Abstract
We aimed to systematically analyze the literature on the use of transcatheter aortic valve replacement (TAVR) to treat active aortic valve infective endocarditis (AV-IE). Surgery is declined in one-third of patients with IE who meet indications because of prohibitive surgical risk. TAVR might be an alternative for selected patients with AV-IE as a bridge-to-surgery or stand-alone therapy. PubMed/MEDLINE, Embase, and Cochrane databases were searched (2002–2022) for studies on TAVR use in active AV-IE. Of 450 identified reports, six met inclusion criteria (all men, mean age 71 ± 12 years, median Society of Thoracic Surgeons (STS) score 27, EuroSCORE 56). All patients were prohibitive surgical risk candidates. Five out of six patients had severe, and one patient had moderate aortic regurgitation on presentation. Five out of six patients had prosthetic valve endocarditis after surgical valve replacement 13 years before (median), and one patient had TAVR a year before hospitalization. All patients had cardiogenic shock as the indication for TAVR. Four patients received balloon-expanding, and two patients received self-expanding TAVR after a median of 19 (IQR 9–25) days from diagnosis of IE. No death or myocardial infarction occurred, but one patient had a stroke within the first 30 days. The median event-free time was 9 (IQR 6–14) months including no death, reinfection, relapse IE, or valve-related rehospitalization. Our review suggests that TAVR can be considered as an adjuvant therapy to medical treatment for selected patients in whom surgery is indicated for treatment of acute heart failure due to aortic valve destruction and incompetence caused by infective endocarditis, but who have a prohibitive surgical risk. Nonetheless, a well-designed prospective registry is urgently needed to investigate the outcomes of TAVR for this off-label indication. No evidence exists for using the TAVR to treat infection-related surgical indications such as uncontrolled infection or control of septic embolization.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Selected patients with aortic valve endocarditis who are prohibitive surgical risk candidates and are failing medical treatment might benefit from TAVR to treat acute heart failure due to aortic valve destruction and incompetence caused by infective endocarditis. |
More evidence is needed to further assess whether there is a role for TAVR in patients with active aortic valve endocarditis. |
We outline a proposal for a prospective multicenter registry to provide more robust evidence for the use of TAVR for this off-label indication. |
Introduction
Rates of classical risk factors for infective endocarditis (IE), such as rheumatic heart disease, have been declining in the USA. However, the prevalence of IE continues to rise as a result of the growing population of elderly patients with degenerative heart disease, adults with congenital heart disease, patients on renal replacement therapy, use of IV drugs, and the widespread utilization of intracardiac devices [1,2,3].
To date, antibiotic therapy and surgical intervention continue to be the standard modalities for the treatment of IE without significant changes in recent decades. Despite antibiotics being the cornerstone of therapy, the success rate in left-sided IE is limited with approximately half of patients requiring surgery [4]. Current American College of Cardiology/American Heart Association (ACC/AHA) [5] and the European Society of Cardiology (ESC) [6] guidelines recommend surgical intervention for patients with IE and symptomatic heart failure, uncontrolled infection, or at high risk of septic emboli. Notably, one-third of the patients who meet these surgical indications cannot undergo surgery because of high operative risk [4, 7]. These patients often require a prolonged hospital stay accompanied by excessively high rates of death and in-hospital complications.
Consequently, novel approaches have been explored to address these high-risk patients. Transcatheter aortic valve replacement (TAVR) has recently been investigated for treatment of aortic valve (AV)-IE [5]. A recent TAVR registry report has suggested that TAVR can safely and successfully be performed in patients with “healed” AV-IE and a moderate or severe AS [8]. However, TAVR has traditionally been contraindicated in the setting of an ongoing infection because of the risk of persistent or relapsing IE, and high death rates in patients who acquired IE following the TAVR procedure [9]. Nonetheless, several authors have demonstrated the feasibility of TAVR as rescue therapy for AV-IE in patients who met indications for surgical aortic valve replacement (SAVR) but had prohibitive surgical risk during their index hospitalization [10,11,12,13,14,15].
Thus far, no systematic review has been performed to evaluate the feasibility of TAVR in active AV-IE. The objective of this study was to systematically analyze current evidence on the safety and efficacy of TAVR for the treatment of active AV-IE. We also propose recommendations for a prospective registry to provide more robust evidence for the potential use of TAVR for this off-label indication.
Materials and Methods
The current study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [16]. Articles were identified by searching the following electronic databases: PubMed/MEDLINE, Embase, and Cochrane databases from January 2002 to May 2022. We used the PubMed-related citations tool. Search terms included “endocarditis” OR “infective endocarditis” OR “prosthetic valve endocarditis” AND “transcatheter aortic valve replacement” OR “transcatheter aortic valve intervention” OR “TAVR” OR “TAVI.” Available medical reference textbooks were also examined by using the key terms noted above. No limits were applied for language, and non-English articles were translated if necessary. The search was amplified with a manual evaluation of the reference lists of relevant articles and by experts’ consultation.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
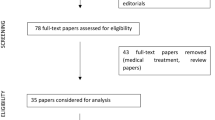
Articles were selected using the following inclusion criteria: (1) reporting on a definite or possible diagnosis of AV-IE based on the modified Duke’s criteria in patients equal to or older than 18 years, (2) TAVR use for the treatment of AV-IE during the index hospitalization. The active AV-IE was defined as the active phase of antibiotic therapy after initial diagnosis of AV-IE. Two reviewers (A.H. and M.B.) performed an eligibility assessment in a standardized manner and independently assessed all relevant reports identified in the search. The quality of the articles was evaluated using the Newcastle–Ottawa Scale, including the third part in case of discordance. No restrictions on the type of study were made. Duplicate articles were excluded, and the most recent or the best-documented data from the same authors were included. The study selection flowchart is displayed in Fig. 1. Of 450 citations obtained after an initial search, seven articles were retrieved following the title and abstract screen. One article was excluded because TAVR was performed for “healed” AV-IE and not during the index hospitalization [8]. A total of six articles were included in the final analysis [10,11,12,13,14,15].
Data extraction was done by two reviewers (A.H. and M.B.) independently using the standardized form to collate details of study design, sample size, patient demographics and baseline features, type of microorganism and antibiotic therapy, hospital course, surgical indication, the timing of the procedure, periprocedural data, follow-up duration, and primary and secondary outcomes. More details are provided in Table 1. Disagreements were resolved by reviewers’ consensus or by contacting experts if an agreement could not be reached.
The primary outcome was defined as 30-day all-cause death. The secondary outcomes included cerebrovascular accident, heart failure rehospitalizations, and “recurrent IE” within the first year following the TAVR procedure. The “recurrent IE” was defined as a “reinfection” if a repeat episode of IE was caused by a microorganism of a different species or same species but different strain as shown by molecular analysis [17, 18]. Patients in whom molecular analysis showed indistinguishable pairs of isolates were defined as having a “relapse” if it occurred within 150 days from the initial episode of IE, or as having a “reinfection” if it occurred more than 150 days from the initial episode of IE according to the prior definition [17].
Data extraction was performed using an Excel spreadsheet, and analyses were performed with a complete data set using the SPSS 28.0 software package for Microsoft Windows.
Results
Table 1 shows patients’ demographics, hospital course, and follow-up data.
Baseline Characteristics, Presenting Features, and Microorganisms
Only six patients (three in the USA and three in Europe) who underwent TAVR for active AV-IE during index hospitalization were identified. All patients were reported between 2012 and 2022. All patients were men with an average age of 71 ± 12 years. Five out of six patients had severe, and one case had moderate aortic regurgitation due to aortic valve destruction and incompetence caused by IE. Five out of six patients had prosthetic valve endocarditis (PVE). Four of those had history of SAVR a median of 13 (IQR 6–28) years before index hospitalization. One patient had undergone TAVR a year before index hospitalization. One patient had a prior history of PVE and a subsequent bacteremia incident 8 months before presentation. Chronic kidney disease and underlying active malignancy were the most common significant comorbidities.
All patients presented with symptomatic heart failure while one patient also developed a stroke due to septic embolization 4 days after the initial presentation. Three patients were initially treated in a peripheral hospital and subsequently transferred to the academic center for further management. All cases were negative for fistula, abscess, and pseudoaneurysm formation except for one patient who had a left ventricular outflow tract aneurysm requiring a deployment of two transcatheter valves to treat aortic insufficiency and for the aneurysm exclusion.
The causative microorganisms were identified in five out of six patients with blood cultures showing growth of Enterococcus faecalis, Diphtheroids, Staphylococcus epidermidis, Staphylococcus aureus, Streptococcus sanguinis, Corynebacterium striatum, and Streptococcus lutetiensis. All patients received an extended course of intravenous broad-spectrum antibiotics with a median duration of 42 (42–70) days, and one case has been started on life-long antibiotic therapy. No precise data could be obtained on the time duration between the first negative blood culture and the timing of the TAVR procedure.
Operative Risk, Surgical Indication, and Timing of the Procedure
The median STS score was 27 (IQR 20–34), and logistic EuroSCORE was 56 (44–68). A multidisciplinary heart team deemed all patients to have a prohibitive surgical risk. All patients developed cardiogenic shock requiring inotropic and pressor support which prompted the authors to consider TAVR as a rescue therapy. Figure 2 shows the number of major clinical events including the development of cardiogenic shock, septic embolization, and end-organ damage at the time of diagnosis of AV-IE and just before TAVR. Of note, multiorgan failure was reversed in all patients following TAVR procedure. The median time from diagnosis to TAVR was 19 (IQR 9–25) days, and the median length of stay was 37 (21–39) days.
Number of major clinical events (cardiogenic shock, septic embolization, acute respiratory failure, and acute renal failure) and a median duration between the time of diagnosis and the time just before TAVR. Of note, all patients have had symptomatic heart failure at the time of diagnosis. Y-axis shows the relative number (%) of major clinical events
Procedural Data and Follow-up Outcome Assessment
All patients underwent TAVR using a transfemoral approach; four patients received a balloon-expanding Edwards SAPIEN-3 valve (Edwards Lifesciences; Irvine, California), and two patients received a self-expanding CoreValve (Medtronic; Minneapolis, Minnesota). Five patients received a single transcatheter valve and one patient needed two Edwards SAPIEN-3 valves to be deployed to exclude a left ventricular outflow tract aneurysm. A cerebral protective device was used in one patient. The authors did not report postprocedural conduction abnormalities requiring permanent pacemaker placement. Postprocedural echocardiography was performed on all patients showing a well-functioning transcatheter valve with no vegetation, or significant valvular or paravalvular regurgitation.
No death or myocardial infarction occurred in these patients within the first 30 days. However, one patient experienced a right common femoral artery occlusion immediately following sheath removal (VARC-2 minor vascular complication), which was successfully treated with self-expanding stent placement. Another patient experienced a stroke 5 days after TAVR with formation of mycotic aneurysms. The patient underwent emergent transcatheter embolization of cerebral aneurysms with a gradual neurological recovery to baseline. The cerebral protective device was not used during this TAVR procedure. The same patient was also found to have a mycotic aneurysm in the femoral artery on the body CT scan which was treated with covered stenting. The median reported survival was 9 (IQR 6–14; min–max 6–18) months. The authors reported no new events during the follow-up period including death, IE-reinfection, IE-residual, or valve-related rehospitalizations.
Discussion
The overall picture of IE is shifting with rising incidence and changing demographics, but mortality remains unacceptably high. While antibiotics are the cornerstone for treatment of AV-IE, there are well accepted indications for urgent SAVR. However, SAVR is not offered to a substantial subset of patients who meet surgical indications because of prohibitive surgical risk. Therefore, we sought to determine whether TAVR might be a reasonable treatment in this high-risk group of patients with active AV-IE.
Patient Selection for Urgent TAVR in Active AV-IE
As shown in our results, there are at least two main subgroups of patients with AV-IE who meet surgical indications but are not offered SAVR. The first group are those with advanced age, poorly controlled comorbidities, prior cardiothoracic surgery, or limited life-expectancy (e.g., patients with cancer). The second subgroup are patients who have an acceptable baseline surgical risk (age and comorbidity profile), but they are declined for SAVR because of too unstable acute condition at the time of surgical evaluation. In both subgroups, TAVR might be an attractive alternative treatment option to SAVR. Yet, the question remains which subset of these high-risk patients would benefit from TAVR, and whether it should be used as a bridge to SAVR or as a stand-alone therapy.
The current review has also demonstrated a signal toward selection bias that deserves attention. Namely, our results showed that all patients who underwent TAVR were men. These results might be biased by limited sample size or even a patient’s preference. Nonetheless, physicians should keep in mind the risk of sex disparities when choosing to proceed with a riskier procedure in men than in women.
TAVR as a Bridge-to-SAVR or Stand-Alone Therapy
Presented findings suggest feasibility of valve-in-valve TAVR in our limited sample of patients with active AV-IE and prior SAVR or TAVR without the need for reoperation during follow-up. Of note is that all of them were deemed to have a prohibitive surgical risk. Their clinical course was complicated by development of cardiogenic shock due to severe valvular dysfunction as the main surgical indication, and they eventually underwent TAVR as a rescue therapy after a median of 19 days after the diagnosis of AV-IE. Importantly, multiorgan failure was reversed entirely after the TAVR in all patients. Thus, it is important to note that if urgent TAVR is considered, it should be done while multiorgan failure is still reversible.
Our results are further supported by Santos-Martínez et al. [8] who showed 1-year mortality of 11% in 56 patients with history of healed AV-IE treated with TAVR which was comparable to propensity-matched patients without history of healed AV-IE treated with TAVR. We should keep in mind that the authors also reported one case of IE-relapse, 18% of sepsis complications, and 43% readmission rate due to heart failure during follow-up. Similar 1-year mortality rates were observed in the Transcatheter Aortic Valve Replacement for Degenerated Transcatheter Aortic Valve (TRANSIT) registry and in the study by Landes et al. in patients with valvular dysfunction following TAVR who underwent re-do TAVR [19, 20]. In contrast, Jawitz et al. [21] reported higher than expected operative mortality for SAVR after early failure of TAVR than in SAVR alone. Importantly, the operative risk rose to 25% when PVE was the main indication for reoperation. The authors also reported higher rates of perioperative complication often requiring concomitant ascending aortic replacement particularly in those with prior CoreValve prostheses [22]. Indeed, studies have shown an added complexity with the extraction of the nitinol cage of the self-expanding valves compared to balloon-expanding valve [23], but also difficulty with TAVR extraction due to neo-endothelizations if implanted more than a year prior [22]. Taken together, current data suggest that we might opt for valve-in-valve TAVR rather than for SAVR in selected cases with degenerated valves.
Of note, we would like to caution the reader to be conservative when directly comparing mortality rates between reported studies for two main reasons. First, all estimates are based on the limited sample size (often 200 patients or fewer) of highly selected individuals with different operative risk. Second, mortality rates are largely affected by the indication for the re-do with PVE carrying the highest mortality rates.
Future Perspectives
Given that the “one size fits all” approach is suboptimal, a prospective registry of patients undergoing TAVR for active AV-IE should be the next step to provide more robust evidence for the questions raised above. Therefore, we propose a set of variables for a future registry to help depict the role of TAVR for this off-label indication (Table 2). Finally, we believe that using a collaborative approach can generate enough data to help modify existing or develop new transcatheter valve platforms and improve the outcome of AV-IE.
Study Limitations
There are several limitations to this study. First, there is a lack of design since the articles are retrospective case reports with limited sample size. As such, they may have been unrepresentative of a broader population of infected stenotic aortic valve patients. Yet, we did not attempt to exclude the articles on the basis of design, size, or follow-up duration. This might have introduced bias in our results, but we wanted to display a widely inclusive review as the first step toward a better cognition of the feasibility or futility of TAVR in patients with active AV-IE. Second, we caution the reader that reports with shorter follow-up and ununiform outcome assessment might have indicated artificially favorable outcomes. Third, a presence of publication bias cannot be excluded because authors would be more likely to report successful outcomes rather than failures, given the high mortality of the underlying condition and the use of TAVR as a bailout option in extreme salvage scenarios. Taken together, we hope that the present study provides a composite view of the existing evidence on the use of TAVR for the management of active AV-IE and sets a foundation for future studies.
Conclusion
The evidence for the use of TAVR in active AV-IE is largely limited. Our review suggests that TAVR can be considered as an adjuvant therapy to medical treatment for selected patients in whom surgery is indicated for the treatment of acute heart failure due to aortic valve destruction and incompetence caused by infective endocarditis, but who have a prohibitive surgical risk. Nonetheless, a well-designed prospective registry is urgently needed to investigate the outcomes of TAVR for this off-label indication. No evidence exists for using the TAVR to treat infection-related surgical indications such as uncontrolled infection or control of septic embolization.
References
Yang X, Chen H, Zhang D, Shen L, An G, Zhao S. Global magnitude and temporal trend of infective endocarditis, 1990–2019: results from the Global Burden of Disease Study. Eur J Prev Cardiol. 2021;29:1277–86.
See I, Gokhale RH, Geller A, et al. National public health burden estimates of endocarditis and skin and soft-tissue infections related to injection drug use: a review. J Infect Dis. 2020;222:S429-s436.
Holland TL, Baddour LM, Bayer AS, Hoen B, Miro JM, Fowler VG. Infective endocarditis. Nat Rev Dis Primers. 2016;2:16059.
Chu VH, Park LP, Athan E, et al. Association between surgical indications, operative risk, and clinical outcome in infective endocarditis: a prospective study from the International Collaboration on Endocarditis. Circulation. 2015;131:131–40.
Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. 2021;143:e35–71.
Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2022;43:561–632.
Mirabel M, Sonneville R, Hajage D, et al. Long-term outcomes and cardiac surgery in critically ill patients with infective endocarditis. Eur Heart J. 2014;35:1195–204.
Santos-Martínez S, Alkhodair A, Nombela-Franco L, et al. Transcatheter aortic valve replacement for residual lesion of the aortic valve following “healed” infective endocarditis. JACC Cardiovasc Interv. 2020;13:1983–96.
Lanz J, Reardon MJ, Pilgrim T, et al. Incidence and outcomes of infective endocarditis after transcatheter or surgical aortic valve replacement. J Am Heart Assoc. 2021;10: e020368.
Shen CP, Munsayac MA, Robinson AA, Stinis CT. Transcatheter aortic valve replacement: a palliative approach to infective endocarditis. BMJ Case Rep. 2022;15e248951.
Albu C, Swaans MJ, ten Berg JM. With the back against the wall: TAVI in a patient with endocarditis. Catheter Cardiovasc Interv. 2013;82:E595–7.
Nguyen C, Cheong AP, Himbert D. Valve-in-valve-in-valve: treating endocarditis of a transcatheter heart valve. Catheter Cardiovasc Interv. 2015;86:E200–4.
Naqvi SY, Salama IG, Narins C, Stuver T. Corynebacterium striatum prosthetic valve endocarditis with severe aortic regurgitation successfully treated with transcatheter aortic valve replacement. BMJ Case Rep. 2018;11:e226881.
Fathi AS, Ali JM, Mann S, Taghavi J, Davies WR, Sudarshan C. Emergency valve-in-valve transcatheter aortic valve implantation for endocarditis degeneration. J Card Surg. 2020;35:713–5.
Brankovic M, Ansari J, Karanam R, Waxman S. Transcatheter aortic valve replacement as a rescue treatment for prosthetic valve endocarditis. JACC Case Rep. 2022;4:1306–10.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339: b2700.
Choi SH, Dagher M, Ruffin F, et al. Risk factors for recurrent Staphylococcus aureus bacteremia. Clin Infect Dis. 2021;72:1891–9.
Chu VH, Sexton DJ, Cabell CH, et al. Repeat infective endocarditis: differentiating relapse from reinfection. Clin Infect Dis. 2005;41:406–9.
Testa L, Agnifili M, Van Mieghem NM, et al. Transcatheter aortic valve replacement for degenerated transcatheter aortic valves: the TRANSIT international project. Circ Cardiovasc Interv. 2021;14:e010440.
Landes U, Webb JG, De Backer O, et al. Repeat transcatheter aortic valve replacement for transcatheter prosthesis dysfunction. J Am Coll Cardiol. 2020;75:1882–93.
Jawitz OK, Gulack BC, Grau-Sepulveda MV, et al. Reoperation after transcatheter aortic valve replacement: an analysis of the Society of Thoracic Surgeons Database. JACC Cardiovasc Interv. 2020;13:1515–25.
Fukuhara S, Brescia AA, Shiomi S, et al. Surgical explantation of transcatheter aortic bioprostheses: results and clinical implications. J Thorac Cardiovasc Surg. 2021;162:539–547.e1.
Mangi AA, Ramchandani M, Reardon M. Surgical removal and replacement of chronically implanted transcatheter aortic prostheses: how I teach it. Ann Thorac Surg. 2018;105:12–4.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Author Contributions
Milos Brankovic (concept and design, data collection, statistical analysis, drafting manuscript), Ashkan Hashemi (data collection, drafting manuscript), Julia Ansari (concept and design, critical appraisal of the manuscript), Abhishek Sharma (concept and design, critical appraisal of the manuscript).
Disclosures
Milos Brankovic, Ashkan Hashemi, Julia Ansari and Abhishek Sharma have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Brankovic, M., Hashemi, A., Ansari, J. et al. Transcatheter Aortic Valve Replacement for Aortic Valve Infective Endocarditis: A Systematic Review and Call for Action. Cardiol Ther 12, 297–306 (2023). https://doi.org/10.1007/s40119-023-00314-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40119-023-00314-9