Abstract
Introduction
Limited data are available regarding the safety and effectiveness of 4-factor prothrombin complex concentrate (4F-PCC) in patients experiencing major hemorrhage or requiring expeditious surgical intervention, both globally and within Japan.
Methods
We executed a prospective, observational post-marketing surveillance study of patients receiving 4F-PCC for the first time between September 19, 2017 and August 15, 2018 in Japan. Patients were subjected to a comprehensive follow-up for a duration of 4 weeks.
Results
Of 1381 eligible patients, 1271 (92%) received a vitamin K antagonist. Among these, 58% were aged ≥ 75 years, 49% manifested atrial fibrillation, 17% presented with valvular heart disease, and 6% exhibited venous thromboembolism. The median (range) international normalized ratio was 2.67 (0.96–27.11) at baseline and 1.21 (0.45–6.61) at first measurement post-administration of 4F-PCC. The most common reason for 4F-PCC administration was intracranial hemorrhage (59.6%), followed by gastrointestinal bleeding (6.6%). Hemostatic effectiveness was achieved in 85.8% of patients. The incidences of adverse drug reactions (ADRs) and serious ADRs were 3.9% and 2.8%, respectively. Thromboembolic events (TEEs) occurred in 20 (1.5%) patients, with a mean onset of 10 days. The majority of TEEs were classified as nervous system disorders (55%). At the time of TEE, only 13% of patients resumed anticoagulant therapy.
Conclusion
The incidence of TEEs following treatment with 4F-PCC did not surpass those observed in phase 3 trials. No novel safety signals were identified. The safety and effectiveness of 4F-PCC in Japanese real-world practice were in harmony with the observations of prior studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Patients presenting with acute hemorrhage require rapid VKA reversal via restoration of vitamin K-dependent coagulation factors. |
While small phase 3 studies have demonstrated that 4-factor prothrombin complex concentrate (4F-PCC) is well tolerated and effective, there have been no large-scale studies. |
What was learned from the study? |
4F-PCC was well tolerated and effective for major bleeding or needing urgent surgery in a real-world setting in Japan. |
Thromboembolic events (TEE) occurred in 1.5% patients, but only 13% restarted anticoagulant therapy at the time of TEE. |
Introduction
Vitamin K antagonists (VKAs) and direct oral anticoagulants (DOACs) exhibit a favorable benefit–risk profile in managing thrombotic events (TEEs). However, they may precipitate or exacerbate acute major bleeding, leading to significant morbidity and mortality [1,2,3,4,5,6]. Current guidelines endorse the use of DOACs over VKAs for stroke prevention in most patients with atrial fibrillation (AF) [7,8,9]. For patients with AF and a history of valvular replacement, mechanical heart valves, or a diagnosis of mostly rheumatic mitral stenosis, VKAs are indicated whereas DOACs are not. In patients with AF and creatinine clearance (CCr) < 15 mL/min, warfarin remains the sole anticoagulant for ischemic stroke prevention. VKA therapy heightens bleeding risk especially during surgical interventions, events are typically minor, but can be life-threatening [10, 11]. A global review of VKA-treated AF found an annual major hemorrhage rate of 1.3–7.2% and an annual intracranial hemorrhage (ICH) rate of 0.1–2.5% [12]. ICH is a major cause of mortality and morbidity in these patients [13, 14]. Prompt VKA reversal is imperative for acute hemorrhage, requiring discontinuation of antithrombotic agents for bleeding cessation, and blood pressure reduction particularly in ICH. Prothrombin complex concentrates (PCCs), containing three or four vitamin K-dependent coagulation factors (3-factor PCC with significant quantities of factors II, IX, X; 4-factor PCCs (4F-PCC) with the addition of factor VII) are recommended [15, 16]. Administration of both 4F-PCC and vitamin K is recommended for fast and reliable reversal [7, 17]. Three phase 3 studies consistently demonstrate effective hemostasis and a notable international normalized ratio (INR) reduction with 4F-PCC [18,19,20], with a total incidence of thromboembolic events (TEEs) possibly related to treatment of 3.8%. The incidence rate is no means insignificant, warranting continued attention, particularly in the Japanese population.
The dosage of 4F-PCC is determined by the prothrombin time-INR (PT-INR) and patient body weight. Phase 3 studies have not investigated usage and dosage for patients with INR < 2.0. However, analysis of the Japanese J-RHYTHM registry suggests INR 1.6–2.6 as the range where thromboembolism and major bleeding risks are minimal [21,22,23], aligning with the current Japanese guideline [7], while the global guidelines advocate PT-INR 2.0–3.0. To date, no extensive study has assessed the safety and effectiveness of 4F-PCC globally or in Japan including patients with INR < 2.0. Thus, we conducted a prospective, observational post-marketing surveillance study to investigate the safety and effectiveness of 4F-PCC in clinical practice collecting real-world data from all patients treated between September 19, 2017 and August 15, 2018 in Japan. Here, we report the results of that analysis.
Methods
Study Design and Patients
This regulatory post-marketing surveillance study, conducted between September 2017 and August 2018, was a prospective, observational, noncontrolled, multicenter, single-arm study. Patients experiencing major bleeding or requiring urgent surgical intervention, and subsequently administered 4F-PCC at 482 Japanese medical centers, underwent a follow-up period of 4 weeks. Given the limited representation of Japanese patients in the 4F-PCC in phase 3 trial [24], this investigation included an all-encompassing surveillance approach, designed to enroll all patients treated with 4F-PCC post-market launch.
The administration doses of 4F-PCC are indicated according to the Japanese label, i.e., based on patient INR and body weight: 25 IU/kg for INR ≥ 2.0 and < 4.0, 35 IU/kg for INR ≥ 4.0 and ≤ 6, or 50 IU/kg for INR > 6.0. The administration protocols for patients with an INR below 2.0 lacked official recognition. In this study, the dose of 4F-PCC was determined at the discretion of the attending physician.
Determinations regarding initiation of 4F-PCC and the resumption of anticoagulant therapy were left to the discretion of the attending physicians. Subgroup classifications included all patients (safety analysis set) and those who had received VKAs.
The study was conducted according to the Declaration of Helsinki (1964 and its later amendments). The study also complied with adhered to the tenets of the Japanese Good Postmarketing Study Practice (GPSP) regulations. The protocol was reviewed and approved by the Japanese regulatory authority, the Pharmaceuticals and Medical Devices Agency (PMDA), and received ethical committee approvals. Comprehensive, written informed consent was obtained from all participating patients. The medical institutions that agreed to provide anonymized data signed a contract with the sponsor (CSL Behring K.K.).
Information was collected via case report forms (CRFs) for patients who had been administered 4F-PCC. While the original target number of patients was 900 as described below, enrollment continued until the conditions of the regulatory approval were satisfied according to the Japanese authority. The registration period was from September 2017 to March 2021 when the conditions of the approval were removed. All sites that administered 4F-PCC to patients after the drug was approved in Japan participated in the study; if a study site had already administered 4F-PCC at the time of contracting with the sponsor, data were collected retrospectively.
Study Endpoints
This was a descriptive study based on surveillance. The primary objective of the study was the incidence of TEEs. Sample size estimation for the primary objective is described Sect. “Statistical Analyses”. The secondary objectives were the incidence of ADRs other than TEEs and unexpected ADRs and hemostatic effectiveness.
ADRs were encoded by attending physicians utilizing the Medical Dictionary for Regulatory Activities Japan (MedDRA/J) version 22.1, and causality and seriousness of ADRs were evaluated at the physician’s discretion. ADRs encompassed all events for which a causal relationship with 4F-PCC could not be excluded, as determined by the attending physician. TEEs, shock, and anaphylaxis were assessed as priority survey items based on the discernment of the PMDA. The timing of anticoagulant therapy resumption and the nature of the drug employed upon therapy recommencement were also scrutinized. Day 1 was operationally defined as the day of administration of 4F-PCC.
Hemostatic effectiveness was assessed at the physician’s discretion. The timing of PT-INR measurement before and after administration of 4F-PCC adhered to routine clinical practice. The INR values measured immediately before the initial 4F-PCC dose were designated as the baseline INR. The initial measured INR values constituted the post-administration INR values for subsequent analysis.
Data Collection, Management, and Analysis
Physicians used CRFs to collect patient data. Observations were made at prespecified time points: during 4F-PCC administration and 4 weeks after administration or discontinuation of 4F-PCC. ADRs were defined as adverse events (AEs) that the investigator assessed as related to 4F-PCC, in which there was at least a reasonable probability of a causal relationship between 4F-PCC and an AE. A serious AE was defined as any AE that (1) resulted in death, (2) was life-threatening, (3) resulted in persistent or significant disability/incapacity, (4) required inpatient hospitalization or prolongation of existing hospitalization, (5) was medically significant, or (6) was a congenital anomaly/birth defect. The safety dataset was the basis of all demographic, baseline, and safety analyses, which included all patients who were registered, received at least one dose of 4F-PCC, and had available CRFs.
Statistical Analyses
On the basis of the observed TEEs incidence rate of 3.8% (9 of 234 patients) in phase 3 trials [18,19,20], a target sample size of 900 was set to detect an incidence of 5.8%. The primary objective of this study was to statistically guarantee that the incidence of TEEs is ≤ 5.8%, which was calculated by multiplying the incidence 3.8% by 1.5. With an assumed true incidence of TEEs of 3.8%, we run simulations with a sample size of 900 evaluable patients; the resulting power to detect (1 − beta) was 0.867, the maximum allowable incidence of adverse events was 4.4% (40 of 900 patients), and the upper limit of the one-sided 95% CI was 5.7%.
Descriptive statistics (number of patients, mean and standard deviation [SD]) were employed to succinctly summarize continuous effectiveness variables. The change in INR from baseline to administration was analyzed using a paired t test. Statistical analyses were conducted using SAS version 9.4 (SAS Institute, Japan).
Results
Patient Disposition
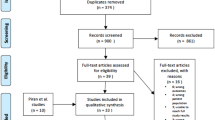
A total of 482 centers actively engaged in this investigative survey. Of 1973 registered patients who were eligible for the study, 1387 provided informed consent, and case report forms were not collected from 6 patients; therefore, data from 1381 patients were analyzed for safety and effectiveness (the overall study populations group). Of these, 1271 (92.0%) patients received a VKA (the VKA treatment group) (Fig. 1). Of 110 patients (8.0%) with records of not receiving a VKA, 7 were treated with DOAC and 103 were not treated with any anticoagulants.
Patient Characteristics at Baseline
Patient baseline characteristics were similar between both groups (Supplementary materials, Table S1). Table 1 shows that 61.4% and 38.6% of patients were male and female, respectively; 57.7% were aged ≥ 75 years and 21.0% were ≥ 85 years; 81.5% had comorbidities. The mean doses of 4F-PCC of both groups were 28 IU/kg. Baseline INR was 2.0 to < 4.0 in the majority of patients (52.6%), and < 1.6 or 1.6 to < 2.0 in substantial numbers (9.7%, 11.1%, respectively). A total of 13 patients (0.9%) received additional administration of 4F-PCC; 10 patients received two doses, 3 patients received three or more doses.
In the VKA treatment group (Supplementary materials, Table S1), AF was the most frequent reason for use of warfarin (48.7%), followed by valvular heart disease (16.5%) and cerebral infarction (8.3%). The proportion of patients with concomitant vitamin K was 72.6%.
Reason for Utilization of 4F-PCC
The most common reasons for the administration of 4F-PCC were ICH at 59.6%, inclusive of 28.8% for non-traumatic ICH and 17.9% for traumatic ICH, followed by gastrointestinal (GI) bleeding at 6.6%, with 14.8% of patients receiving 4F-PCC for urgent surgical interventions and/or invasive procedures. A marginal 1.2% of cases exhibited overextension of PT-INR (Table 2).
Effectiveness
In patients who received a VKA, hemostatic effectiveness was achieved 1091/1271 (85.8%), not achieved in 8 (0.6%), and unknown in 172 (13.5%) patients. Treatment with 4F-PCC significantly reduced INR (p < 0.001); median (range) INRs at baseline and post-administration were 2.67 (0.96–22.11) and 1.21 (0.45–6.61), respectively (Fig. 2). The median (interquartile range) time between 4F-PCC administration and the first INR measurement was 2.34 (0.97–12.0) h. The first measured INRs was ≤ 1.30 in 67.2% of patients and < 1.50 in 84.7% of patients.
Safety
ADRs were reported in 54 (3.9%) patients; and serious ADRs were reported in 38 (2.8%) patients. The most common serious ADRs were cerebral infarction (n = 6, 0.4%), embolic stroke, deep vein thrombosis, and abnormal hepatic function (n = 3 for each, 0.2%).
Assessing priority survey items, shock and anaphylaxis were reported in one and no patients, respectively. A total of 31 TEEs reported in 20 (1.5%) patients (Table 3).
Characteristics of TEEs
The characteristics of the TEEs are presented in Table 4. The majority of TEEs were categorized as nervous system disorders (55%); further details are available in the supplementary materials (Table S2); and 35.5% of events developed ≥ 8 days and 61.2% developed ≥ 4 days post-administrations of 4F-PCC. Only 12.9% of patients were receiving anticoagulant therapy at the time of the TEE event.
Baseline characteristics of patients who experienced TEEs are presented in Table 5. Among patients with TEEs, the mean dose of 4F-PCC used was 33 IU/kg, and 65.0% received concomitant vitamin K. Overall, the characteristics of patients who experienced TEEs were comparable with those of the overall population (Table 1).
Resumption of Anticoagulant Therapy After 4F-PCC administration
In the VKA treatment group, 62.8% of patients resumed anticoagulants. Of these, 25.3% resumed anticoagulant use within 3 days of 4F-PCC administration, 54.0% resumed use within 7 days, and 80.5% resumed use within 14 days. The following anticoagulants were used: warfarin (60.7%), heparin (10.0%), and DOACs. Similarly, anticoagulant use was resumed in 56.9% of patients with ICH; 17.1% resumed use within 3 days, 46.2% resumed use within 7 days, and 74.3% resumed use within 14 days.
Discussion
In this study, information concerning 1381 patients between September 2017 and August 2018 was amassed through a comprehensive surveillance initiative, designed to encompass all individuals subjected to 4F-PCC treatment following its market launch in Japan. Preceding multinational phase 3 trials delineated the experiences of 100 and 80 patients who received 4F-PCC [19, 20]. In a Japanese phase 3 study, a cohort of 11 patients received 4F-PCC [24]. To our knowledge, the present study is the largest one investigating the safety and effectiveness of 4F-PCC both globally and within Japan.
4F-PCC treatment effectively reduced INR to levels below 1.3, demonstrating commendable hemostatic efficacy, when used concomitantly with vitamin K, as observed in the majority of patients. The results are consistent with findings from the two large global phase 3 trials [19, 20]. Treatment with 4F-PCC displayed hemostatic effectiveness in 85.8% of patients, which is similar to the observations in the phase 3 trials [19, 20]. A comparative analysis showed that Japanese patients in the current study exhibited advanced age (74.3 vs. 69.8 years) and a lower body mass index (21.9 vs. 27.7 kg/m2) compared with a phase 3 study [19]. Consequently, patients in the current study appeared to be at higher risk of bleeding than those in the phase 3 studies.
Comparative evidence from global phase 3 trials with fresh frozen plasma demonstrated the non-inferiority and superiority of 4F-PCC over plasma in achieving effective hemostasis and the rapid attainment of the INR target [19, 20]. Consequently, the current Japanese guidelines recommend the administration of 4F-PCC plus vitamin K over plasma [7]. Nevertheless, the observed 3.8% incidence rate of TEEs in global phase 3 trials is by no means insignificant, warranting continued attention, particularly in the Japanese population. Importantly, this study is the first adequately statistically powered assessment of incidence of TEEs. The data revealed that the incidence of TEEs (1.5%) in this study was numerically lower than the global phase 3 trials [19, 20]. This large-scale investigation is provided on the management of patients undergoing 4F-PCC treatment, with particular emphasis on TEEs the real-world setting in Japan.
A parallel observation was noted in cases involving the reversal of other anticoagulant therapies using andexanet alfa, a specific reversal agent for factor Xa (FXa) inhibitor activity [25, 26], a phenomenon partially documented in Japanese patients with acute major bleeding [27]. Similarly, analogous trends were observed in Asian populations treated with idarucizumab, a monoclonal antibody fragment used for dabigatran reversal [28, 29]. The low rates of TEEs associated with anticoagulant therapy reversal may be attributed to differences in ethnicity. Notably, the study populations were predominantly composed of patients with AF who appeared to discontinue anticoagulants at the time of TEE. This may suggest differences in ethnicity in the susceptibility to TEE in patients with AF. The divergence in risk factors for thromboembolism on ethnicity and region is underscored by the use of CHADS2 score in Japan for thromboembolic risk stratification in patients with nonvalvular AF, as opposed to the global use of the CHA2DS2-VASc score [30]. This notion is further supported by a comprehensive meta-analysis of genome-wide association studies for AF revealing the existence of ethnicity specific loci associated with AF [31].
The majority of TEEs were categorized as nervous system disorders (55%) and the remainder were coronary and peripheral vessels. Notably, over 35% of TEEs manifested at least 8 days after 4F-PCC administration. The total number of patients who received multiple doses was 0.9%, but TEEs were not observed in patients with multiple dosing, so we conclude that multiple doses do not affect the occurrence of TEE. It should be noted that 87.1% of TEEs occurred in patients who were not receiving anticoagulant therapy at the time of the event. A post hoc analysis comparing treatment-related TEEs following VKA reversal using 4F-PCC and plasma demonstrated similar percentages of TEEs deemed related to treatment in both groups [32]. Most patients were not receiving anticoagulants at the time of the event, and the failure to reinitiate anticoagulants treatment in patients with pre-existing thromboembolic risks, coupled with their heightened risk due to acute illness, may have contributed to observed rate of thromboembolism. Indeed, in some patients, anticoagulants might be indicated at the time of the event.
Currently, medications including 4F-PCC are sanctioned in Japan, the USA, and the EU for the treatment of patients with life-threatening or uncontrolled bleeding linked to anticoagulants. Nonetheless, disparities in epidemiologic characteristics and stroke management practices exist between Japan and Western countries, notably the higher prevalence of ICH in Japan [33,34,35]. While the majority of patients in this study received 4F-PCC for ICH, global phase 3 trials predominantly featured patients with GI bleeding [18,19,20]. Importantly, patients with ICH did not experience a higher rate of TEEs, defying expectations due to post-ICH immobilization. It has been proposed that VKA-associated ICH could be partially attributed to genetic differences affecting warfarin metabolism or treatment response [36, 37]. However, the elevated incidence of ICH in Asian populations does not seem to be linked to drug metabolism, as evidenced by observations with andexanet alfa for FXa inhibitors in Japanese patients [27]
Based on evidence from Japanese registries, the Japan guideline recommends maintaining an optimal INR range of 1.6–2.6 under warfarin treatment [7]. It is noteworthy that, for this target range, physicians are advised to target a PT-INR of 2.0, rather than lower values such as 1.6 or 1.7 to mitigate the risk of TEE. However, the official indication for usage and dosage of 4F-PCC in patients with INR < 2.0 is currently absent. This study provided an opportunity to assess the safety and effectiveness of 4F-PCC in 215 patients with INR < 2.0. The data revealed that the mean dose of 4F-PCC in patients with INR < 2.0 was 24.5 IU/kg, and the effects appeared comparable to those in patients with INR 2 to 4. A prior pharmacometric simulation model suggested that lower 4F-PCC doses (15–20 IU/kg) for INR < 2.0 might be considered [38]. Retrospective studies from US groups also suggested the usefulness of administering 15–25 IU/kg of 4F-PCC for INR < 2.0 [39, 40]. The current study expands these important earlier observations, reinforcing the rationale for utilizing 4F-PCC in patients with INR < 2.0.
This regulatory-mandated all-case post-marketing surveillance study is an observational study and it therefore has inherent limitations. First, the single-cohort design prevented comparisons with conventional therapy. Second, possible misclassifications of events and hemostatic effectiveness cannot be ruled out because these were assessed by treating physicians and were not confirmed by an independent adjudication committee. Third, the dose of 4F-PCC and the time of INR measurement particularly for post-4F-PCC administration was variable because data originated from daily medical practice. Finally, relative risk estimates or absolute incidence rates cannot be confirmed as completely unbiased.
Conclusion
This prospective, observational study elucidated the favorable tolerability and efficacy of 4F-PCC, with a prompt reduction of INR and substantial hemostatic efficacy in the real-world setting for a large dataset of patients requiring urgent VKA reversal because of an acute major bleed or an urgent surgery/invasive procedure. TEEs manifested in 1.5% patients, yet a mere 13% recommenced anticoagulant therapy during the occurrence of TEE. This underscores the pivotal significance of expeditiously reinstating anticoagulant therapy to avert TEEs when clinically warranted.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Toyoda K, Arihiro S, Todo K, et al. Trends in oral anticoagulant choice for acute stroke patients with nonvalvular atrial fibrillation in Japan: the SAMURAI-NVAF study. Int J Stroke. 2015;10:836–42.
Shehab N, Sperling LS, Kegler SR, Budnitz DS. National estimates of emergency department visits for hemorrhage-related adverse events from clopidogrel plus aspirin and from warfarin. Arch Intern Med. 2010;170:1926–33.
Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51.
Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–91.
Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92.
Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–104.
Ono K, Iwasaki Y, Akao M, et al. JCS/JHRS 2020 Guideline on pharmacotherapy of cardiac arrhythmias. Circ J. 2022;86:1790–924.
Chen A, Stecker E, Warden BA, et al. Direct oral anticoagulant use: a practical guide to common clinical challenges. J Am Heart Assoc. 2020;9:e017559. https://doi.org/10.1161/JAHA.120.017559.
Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498.
Schulman S, Beyth RJ, Kearon C, Levine MN, American College of Chest Physicians. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest. 2008;133:257S–298S.
Fitzmaurice DA, Blann AD, Lip GY. Bleeding risks of antithrombotic therapy. BMJ. 2002;325:828–31.
Lip GY, Andreotti F, Fauchier L, et al. Bleeding risk assessment and management in atrial fibrillation patients: a position document from the European Heart Rhythm Association, endorsed by the European Society of Cardiology Working Group on Thrombosis. Europace. 2011;13:723–46.
Fang MC, Go AS, Chang Y, et al. Death and disability from warfarin-associated intracranial and extracranial hemorrhages. Am J Med. 2007;120:700–5.
Fang MC, Go AS, Chang Y, et al. Thirty-day mortality after ischemic stroke and intracranial hemorrhage in patients with atrial fibrillation on and off anticoagulants. Stroke. 2012;43:1795–9.
Ageno W, Gallus AS, Wittkowsky A, et al. Oral anticoagulant therapy: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e44S–e88S.
Holland L, Warkentin TE, Refaai M, et al. Suboptimal effect of a three-factor prothrombin complex concentrate (Profilnine-SD) in correcting supratherapeutic international normalized ratio due to warfarin overdose. Transfusion. 2009;49:1171–7.
Greenberg SM, Ziai WC, Cordonnier C, et al. 2022 guideline for the management of patients with spontaneous intracerebral hemorrhage: a guideline from the American Heart Association/American Stroke Association. Stroke. 2022;53:e282–361.
Pabinger I, Brenner B, Kalina U, et al. Prothrombin complex concentrate (Beriplex PN) for emergency anticoagulation reversal: a prospective multinational clinical trial. J Thromb Haemost. 2008;6:622–31.
Sarode R, Milling TJ Jr, Refaai MA, et al. Efficacy and safety of a 4-factor prothrombin complex concentrate in patients on vitamin K antagonists presenting with major bleeding: a randomized, plasma-controlled, phase IIIb study. Circulation. 2013;128:1234–43.
Goldstein JN, Refaai MA, Milling TJ Jr, et al. Four-factor prothrombin complex concentrate versus plasma for rapid vitamin K antagonist reversal in patients needing urgent surgical or invasive interventions: a phase 3b, open-label, non-inferiority, randomised trial. Lancet. 2015;385:2077–87.
Inoue H, Okumura K, Atarashi H, et al. Target international normalized ratio values for preventing thromboembolic and hemorrhagic events in Japanese patients with non-valvular atrial fibrillation: results of the J-RHYTHM Registry. Circ J. 2013;77:2264–70.
Yamashita T, Inoue H, Okumura K, et al. Warfarin anticoagulation intensity in Japanese nonvalvular atrial fibrillation patients: a J-RHYTHM Registry analysis. J Cardiol. 2015;65:175–7.
Kodani E, Atarashi H, Inoue H, et al. Target intensity of anticoagulation with warfarin in Japanese patients with valvular atrial fibrillation: subanalysis of the J-RHYTHM Registry. Circ J. 2015;79:325–30.
Kushimoto S, Fukuoka T, Kimura A, et al. Efficacy and safety of a 4-factor prothrombin complex concentrate for rapid vitamin K antagonist reversal in Japanese patients presenting with major bleeding or requiring urgent surgical or invasive procedures: a prospective, open-label, single-arm phase 3b study. Int J Hematol. 2017;106:777–86.
Connolly SJ, Crowther M, Eikelboom JW, et al. Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. N Engl J Med. 2019;380:1326–35.
Milling TJ Jr, Middeldorp S, Xu L, et al. Final study report of andexanet alfa for major bleeding with factor Xa inhibitors. Circulation. 2023;147:1026–38.
Toyoda K, Arakawa S, Ezura M, et al. Andexanet alfa for the reversal of factor Xa inhibitor activity: prespecified subgroup analysis of the ANNEXA-4 study in Japan. J Atheroscler Thromb. 2023. https://doi.org/10.5551/jat.64223.
Yasaka M, Yokota H, Suzuki M, et al. Idarucizumab for emergency reversal of the anticoagulant effects of dabigatran: final results of a Japanese postmarketing surveillance study. Cardiol Ther. 2023. https://doi.org/10.1007/s40119-023-00333-6.
Dai JW, Wang CH, Chu CL, et al. Effectiveness and safety of dabigatran reversal with idarucizumab in the Taiwanese population: a comparison based oneligibility for inclusion in clinical trials. Medicina (Kaunas). 2023;59:881.
Okumura K, Tomita H, Nakai M, et al. Risk factors associated with ischemic stroke in Japanese patients with nonvalvular atrial fibrillation. JAMA Netw Open. 2020;3(4):e202881.
Roselli C, Chaffin MD, Weng L, et al. Multi-ethnic genome-wide association study for atrial fibrillation. Nat Genet. 2018;50(9):1225–33.
Milling TJ Jr, Refaai MA, Sarode R, et al. Safety of a four-factor prothrombin complex concentrate versus plasma for vitamin K antagonist reversal: an integrated analysis of two phase IIIb clinical trials. Acad Emerg Med. 2016;23:466–75.
Shen AY, Yao JF, Brar SS, et al. Racial/ethnic differences in the risk of intracranial hemorrhage among patients with atrial fibrillation. J Am Coll Cardiol. 2007;50:309–15.
Toyoda K. Pharmacotherapy for the secondary prevention of stroke. Drugs. 2009;69:633–47.
van Asch CJ, Luitse MJ, Rinkel GJ, et al. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9:167–76.
Rieder MJ, Reiner AP, Gage BF, et al. Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N Engl J Med. 2005;352:2285–93.
Takahashi H, Wilkinson GR, Nutescu EA, et al. Different contributions of polymorphisms in VKORC1 and CYP2C9 to intra- and inter-population differences in maintenance dose of warfarin in Japanese Caucasians and African-Americans. Pharmacogenet Genom. 2006;16:101–10.
Sarode R, Fukutake K, Yasaka M, et al. Pharmacometric modeling to explore 4F-PCC dosing strategies for VKA reversal in patients with INR below 2. Blood Adv. 2020;4:4208–16.
Zemrak WR, Smith KE, Rolfe SS, et al. Low-dose prothrombin complex concentrate for warfarin-associated intracranial hemorrhage with INR less than 2.0. Neurocrit Care. 2017;27:334–40.
Rivosecchi RM, Durkin J, Okonkwo DO, et al. Safety and efficacy of warfarin reversal with four-factor prothrombin complex concentrate for subtherapeutic INR in intracerebral hemorrhage. Neurocrit Care. 2016;25:359–64.
Acknowledgements
We thank Masashi Nakayama, a former employee of CSL Behring K.K. for conceptualization and the acquisition of data.
Medical Writing Assistance
Under the direction of the authors, Meridian HealthComms provided medical writing assistance in accordance with Good Publication Practice (GPP 2022), funded by CSL Behring K.K.
Funding
This research and the journal’s Rapid Service Fee were funded by CSL Behring K.K. Study design, data collection, analysis, and interpretation, and the decision to submit the article for publication were also supported by CSL Behring K.K.
Author information
Authors and Affiliations
Contributions
Masahiro Yasaka: data interpretation. Michiyasu Suzuki: data interpretation. Shigeki Kushimoto: data interpretation. Ayako Kiyonaga: data analysis, data interpretation. Antoinette Mangione: data interpretation, Yuki Niwa: data interpretation. Naoki Terasaka: data interpretation, drafting the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Ayako Kiyonaga, Antoinette Mangione, Yuki Niwa, Naoki Terasaka are full-time employees of CSL Behring. Masahiro Yasaka has received lecture, advisory, and travel fees from Bristol-Myers Squibb, Nippon Boehringer Ingelheim, Bayer, Daiichi Sankyo, and CSL Behring, as well as scholarship funds or nonrestricted grants from Nippon Boehringer Ingelheim. Michiyasu Suzuki has received lecture fees from Nippon Boehringer Ingelheim and CSL Behring. Shigeki Kushimoto has received lecture fee from CSL Behring.
Ethical Approval
The study was conducted according to the Declaration of Helsinki (1964 and its later amendments). The study was also complied in accordance with Japanese Good Postmarketing Study Practice (GPSP) regulations. The protocol was reviewed and approved by the Japanese regulatory authority, PMDA, and by the ethics committees; written informed consent was obtained from all patients.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Yasaka, M., Suzuki, M., Kushimoto, S. et al. Real-World Safety and Effectiveness of a 4-Factor Prothrombin Complex Concentrate in Japanese Patients Experiencing Major Bleeding: A Post-marketing Surveillance Study. Cardiol Ther 13, 221–232 (2024). https://doi.org/10.1007/s40119-024-00357-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40119-024-00357-6