Kim has served a scientific committee member for Janssen Pharmaceutical Companies of Johnson & Johnson; has received research grants / support from Janssen Pharmaceutical Companies of Johnson & Johnson, Bellerophon, Eiger, Gossamer Bio, Lung Biotechnology, SoniVie, and Altavant; has received consultant fees from Bayer, Merck, United Therapeutics, Pulnovo, and Polarean; and speaker fees from Janssen Pharmaceutical Companies of Johnson & Johnson and Bayer.
Ethical Approval
OPUS and OrPHeUS were executed in accordance with Good Pharmacoepidemiology Practices [18] and the 2008 Declaration of Helsinki ethical principles. Ethical approval was received from independent ethics committees/institutional review boards of participating centers (Supplementary Material I). The protocols were reviewed by the US FDA with written informed consent obtained from all patients in OPUS (informed consent was not required in OrPHeUS as an Institutional Review Board (IRB) waiver was obtained). OPUS’ Informed Consent Form included a confidentiality clause that all records and documents pertaining to the participation of patients in the OPUS registry would be held strictly confidential and their names would not be reported in any publications resulting from the OPUS registry. IRB approvals were provided by the Western IRB and Quorum (now Advarra) (OPUS registry; Western IRB approval number 2014‐0816, Quorum Review File number 29120/Advarra Pro00035124) and WCG‐IRB (OrPHeUS study; IRB numbers 2017‐8051 and 2017‐2348).
Additional information
Prior Presentation: Data were presented in part as an oral presentation at CHEST, October 2019 (McLaughlin et al. CHEST 2019; 156(S4):A874-6), as a poster at the virtual ATS congress, August 2020 (Lammi et al. AJRCCM 2020; 201:A2914) and also as an encore poster presentation at the virtual ACR congress, November 2020 (Lammi et al. Arthritis Rheumatol 2020; 72(suppl 10):A1381).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Channick, R., Chin, K.M., McLaughlin, V.V. et al. Macitentan in Pulmonary Arterial Hypertension Associated with Connective Tissue Disease (CTD-PAH): Real-World Evidence from the Combined OPUS/OrPHeUS Dataset. Cardiol Ther 13, 315–339 (2024). https://doi.org/10.1007/s40119-024-00361-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40119-024-00361-w