Abstract
The established benefits of cooling along with development of sophisticated methods to safely and precisely induce, maintain, monitor, and reverse hypothermia have led to the development of targeted temperature management (TTM). Early trials in human subjects showed that hypothermia conferred better neurological outcomes when compared to normothermia among survivors of cardiac arrest, leading to guidelines recommending targeted hypothermia in this patient population. Multiple studies have sought to explore and compare the benefit of hypothermia in various subgroups of patients, such as survivors of out-of-hospital cardiac arrest versus in-hospital cardiac arrest, and survivors of an initial shockable versus non-shockable rhythm. Larger and more recent trials have shown no statistically significant difference in neurological outcomes between patients with targeted hypothermia and targeted normothermia; further, aggressive cooling is associated with a higher incidence of multiple systemic complications. Based on this data, temporal trends have leaned towards using a lenient temperature target in more recent times. Current guidelines recommend selecting and maintaining a constant target temperature between 32 and 36 °C for those patients in whom TTM is used (strong recommendation, moderate-quality evidence), as soon as possible after return of spontaneous circulation is achieved and airway, breathing (including mechanical ventilation), and circulation are stabilized. The comparative benefit of lower (32–34 °C) versus higher (36 °C) temperatures remains unknown, and further research may help elucidate this. Any survivor of cardiac arrest who is comatose (defined as unarousable unresponsiveness to external stimuli) should be considered as a candidate for TTM regardless of the initial presenting rhythm, and the decision to opt for targeted hypothermia versus targeted normothermia should be made on a case-by-case basis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Survivors of cardiac arrest bear a substantial risk of neurological injury via hypoxic ischemic insult and reperfusion damage. |
Any survivor of cardiac arrest who is comatose should be considered as a candidate for targeted temperature management (TTM). |
TTM requires a multidisciplinary team and multiple clinical decisions. |
On the basis of current data, there does not seem to be evidence to pick targeted hypothermia over targeted normothermia. |
The decision to opt for targeted hypothermia versus targeted normothermia should be tailored on the basis of severity of illness. |
Introduction
The earliest approach to using hypothermia for the treatment of comatose patients dates to the early 1900s, when Russian physicians used snow to cool patients with cardiac arrest to revive them [1, 2]. With the introduction and more widespread use of cardiopulmonary bypass in the mid-to-late-twentieth century, surgeons observed that patients who had their head immersed in ice water during bypass had more favorable neurological outcomes after surgery when compared to those who did not undergo a similar cooling process. Further, Williams and Spencer published a case series of patients who had better neurological outcomes with therapeutic hypothermia after intraoperative cardiac arrest [3]. Unfortunately, despite advances in targeted hypothermia and established neuroprotective benefits of hypothermia in porcine and canine subjects, this practice failed to have widespread use until the publication of early clinical trials that demonstrated benefits of hypothermia in patients who had suffered cardiac arrest [4, 5]. The last century saw advances in more sophisticated and controlled methods to induce, monitor, and reverse iatrogenic hypothermia, leading to the development of a regimented series of guideline-directed steps for therapeutic cooling known as targeted temperature management (TTM) (Fig. 1). The current American Heart Association guidelines have a Class I recommendation for TTM to a core body temperature between 32 and 36 °C for at least 24 h for unresponsive patients after out-of-hospital cardiac arrest (OHCA) and in-hospital cardiac-arrest (IHCA) [6]. The European Resuscitation Council also recommends TTM with a target temperature between 32 and 36 °C for at least 24 h for adults who remain comatose or unresponsive after the return of spontaneous circulation (ROSC) for both OHCA and IHCA [7]. However, the recently published TTM2 (Hypothermia versus Normothermia after Out-of-Hospital Cardiac Arrest) trial [8] showed no reduction of mortality at 6 months and showed a higher risk of cardiac arrythmia in the targeted hypothermia group when compared to a normothermia group. Further comparative benefit of lower (32–34 °C) versus higher (36 °C) temperatures remains unclear, and future research may help to elucidate this. In this narrative review we have sought to fully appraise the available literature and identify knowledge gaps. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Cardiac Arrest and the Comatose Patient
Epidemiology of Sudden Cardiac Arrest
Cardiac arrest is the cessation of cardiac mechanical activity, as confirmed by the absence of signs of circulation [9]. If resuscitation attempts are unsuccessful, this situation is referred to as sudden cardiac death. Cardiac arrest may occur from external causes (e.g., drowning, trauma, asphyxia, electrocution, severe hypothermia, and drug overdose) or any medical causes that result in a metabolic, hypoxic, or thromboembolic insult that is large enough to cause cessation of cardiac mechanical activity (severe hypoxia, hypovolemia, severe acidosis, hyper- or hypokalemia, pulmonary embolus, coronary artery thrombosis, or tension pneumothorax) [10].
Per the American Heart Association’s “Heart and Stroke Statistics – 2022 Update” [11], there are more than 356,000 OHCA annually in the USA, which translates to an estimated 1000 OHCAs a day. Survival to hospital discharge after emergency medical services (EMS)-treated cardiac arrest is very low at about 10%. Even among patients who survive, incidence of post-arrest symptoms is high, with the most reported sequelae being severe cognitive deficits (13%), anxiety and depression (15%), post-traumatic stress symptoms (28%), and severe fatigue (52%). These long-term neurocognitive and functional limitations are associated with increased dependency, reduced quality of life, and shortened life span. Incidence of EMS-assessed OHCA for 2015 in adults was 140.7 individuals per 100,000 population. Incidence of EMS-treated OHCA in adults for 2015 was lower at 73.0 individuals per 100,000 population. Conversely, the incidence of adult IHCA was a mean of 10.16 [standard deviation (SD), 26.08] per 1000 hospital admissions and 1.99 (SD, 1.57) per 1000 inpatient days [9, 12,13,14,15,16]. Mean survival for patients who suffer IHCA is understandably higher when compared to those who suffer OHCA but is still low at around 25% [17].
Shockable and Non-shockable Rhythms
Differentiating shockable and non-shockable rhythms is essential to understanding the clinical trials and the evolution of guidelines for TTM in post-arrest patients. Aberrant electrical activity that causes cessation of meaningful cardiac function is mostly due to non-perfusing ventricular tachycardia (VT), ventricular fibrillation (VF), or pulseless electrical activity (PEA), and complete cessation of all cardiac electrical activity (asystole). Of these rhythms, VF and VT should be immediately treated with a defibrillation shock [6, 18] that renders cardiomyocytes refractory to the propagating aberrant wave fronts, allowing the heart to re-establish normal sinus rhythm and thus terminating the potentially fatal ventricular arrhythmia. As the problem in PEA is due to lack of response of myocardial tissue to electrical impulses, this rhythm does not respond to defibrillation. Further, inappropriately shocking PEA may cause loss of organized electrical activity and precipitate asystole. Victims of sudden cardiac arrest who present with asystole as the initial rhythm have an extremely poor prognosis (10% survive to admission and 0–2% survive to hospital discharge) [19]. Given the complete cessation of cardiac electrical activity, delivering defibrillation shocks is meaningless in asystole as there is no aberrant electrical activity left to terminate.
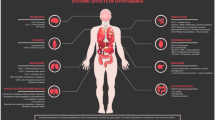
Overarching critical care goals of any comatose survivor of cardiac arrest necessitating TTM should be aimed at minimizing the risk of neurological injury. Any survivor of cardiac arrest who is comatose should be considered as a candidate for TTM regardless of the initial presenting rhythm [20]. All comatose patients should have post-arrest care per advanced cardiac life support guidelines, including intubation for airway protection and mechanical ventilation [6]. Hemodynamic support should be provided with intravenous fluids, inotropes, vasopressors, and mechanical circulatory support when appropriate. Prompt revascularization should be considered if coronary ischemia is suspected as the cause of cardiac arrest. Other disease-specific medical management such as monitoring of blood glucose, administration of antibiotics for suspected infection, etc. should be started as appropriate (Figs. 2, 3).
Elements of high-quality targeted temperature management delivery for cardiac arrest in the cardiac intensive care unit. CO cardiac output, DIC disseminated intravascular coagulopathy, EEG electroencephalogram, IV intravenous, NMB neuromuscular blockade, SVI stroke volume index, SVR systemic vascular resistance, TTM targeted temperature management
Neuroprotective Effects of Hypothermia
Survivors of cardiac arrest bear a substantial risk of neurological injury via hypoxic ischemic insult and reperfusion damage leading to inflammation, excitotoxicity, apoptosis, free radical production, blood–brain barrier disruption, cerebral edema, intraparenchymal hemorrhage and thermomoulding, among others [21]. These adverse effects can be directly mitigated by hypothermia. Neural oxygen consumption and glucose metabolism reduces by 5% for each degree Celsius drop in temperature [22, 23]. This preserves high phosphate substrates such as adenosine triphosphate and maintains a stable pH in the brain and is thought to be the main mechanism through which hypothermia confers neuroprotection. Furthermore, hypothermia may also positively influence neurogenesis, gliogenesis, and angiogenesis after injury [22] via mechanisms that remain incompletely understood, thereby decreasing the extent of damage and promoting recovery from the initial ischemic insult. Hypothermia also preserves the integrity of the blood–brain barrier and modulates aquaporins to preserve the brain’s water balance, reducing the incidence and extent of edema. Thus, hypothermia influences multiple aspects of brain physiology at molecular and cellular levels that collectively confer neuroprotection in the acute, subacute, and chronic stages of ischemia.
Cardioprotective Effects of Hypothermia
There is evidence to suggest that reducing body temperature by as little as 2–5 °C confers some cardioprotective benefits via similar mechanisms that confer neuroprotective benefits. Hypothermia is able to attenuate inflammatory cell infiltration, apoptosis, and expression of pro-inflammatory cytokines in cardiomyocytes that are already injured from an ischemic insult [24, 25]. Mild hypothermia has also been shown to reduce free radical production, production of excitatory amino acids and neutrophil chemotaxis in early ischemia, and favorably modulate apoptotic, necrotic, and inflammatory pathways that lead to cell death in the post-reperfusion period. All these effects have been shown to have cardioprotective benefits at a molecular and cellular level in the short-term as well as long-term after an initial ischemic insult [24,25,26].
Early Clinical Trials Showing Benefit of Hypothermia in Humans
The benefit of induced hypothermia was first demonstrated in two landmark randomized controlled trials that were published in 2002 [4, 5] (Table 1). The Hypothermia After Cardiac Arrest (HACA) study included 275 patients from nine centers across Europe and found that hypothermia with a target temperature of 32–34 °C improved survival to discharge with a good neurological outcome [55% vs. 39%; relative risk (RR), 1.40; 95% confidence interval (CI) 1.08–1.81] as well as 6-month mortality (41% vs. 55%; RR 0.74; 95% CI 0.58–0.95) [4]. A trial by Bernard et al. showed that the difference between good neurological outcomes (defined as no or only moderate disability at discharge) was better in the hypothermia group when compared to the normothermia group (49% and 26% respectively, p = 0.046), with an unadjusted odds ratio for a good outcome in the hypothermia group as compared to the normothermia group of 2.65 (95% CI 1.02–6.88; p = 0.046). When adjusted for age and time to ROSC, the odds ratio for a good outcome in the hypothermia group as compared to the normothermia group was 5.25 (95% CI 1.47–18.76; p = 0.011) [5].
In addition to hyperthermia being associated with less favorable neurological outcomes [28], the promising results of the aforementioned trials rapidly led to adoption of a strategy of induced hypothermia rather than prevention of hyperthermia as a Class I indication in survivors of cardiac arrest, especially in victims of OHCA due to a shockable rhythm [29, 30].
Limitations of Early Studies and Subsequent Trials
Both the early trials [4, 5] had several limitations. In addition to being small studies, treating physicians were unblinded without any standardized neuroprognostication and withdrawal of care protocols, raising concerns regarding premature withdrawal of care in patients treated with normothermia. Further, in the HACA study, the mean temperature in the normothermia arm was 37.5 °C, which raises questions regarding whether difference in outcomes was potentially driven by increased neurological injury from hyperthermia in the control arm. Finally, it remained unclear whether the benefit of hypothermia could be extrapolated to patients with non-shockable rhythms or IHCA as they were excluded.
Given that there was now an established benefit of hypothermia among survivors of cardiac arrest with an initial shockable rhythm, the TTM trial was designed to study the optimal target temperature during cooling protocol [31]. This trial also partially addressed the shortcomings of the early trials described above. The TTM trial was a large, international trial wherein 950 unconscious adults after OHCA of presumed cardiac cause were randomized to TTM at either 33 °C or 36 °C. The primary outcome was all-cause mortality through the end of the trial. Secondary outcomes included a composite of poor neurologic function or death at 180 days, as evaluated with the Cerebral Performance Category scale and the modified Rankin scale. Although the representation of non-shockable OHCA in the TTM trial was only 20%, an important strength of this trial was that neuroprognostication was standardized across both treatment arms. Early withdrawal of care was strongly discouraged for at least 72 h after return of normothermia in both groups. The authors found that in unconscious survivors of OHCA of presumed cardiac cause, hypothermia at a targeted temperature of 33 °C did not confer a benefit as compared with a targeted temperature of 36 °C. A more recent trial that sought to study an optimal target temperature after cardiac arrest was the FROST-1 (A Pilot Multicenter Randomized Trial on the Effectiveness of Different Levels of Cooling in Comatose Survivors of Out-of-hospital Cardiac Arrest) trial [32]. In this trial, 150 patients were randomly assigned to one of three temperature groups—32 °C, 33 °C, or 34 °C. There were no statistically significant differences in the primary end point of survival with good neurological outcomes at 90 days in patients with OHCA and a shockable rhythm. Given the relatively low number of subjects, it is possible that the trial may be underpowered. However, taken together, both the TTM and FROST-1 trials showed that a lower temperature target is not superior to a more lenient temperature that still maintains hypothermia.
Role of TTM in Non-shockable Rhythms
Of all EMS-treated adult OHCAs in 2019, nearly 20% were due to an initial non-perfusing VT or VF arrest (detected as a shockable rhythm by an automated external defibrillator) [9]. Further, it is estimated that more than 80% of in patients with IHCA in the USA have an initial non-shockable rhythm at the time of cardiac arrest [33]. This clearly identifies the magnitude of cardiac arrest due to initial non-shockable rhythms, which was unfortunately either excluded or underrepresented in the trials described above. A few retrospective studies explored this problem and showed that TTM is associated with favorable neurologic outcome and survival in patients resuscitated after cardiac arrest due to non-shockable rhythms [34, 35]. The first trial to explore the role of TTM in non-shockable rhythms was the HYPERION (Targeted Temperature Management for Cardiac Arrest with Non-shockable Rhythm) trial. This trial randomized 584 comatose survivors of OHCA or IHCA due to an initial non-shockable rhythm to undergo targeted hypothermia to 33 °C versus normothermia at 37 °C. The primary end point of this trial was survival with a favorable neurological outcome at 90 days, and this was significantly higher in patients who received hypothermia when compared to the normothermia arm (95% CI 0.1–8.9, p = 0.04). However, there was no statistically significant difference in mortality between both arms. Major drawbacks of the trial were a fragility index of 1, and systematic differences between the treatment and control groups about neuroprognostication that may have impacted the incidence of the primary end point [36].
TTM in IHCA
In general, patients with IHCA have more favorable neurological as well as mortality outcomes when compared to victims of OHCA. This is probably due to rapid detection of IHCA in a closely monitored setting and immediate response by highly trained medical professionals in the hospital when compared to OHCAs, wherein patients may have unwitnessed arrest with prolonged downtime prior to detection and response from bystanders who may not always be medically trained. The only trial dedicated to studying the role of TTM in this patient population was conducted in children and found that hypothermia did not confer a significant benefit in survival with a favorable functional outcome at 1 year when compared to normothermia in comatose children who had survived IHCA [37]. Among adults, a multicenter observational cohort study by Chan et al. within the national Get With the Guidelines-Resuscitation registry identified 26,183 patients with IHCA and shockable as well as non-shockable rhythm and found that hypothermia was associated with a lower likelihood of survival to hospital discharge and a lower likelihood of favorable neurological survival [38]. However, this was an observational study; in the subsequently published HYPERION trial discussed above, approximately 25% patients with an initial non-shockable rhythm had suffered IHCA and in fact had better outcomes when compared to patients with OHCA. The benefit of TTM in IHCA compared to OHCA was not statistically significant, likely due to the small size of the trial.
Novel Approaches to Targeted Temperature Management
The recently published TTM2 (Hypothermia versus Normothermia after Out-of-Hospital Cardiac Arrest) trial [8] randomly assigned 1850 adults with coma who had an OHCA of presumed cardiac or unknown cause to undergo targeted hypothermia at 33 °C followed by controlled rewarming, or targeted normothermia with early treatment of fever (defined as body temperature ≥ 37.8 °C). The trial was conducted at 61 sites across Australia, Europe, and the USA which adds to the generalizability of the data. Overall, there was no difference in survival at 6 months (50% vs. 48%, p = 0.37) and no difference in survival with severe disability on the modified Rankin scale (55% vs. 55%; RR 1.00; 95% CI 0.92–1.09) in both groups. There was also no difference in health-related quality of life between the two groups.
The TTM2 trial has several strengths. It is the largest trial to date to study TTM. Apart from the randomized intervention, both the groups received similar treatment including mechanical ventilator support, sedation, and neuromuscular blockade. Neurological prognostication was assessed using a standardized protocol. Withdrawal of care before 96 h was discouraged unless further treatment was deemed unethical or if there was evidence of brain death. The physicians were not blinded but the outcome assessors were blinded, thereby reducing bias. Follow-up rate was close to 100% and there were minimal protocol deviations.
The TTM2 trial had a few limitations. More than 90% of the patients had a witnessed cardiac arrest and 80% received bystander CPR. A high proportion (72% in the hypothermia arm and 75% in the normothermia arm) had an initial shockable rhythm, suggesting that the findings may not be generalizable to patients with OHCA and an initial non-shockable rhythm. Further, since only patients with OHCA were included, findings may not be generalizable to patients with IHCA. Active cooling was used among 46% of the patients in the normothermia group and underlines the high prevalence of fever and highlights the importance of temperature management in the post cardiac arrest setting. The median duration to achieve a target temperature of 34 °C was 3 h after randomization. The mean duration from point of contact to randomization was 136 min. Thus, the average time to achieve the target temperature was approximately 5 h and the timeframe resembles the real-world clinical experience. It remains to be seen if more rapid cooling would be of clinical benefit although prior studies on prehospital cooling have failed to show a clinical benefit in terms of neuroprotection [39, 40].
Similar results were reported in the CAPITAL CHILL (Effect of Moderate vs Mild Therapeutic Hypothermia on Mortality and Neurologic Outcomes in Comatose Survivors of Out-of-Hospital Cardiac Arrest: The CAPITAL CHILL Randomized Clinical Trial) trial [41], published shortly after the TTM2 trial. This was a single-center, double-blinded, randomized, clinical superiority trial carried out in a tertiary cardiac care center in eastern Ontario, Canada. A total of 389 patients with OHCA were enrolled and randomly assigned to temperature management with a target body temperature of 31 °C (n = 193) or 34 °C (n = 196) for a period of 24 h. The primary outcome was all-cause mortality or poor neurologic outcome at 180 days, which was assessed using the Disability Rating Scale. Poor neurologic outcome was defined as a score greater than 5 [range 0–29, with 29 being the worst outcome (vegetative state)]. The primary outcome occurred in 89 of 184 patients (48.4%) in the 31 °C group and in 83 of 183 patients (45.4%) in the 34 °C group [risk difference 3.0% (95% CI 7.2–13.2%); RR 1.07 (95% CI 0.86–1.33); p = 0.56]. Furthermore, the median length of stay in the intensive care unit was longer in the 31 °C group (10 vs. 7 days; p = 0.004); this was the only one of 19 reported secondary outcomes that was statistically significant. The authors concluded that there was no significant reduction in the rate of death or poor neurological outcome between the two arms of the study. However, this was a small study and likely underpowered in its ability to detect a significant difference between both groups.
High-Quality TTM
In 2009, representatives of five international critical care societies introduced the concept of TTM, replacing the prior concept of TH [42]. The recommendation highlights the importance of the phases of induction, rewarming, and normothermia. The Cool It protocol designed in 2011 has established hypothermia as the standard of care for OHCA across a regional network of hospitals transferring patients to a central TH-capable hospital [43]. Data on the optimal time to initiate TTM, ideal method, duration of cooling, and the rewarming rate is varied. Herein we will summarize the current evidence on the components of high-quality TTM.
Timing of Initiation
TTM should be initiated as early as possible to minimize reperfusion injury after achieving ROSC in cardiac arrest [44,45,46,47]. Two contemporary randomized trials studied prehospital cooling using cold intravenous fluids [39, 40]. Both trials showed a higher incidence of early re-arrests and pulmonary edema in the prehospital cooling group. Randomized trials studying intra-arrest TTM by administering cold fluids during CPR did not show a superior outcome with an early initiation of TTM during CPR [48, 49]. Intra-arrest TTM using a transnasal cooling device to locally reduce brain temperature without reducing systemic temperature has shown some potential benefit among patients with OHCA who had an initial shockable rhythm [50, 51]. Although evidence of intranasal cooling devices is limited, it does suggest that early lowering of local brain temperature after cardiac arrest may aid in neuroprotection, opening potential avenues for future research.
Target Temperature
Prior randomized controlled trials discussed above have studied various target temperatures ranging from 32 to 36 °C. In a study by Casamento et al., TTM was strictly maintained at the target values using surface cooling and temperature feedback mechanisms, and there was no difference in outcomes between the two targets of 33 °C and 36 °C [52]. Similar results were seen in a single-center retrospective cohort study by Johnson et al. [53], and a Swedish national registry study [54]. In the recent TTM2 trial, among patients with coma after OHCA, TTM did not reduce death at 6 months compared to targeted normothermia [8], discussed below.
Cooling Techniques
Currently available cooling techniques include (a) conventional cooling techniques, (b) surface cooling systems, and (c) intravascular cooling techniques.
-
(a)
Conventional cooling techniques: cold saline, ice bags, and crushed ice have been used as conventional techniques to induce hypothermia. Although these techniques are effective in inducing hypothermia, they are not as effective in maintaining target temperature like surface cooling systems and intravascular cooling techniques described below [55, 56].
-
(b)
Surface cooling systems: surface cooling systems work by circulating cold fluid or cold air in a blanket or cooling pads that are applied externally to the patient. Surface cooling offers the advantages of ease of application and rapid initiation of treatment. However, it carries the risk of skin burns and dermal irritation [57]. Shivering is more common with this cooling technique and maintenance of temperature is challenging with surface cooling systems [58].
-
(c)
Intravascular cooling techniques: intravascular cooling techniques include placement of a central venous catheter into an internal jugular, subclavian, or femoral vein. Temperature is maintained by circulating cold saline through a closed loop of the catheter’s balloon [59]. Intravascular devices offer several advantages over other methods including better temperature control during rewarming and less shivering [60]. Despite these advantages, there is no difference in clinical outcomes when compared to surface cooling devices [61]. Intravascular catheter placement also carries the risk of catheter-related bloodstream infection, and catheter-related thrombosis. A combination of core-cooling and surface-cooling methods is generally used.
Duration of Cooling
After achieving target temperature, the cooling phase should last for at least 20 h. In a randomized controlled trial of 355 patients, there was a small improvement in neurological outcomes at 6 months with TTM to 33 °C for 48 h when compared to 24 h, but this difference was not statistically significant. Further, there was increased incidence of adverse outcomes related to hypothermia in the 48-h arm of the study. The study was small and may have had limited power to detect clinically important differences, and put together, data from this trial suggests no benefit from prolonged cooling in adults [62]. Improved survival and neurodevelopmental outcomes are seen among neonates with moderate to severe hypoxic ischemic encephalopathy who receive TTM for 72 h [63]. However, data on prolonged TTM among adults is limited and should be avoided.
Duration of Rewarming
Rewarming should be slow, at a rate of 0.15–0.25 °C/h controlled by rewarming devices as spontaneous rewarming is unpredictable and adds the risk of post-TTM hyperthermia [20, 64]. It is important to control body temperature and prevent post-TTM fever, especially in the first 48 h after rewarming, as fever and high temperature are associated with detrimental clinical outcomes [64,65,66]. A shorter rewarming strategy may be used in patients with shorter duration of CPR and immediate ROSC, and a prolonged rewarming strategy should be considered in patients with moderate to severe cerebral ischemia.
Supportive Clinical Care to Achieve High-Quality TTM
Sedation, Analgesia, and Prevention of Shivering
All patients undergoing TTM should receive appropriate sedation and analgesia [67]. Studies have not compared superiority of a particular sedative regimen; however, clinicians should use short-acting agents like propofol and remifentanil to avoid accumulation of sedative drugs and their metabolites, which may delay awakening [68, 69]. Sedatives and antipyretics should be started at the time of initiating TTM and should be stopped only after achieving normothermia, i.e., temperature of 37 °C [70]. Shivering is commonly encountered as a physiological response to cooling. It can lead to heat generation which may result in a delay in achieving target temperature, temperature variation during the maintenance phase and faster rewarming, and must be treated aggressively for these reasons [71, 72]. Shivering can be treated with antipyretics, low dose sedative, and α2-agonists such as dexmedetomidine. A bolus of intravenous magnesium can help to raise shivering threshold [70]. If these measures are not effective, neuromuscular blockers (NMBs) may be considered [73]. There are conflicting opinions on the role of NMBs in improvement of clinical outcomes. NMBs have altered pharmacokinetic and pharmacodynamic responses at lower body temperatures, and monitoring can be unreliable. Dosing and administration strategy remains unclear. Several studies have been conducted to study the use of NMBs to prevent shivering in patients post cardiac arrest undergoing TTM; however, given the lack of conclusive evidence, it remains an off-label use [74,75,76,77].
Seizure Prophylaxis
Detection and management of seizures is a critical component of post cardiac arrest neurologic care because 25–50% have electroencephalographic (EEG) activity on the ictal-interictal continuum suggestive of non-convulsive seizures [78]. This is related to ischemic brain injury and is a potential contributor to secondary brain injury. EEG findings have prognostic value, and detection of EEG abnormalities can alter clinical care [79]. Based on these findings, consensus clinical guidelines recommend frequent or continuous EEG monitoring for comatose patients after cardiac arrest [80].
Ventilation
Ventilation should be targeted to maintain a partial pressure of carbon dioxide of 35–45 mmHg, as hypocapnia has been associated with decreased cerebral perfusion and worse clinical outcomes [81, 82]. Oxygenation should be targeted to avoid hypoxia (partial pressure of oxygen < 60 mmHg) or hyperoxia (partial pressure of oxygen > 300 mmHg). Current recommendations suggest titrating FiO2 to maintain oxygen saturation at greater than 94% [80].
Glycemic Control
Hypothermia decreases pancreatic insulin secretion and increases insulin resistance, resulting in hyperglycemia [83]. The optimal glycemic target during hypothermia remains debated, and avoidance of hypoglycemia is the key as it can exacerbate neurological damage [84].
Hypokalemia
Hypokalemia commonly occurs during the cooling phase of TTM as a result of intracellular potassium shift and kaliuresis [85]. Electrolytes should be monitored every 4–6 h and should be repleted appropriately. Potassium repletion should be held 4 h prior to rewarming because reversal of cellular shift may result in hyperkalemia [86].
Infection
Infections frequently occur among patients post cardiac arrest as a result of a combination of exposure from aspiration, emergent intubation, mechanical ventilation, and catheter-related bloodstream infections. Gram-negative pathogens are most common causes of nosocomial infections after cardiac arrest, and TTM does not impact the microbiological profile [87, 88].
Cardiac Electrophysiological Effects
TTM is associated with conduction abnormalities, and sinus bradycardia is the most common arrythmia. PR-interval prolongation and junctional rhythm is occasionally seen during the cooling phase. Hypothermia can also prolong QTc although there is no reported association with torsades de pointes. Osborn or J-waves may be present in up to 20% of patients in a temperature-dependent fashion. No specific intervention is required for conduction abnormalities as most will resolve after rewarming by 1–2 °C [89].
Hemodynamic Effect
A post hoc analysis of the TTM trial studied the hemodynamic effects of hypothermia using data from serial right heart catheterization and transthoracic echocardiography. Primary end point was systemic vascular resistance index after 24 h of cooling and secondary end points included mean systemic vascular resistance index, cardiac index, systolic function, and lactate levels. Patients cooled to 33 °C had a significant increase in systemic vascular resistance index compared with those cooled to 36 °C [2595 (95% confidence interval, 2422–2767) versus 1960 (95% confidence interval, 1787–2134) dynes m2/s/cm5; p < 0.0001, respectively] after 24 h of cooling with an overall difference of 556 dynes m2/s/cm5 [p(group) < 0.0001]. Further, cooling to 33 °C was associated with decreased cardiac index [− 0.4 L/min/m2; p(group) < 0.0001], decreased heart rate [p(group) = 0.01], and stroke volume index [p(group) = 0.004] compared with cooling to 36 °C. Left ventricular ejection fraction (p = 0.39) and peak systolic myocardial velocity (p = 0.62) did not differ between TTM groups. Lactate levels were significantly higher in patients cooled to 33 °C (p = 0.0008) [90].
Current Guidelines
The 2021 European Resuscitation Council and European Society of Intensive Care Medicine guidelines for post-resuscitation care [7] and the International Liaison Committee on Resuscitation Advanced Life Support Task Force [91] recommend selecting and maintaining a constant target temperature between 32 and 36 °C for those patients in whom TTM is used (strong recommendation, moderate-quality evidence), as soon as possible after ROSC is achieved and airway, breathing (including mechanical ventilation), and circulation are stabilized. The comparative benefit of lower (32–34 °C) versus higher (36 °C) temperatures remains unknown, and further research may help elucidate this. On the basis of the above trials, there is a recommendation for TTM in survivors of OHCA due to initial shockable rhythms (strong recommendation, low-quality evidence) as well as non-shockable rhythms (weak recommendation, very-low-quality evidence). TTM for comatose survivors of IHCA is recommended regardless of initial rhythm (weak recommendation, very-low-quality evidence). If TTM is used, duration should be at least 24 h (weak recommendation, very-low-quality evidence), and there are recommendations against routine use of prehospital cooling with rapid infusion of large volumes of cold intravenous fluid immediately after ROSC (strong recommendation, moderate-quality evidence). Fever should be prevented in all patients after rewarming to normothermia.
Anecdotal data suggest that TTM to 36 °C for 24 h should be used in uncomplicated patients with evidence of mild to moderate brain injury (coma with preservation of some motor response, no malignant EEG patterns, and no evidence of cerebral edema on CT scan). Special precautions should be taken while caring for patients with active non-compressible bleeding, who should generally be managed with targeted normothermia rather than a lower target as hypothermia causes coagulopathy. If tolerated, patients with evidence of severe brain injury (loss of motor response or brainstem reflexes, malignant EEG patterns, or early changes on CT suggesting the development of cerebral edema) should undergo TTM to 33 °C. Although there is no data on benefit of one temperature over the other, lower temperature may theoretically reduce cerebral edema, seizure activity, and metabolic demand, thereby benefitting patients with these complications [8, 31, 36, 92].
Multidisciplinary Approach
Post-resuscitation care and TTM require a coordinated multidisciplinary approach with participation of practitioners from multiple specialties, nurses, technicians, and other support staff. Effective coordination of care can be achieved by developing institutional protocols designating different roles and incorporating bundled approaches to facilitate effective administration of a time-sensitive intervention such as TTM. Participation of multidisciplinary teams can also facilitate standardized neuroprognostication, consistent decision-making regarding patient selection for TTM initiation, withdrawal of care and end-of-life decisions when appropriate per local practice patterns and institutional protocols.
Future Directions
There are several knowledge gaps in the existing literature. Future studies should focus on the occurrence of acute kidney injury, gastrointestinal complications, hypotension, thromboembolic events, and impact on the duration of mechanical ventilation. In the TTM2 trial, the mean duration from the initial contact to randomization was 136 min. The median time to achieve the target temperature of 34 °C was 3 h after randomization. Collectively, this amounted to a total duration of 5 h to achieve the target temperature. This delay in achieving target temperature likely represents a real-world scenario of cardiac arrest survivors. Future trials should investigate if more rapid cooling offers a clinical benefit although prior studies on prehospital cooling have failed to show a mortality benefit [39, 40]. Apart from the time to achieve targeted temperature, several other factors including cooling duration and use of an anti-shivering protocol may affect clinical outcomes. The influence of cooling duration on efficacy in cardiac arrest patients (ICECAP trial, ClinicalTrials.gov Identifier NCT04217551) is currently underway. Similarly, effect of anti-shivering protocol with neuromuscular blockade is currently being studied using a before-after-retrospective design (ClinicalTrials.gov Identifier NCT05264246). We eagerly await the results of these studies.
Conclusion
TTM is a complex intervention that requires a multidisciplinary team and multiple clinical decisions. Homogeneity in practice and minimization of errors can be achieved by using standardized institutional protocols that can effectively guide a multidisciplinary team in rendering high-quality care to achieve neuroprotection after cardiac arrest with rigorous quality control and quality assurance measures to assess internal adherence to the above measures. On the basis of current data, there does not seem to be evidence to pick TTM over targeted normothermia, and temporal trends have leaned towards using a lenient temperature target especially since the publication of TTM trial [93]. TTM should be considered as a tool for neuroprotection in all comatose survivors of cardiac arrest, and the decision to opt for targeted hypothermia versus targeted normothermia should be tailored on the basis of severity of illness on a case-by-case basis.
References
Liss HP. A history of resuscitation. Ann Emerg Med. 1986;15(1):65–72. https://doi.org/10.1016/s0196-0644(86)80490-5.
Perman SM, Goyal M, Neumar RW, Topjian AA, Gaieski DF. Clinical applications of targeted temperature management. Chest. 2014;145(2):386–93. https://doi.org/10.1378/chest.12-3025.
Williams GR, Spencer FC. The clinical use of hypothermia following cardiac arrest. Ann Surg. 1958;148(3):462–8. https://doi.org/10.1097/00000658-195809000-00014.
Group HaCAS. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549–56. https://doi.org/10.1056/NEJMoa012689.
Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346(8):557–63. https://doi.org/10.1056/NEJMoa003289.
Panchal AR, Bartos JA, Cabañas JG, et al. Part 3: Adult basic and advanced life support: 2020 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2020;142(16_suppl_2):S366–468. https://doi.org/10.1161/CIR.0000000000000916.
Nolan JP, Sandroni C, Böttiger BW, et al. European Resuscitation Council and European Society of Intensive Care Medicine guidelines 2021: post-resuscitation care. Intensive Care Med. 2021;47(4):369–421. https://doi.org/10.1007/s00134-021-06368-4.
Dankiewicz J, Cronberg T, Lilja G, et al. Hypothermia versus normothermia after out-of-hospital cardiac arrest. N Engl J Med. 2021;384(24):2283–94. https://doi.org/10.1056/NEJMoa2100591.
Virani SS, Alonso A, Aparicio HJ, et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143(8):e254–743. https://doi.org/10.1161/CIR.0000000000000950.
Perkins GD, Jacobs IG, Nadkarni VM, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update of the Utstein Resuscitation Registry Templates for Out-of-Hospital Cardiac Arrest: a statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian and New Zealand Council on Resuscitation, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa, Resuscitation Council of Asia); and the American Heart Association Emergency Cardiovascular Care Committee and the Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Circulation. 2015;132(13):1286–300. https://doi.org/10.1161/CIR.0000000000000144.
Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics-2022 update: a report from the American Heart Association. Circulation. 2022;145(8):e153–639. https://doi.org/10.1161/CIR.0000000000001052.
US Census Bureau. https://www.census.gov. Accessed 14 May 2022.
American Hospital Association (AHA). https://www.ahadataviewer.com. Accessed 14 May 2022.
Wissenberg M, Lippert FK, Folke F, et al. Association of national initiatives to improve cardiac arrest management with rates of bystander intervention and patient survival after out-of-hospital cardiac arrest. JAMA. 2013;310(13):1377–84. https://doi.org/10.1001/jama.2013.278483.
Hasselqvist-Ax I, Riva G, Herlitz J, et al. Early cardiopulmonary resuscitation in out-of-hospital cardiac arrest. N Engl J Med. 2015;372(24):2307–15. https://doi.org/10.1056/NEJMoa1405796.
Hansen CM, Kragholm K, Granger CB, et al. The role of bystanders, first responders, and emergency medical service providers in timely defibrillation and related outcomes after out-of-hospital cardiac arrest: results from a statewide registry. Resuscitation. 2015;96:303–9. https://doi.org/10.1016/j.resuscitation.2015.09.002.
Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146–603. https://doi.org/10.1161/CIR.0000000000000485.
Merchant RM, Topjian AA, Panchal AR, et al. Part 1: executive summary: 2020 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2020;142(16_suppl_2):S337–57. https://doi.org/10.1161/CIR.0000000000000918.
Chan PS, Jain R, Nallmothu BK, Berg RA, Sasson C. Rapid response teams: a systematic review and meta-analysis. Arch Intern Med. 2010;170(1):18–26. https://doi.org/10.1001/archinternmed.2009.424.
Holzer M. Targeted temperature management for comatose survivors of cardiac arrest. N Engl J Med. 2010;363(13):1256–64. https://doi.org/10.1056/NEJMct1002402.
Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med. 2009;37(7 Suppl):S186-202. https://doi.org/10.1097/CCM.0b013e3181aa5241.
Yenari MA, Han HS. Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat Rev Neurosci. 2012;13(4):267–78. https://doi.org/10.1038/nrn3174.
Yenari M, Kitagawa K, Lyden P, Perez-Pinzon M. Metabolic downregulation: a key to successful neuroprotection? Stroke. 2008;39(10):2910–7. https://doi.org/10.1161/STROKEAHA.108.514471.
Delgado GA, Truesdell AG, Abbott JD. Therapeutic hypothermia for myocardial protection in ST elevation myocardial infarction. J Clinic Experiment Cardiol. 2011;S5:2.
Meybohm P, Gruenewald M, Albrecht M, et al. Hypothermia and postconditioning after cardiopulmonary resuscitation reduce cardiac dysfunction by modulating inflammation, apoptosis and remodeling. PLoS ONE. 2009;4(10):e7588. https://doi.org/10.1371/journal.pone.0007588.
Tissier R, Chenoune M, Ghaleh B, Cohen MV, Downey JM, Berdeaux A. The small chill: mild hypothermia for cardioprotection? Cardiovasc Res. 2010;88(3):406–14. https://doi.org/10.1093/cvr/cvq227.
Deye N, Cariou A, Girardie P, Pichon N, Megarbane B, Midez P, Tonnelier JM, Boulain T, Outin H, Delahaye A, Cravoisy A, Mercat A, Blanc P, Santré C, Quintard H, Brivet F, Charpentier J, Garrigue D, Francois B, Quenot JP, Vincent F, Gueugniaud PY, Mira JP, Carli P, Vicaut E, Baud FJ. Clinical and Economical Impact of Endovascular Cooling in the Management of Cardiac Arrest (ICEREA) study group. Endovascular Versus External Targeted Temperature Management for Patients With Out-of-Hospital Cardiac Arrest: A Randomized, Controlled Study. Circulation. 2015;132(3):182–93. https://doi.org/10.1161/CIRCULATIONAHA.114.012805. Epub 2015 Jun 19. Erratum in: Circulation. 2016 Feb 23;133(8):e418. PMID: 26092673.
Zeiner A, Holzer M, Sterz F, et al. Hyperthermia after cardiac arrest is associated with an unfavorable neurologic outcome. Arch Intern Med. 2001;161(16):2007–12. https://doi.org/10.1001/archinte.161.16.2007.
Neumar RW, Nolan JP, Adrie C, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation. 2008;118(23):2452–2483. https://doi.org/10.1161/CIRCULATIONAHA.108.190652.
Peberdy MA, Callaway CW, Neumar RW, et al. Part 9: post-cardiac arrest care: 2010 American Heart Association Guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010;122(18 Suppl 3):S768–86. https://doi.org/10.1161/CIRCULATIONAHA.110.971002.
Nielsen N, Wetterslev J, Cronberg T, et al. Targeted temperature management at 33 °C versus 36 °C after cardiac arrest. N Engl J Med. 2013;369(23):2197–206. https://doi.org/10.1056/NEJMoa1310519.
Lopez-de-Sa E, Juarez M, Armada E, et al. A multicentre randomized pilot trial on the effectiveness of different levels of cooling in comatose survivors of out-of-hospital cardiac arrest: the FROST-I trial. Intensive Care Med. 2018;44(11):1807–15. https://doi.org/10.1007/s00134-018-5256-z.
Rasmussen TP, Bullis TC, Girotra S. Targeted temperature management for treatment of cardiac arrest. Curr Treat Options Cardiovasc Med. 2020;22(11):39. https://doi.org/10.1007/s11936-020-00846-6.
Testori C, Sterz F, Behringer W, et al. Mild therapeutic hypothermia is associated with favourable outcome in patients after cardiac arrest with non-shockable rhythms. Resuscitation. 2011;82(9):1162–7. https://doi.org/10.1016/j.resuscitation.2011.05.022.
Lundbye JB, Rai M, Ramu B, et al. Therapeutic hypothermia is associated with improved neurologic outcome and survival in cardiac arrest survivors of non-shockable rhythms. Resuscitation. 2012;83(2):202–7. https://doi.org/10.1016/j.resuscitation.2011.08.005.
Lascarrou JB, Merdji H, Le Gouge A, et al. Targeted temperature management for cardiac arrest with non-shockable rhythm. N Engl J Med. 2019;381(24):2327–37. https://doi.org/10.1056/NEJMoa1906661.
Moler FW, Silverstein FS, Holubkov R, et al. Therapeutic hypothermia after in-hospital cardiac arrest in children. N Engl J Med. 2017;376(4):318–29. https://doi.org/10.1056/NEJMoa1610493.
Chan PS, Berg RA, Tang Y, Curtis LH, Spertus JA, American Heart Association’s Get With the Guidelines–Resuscitation Investigators. Association between therapeutic hypothermia and survival after in-hospital cardiac arrest. JAMA. 2016;316(13):1375–82. https://doi.org/10.1001/jama.2016.14380.
Bernard SA, Smith K, Cameron P, et al. Induction of therapeutic hypothermia by paramedics after resuscitation from out-of-hospital ventricular fibrillation cardiac arrest: a randomized controlled trial. Circulation. 2010;122(7):737–42. https://doi.org/10.1161/CIRCULATIONAHA.109.906859.
Kim F, Nichol G, Maynard C, et al. Effect of prehospital induction of mild hypothermia on survival and neurological status among adults with cardiac arrest: a randomized clinical trial. JAMA. 2014;311(1):45–52. https://doi.org/10.1001/jama.2013.282173.
Le May M, Osborne C, Russo J, et al. Effect of moderate vs mild therapeutic hypothermia on mortality and neurologic outcomes in comatose survivors of out-of-hospital cardiac arrest: the CAPITAL CHILL randomized clinical trial. JAMA. 2021;326(15):1494–503. https://doi.org/10.1001/jama.2021.15703.
Nunnally ME, Jaeschke R, Bellingan GJ, et al. Targeted temperature management in critical care: a report and recommendations from five professional societies. Crit Care Med. 2011;39(5):1113–25. https://doi.org/10.1097/CCM.0b013e318206bab2.
Mooney MR, Unger BT, Boland LL, et al. Therapeutic hypothermia after out-of-hospital cardiac arrest: evaluation of a regional system to increase access to cooling. Circulation. 2011;124(2):206–14. https://doi.org/10.1161/CIRCULATIONAHA.110.986257.
Abella BS, Zhao D, Alvarado J, Hamann K, Vanden Hoek TL, Becker LB. Intra-arrest cooling improves outcomes in a murine cardiac arrest model. Circulation. 2004;109(22):2786–91. https://doi.org/10.1161/01.CIR.0000131940.19833.85.
Wolff B, Machill K, Schumacher D, Schulzki I, Werner D. Early achievement of mild therapeutic hypothermia and the neurologic outcome after cardiac arrest. Int J Cardiol. 2009;133(2):223–8. https://doi.org/10.1016/j.ijcard.2007.12.039.
Haugk M, Testori C, Sterz F, et al. Relationship between time to target temperature and outcome in patients treated with therapeutic hypothermia after cardiac arrest. Crit Care. 2011;15(2):R101. https://doi.org/10.1186/cc10116.
Lee BK, Jeung KW, Jung YH, et al. Relationship between timing of cooling and outcomes in adult comatose cardiac arrest patients treated with targeted temperature management. Resuscitation. 2017;04(113):135–41. https://doi.org/10.1016/j.resuscitation.2016.12.002.
Debaty G, Maignan M, Savary D, et al. Impact of intra-arrest therapeutic hypothermia in outcomes of prehospital cardiac arrest: a randomized controlled trial. Intensive Care Med. 2014;40(12):1832–42. https://doi.org/10.1007/s00134-014-3519-x.
Bernard SA, Smith K, Finn J, et al. Induction of therapeutic hypothermia during out-of-hospital cardiac arrest using a rapid infusion of cold saline: the RINSE Trial (Rapid Infusion of Cold Normal Saline). Circulation. 2016;134(11):797–805. https://doi.org/10.1161/CIRCULATIONAHA.116.021989.
Castrén M, Nordberg P, Svensson L, et al. Intra-arrest transnasal evaporative cooling: a randomized, prehospital, multicenter study (PRINCE: Pre-ROSC IntraNasal Cooling Effectiveness). Circulation. 2010;122(7):729–36. https://doi.org/10.1161/CIRCULATIONAHA.109.931691.
Nordberg P, Taccone FS, Truhlar A, et al. Effect of trans-nasal evaporative intra-arrest cooling on functional neurologic outcome in out-of-hospital cardiac arrest: the PRINCESS randomized clinical trial. JAMA. 2019;321(17):1677–85. https://doi.org/10.1001/jama.2019.4149.
Casamento A, Minson A, Radford S, et al. A comparison of therapeutic hypothermia and strict therapeutic normothermia after cardiac arrest. Resuscitation. 2016;09(106):83–8. https://doi.org/10.1016/j.resuscitation.2016.06.019.
Johnson NJ, Danielson KR, Counts CR, Ruark K, Scruggs S, Hough CL, Maynard C, Sayre MR, Carlbom DJ. Targeted Temperature Management at 33 Versus 36 Degrees: A Retrospective Cohort Study. Crit Care Med. 2020;48(3):362–9. https://doi.org/10.1097/CCM.0000000000004159.
Abazi L, Awad A, Nordberg P, et al. Long-term survival in out-of-hospital cardiac arrest patients treated with targeted temperature control at 33 °C or 36 °C: a national registry study. Resuscitation. 2019;10(143):142–7. https://doi.org/10.1016/j.resuscitation.2019.08.029.
Larsson IM, Wallin E, Rubertsson S. Cold saline infusion and ice packs alone are effective in inducing and maintaining therapeutic hypothermia after cardiac arrest. Resuscitation. 2010;81(1):15–9. https://doi.org/10.1016/j.resuscitation.2009.09.012.
Merchant RM, Abella BS, Peberdy MA, et al. Therapeutic hypothermia after cardiac arrest: unintentional overcooling is common using ice packs and conventional cooling blankets. Crit Care Med. 2006;34(12 Suppl):S490–4. https://doi.org/10.1097/01.CCM.0000246016.28679.36.
Varon J, Acosta P, Wintz R, Mendoza N. Unusual side effect from hydrogel pads during therapeutic hypothermia. Resuscitation. 2008;78(3):248–9. https://doi.org/10.1016/j.resuscitation.2008.03.223.
Mayer SA, Kowalski RG, Presciutti M, et al. Clinical trial of a novel surface cooling system for fever control in neurocritical care patients. Crit Care Med. 2004;32(12):2508–15. https://doi.org/10.1097/01.ccm.0000147441.39670.37.
Zoll. IVTM™ Intravascular Temperature Management. https://www.zoll.com/-/media/uploadedfiles/public_site/products/catheters/catheters_spec_sheet.ashx. Accessed 14 May 2022.
Lyden PD, Allgren RL, Ng K, et al. Intravascular cooling in the treatment of stroke (ICTuS): early clinical experience. J Stroke Cerebrovasc Dis. 2005;14(3):107–14. https://doi.org/10.1016/j.jstrokecerebrovasdis.2005.01.001.
Gillies MA, Pratt R, Whiteley C, Borg J, Beale RJ, Tibby SM. Therapeutic hypothermia after cardiac arrest: a retrospective comparison of surface and endovascular cooling techniques. Resuscitation. 2010;81(9):1117–22. https://doi.org/10.1016/j.resuscitation.2010.05.001.
Kirkegaard H, Søreide E, de Haas I, et al. Targeted temperature management for 48 vs 24 hours and neurologic outcome after out-of-hospital cardiac arrest: a randomized clinical trial. JAMA. 2017;318(4):341–50. https://doi.org/10.1001/jama.2017.8978.
Tagin MA, Woolcott CG, Vincer MJ, Whyte RK, Stinson DA. Hypothermia for neonatal hypoxic ischemic encephalopathy: an updated systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2012;166(6):558–66. https://doi.org/10.1001/archpediatrics.2011.1772.
Picetti E, Antonini MV, Bartolini Y, et al. Delayed fever and neurological outcome after cardiac arrest: a retrospective clinical study. Neurocrit Care. 2016;24(2):163–71. https://doi.org/10.1007/s12028-016-0251-0.
Bro-Jeppesen J, Hassager C, Wanscher M, et al. Post-hypothermia fever is associated with increased mortality after out-of-hospital cardiac arrest. Resuscitation. 2013;84(12):1734–40. https://doi.org/10.1016/j.resuscitation.2013.07.023.
Povlishock JT, Wei EP. Posthypothermic rewarming considerations following traumatic brain injury. J Neurotrauma. 2009;26(3):333–40. https://doi.org/10.1089/neu.2008.0604.
Gaieski DF, Band RA, Abella BS, et al. Early goal-directed hemodynamic optimization combined with therapeutic hypothermia in comatose survivors of out-of-hospital cardiac arrest. Resuscitation. 2009;80(4):418–24. https://doi.org/10.1016/j.resuscitation.2008.12.015.
Paul M, Bougouin W, Dumas F, et al. Comparison of two sedation regimens during targeted temperature management after cardiac arrest. Resuscitation. 2018;07(128):204–10. https://doi.org/10.1016/j.resuscitation.2018.03.025.
Hostler D, Northington WE, Callaway CW. High-dose diazepam facilitates core cooling during cold saline infusion in healthy volunteers. Appl Physiol Nutr Metab. 2009;34(4):582–6. https://doi.org/10.1139/H09-011.
Choi HA, Ko SB, Presciutti M, et al. Prevention of shivering during therapeutic temperature modulation: the Columbia anti-shivering protocol. Neurocrit Care. 2011;14(3):389–94. https://doi.org/10.1007/s12028-010-9474-7.
Dell’Anna AM, Taccone FS, Halenarova K, Citerio G. Sedation after cardiac arrest and during therapeutic hypothermia. Minerva Anestesiol. 2014;80(8):954–62.
Badjatia N, Strongilis E, Gordon E, et al. Metabolic impact of shivering during therapeutic temperature modulation: the Bedside Shivering Assessment Scale. Stroke. 2008;39(12):3242–7. https://doi.org/10.1161/STROKEAHA.108.523654.
May TL, Riker RR, Fraser GL, et al. Variation in sedation and neuromuscular blockade regimens on outcome after cardiac arrest. Crit Care Med. 2018;46(10):e975–80. https://doi.org/10.1097/CCM.0000000000003301.
Lee BK, Cho IS, Oh JS, et al. Continuous neuromuscular blockade infusion for out-of-hospital cardiac arrest patients treated with targeted temperature management: a multicenter randomized controlled trial. PLoS One. 2018;13(12): e0209327. https://doi.org/10.1371/journal.pone.0209327.
Moskowitz A, Andersen LW, Rittenberger JC, et al. Continuous neuromuscular blockade following successful resuscitation from cardiac arrest: a randomized trial. J Am Heart Assoc. 2020;9(17): e017171. https://doi.org/10.1161/JAHA.120.017171.
Salciccioli JD, Cocchi MN, Rittenberger JC, et al. Continuous neuromuscular blockade is associated with decreased mortality in post-cardiac arrest patients. Resuscitation. 2013;84(12):1728–33. https://doi.org/10.1016/j.resuscitation.2013.06.008.
Takiguchi T, Ohbe H, Nakajima M, et al. Intermittent versus continuous neuromuscular blockade during target temperature management after cardiac arrest: a nationwide observational study. J Crit Care. 2021;04(62):276–82. https://doi.org/10.1016/j.jcrc.2021.01.002.
Solanki P, Coppler PJ, Kvaløy JT, et al. Association of antiepileptic drugs with resolution of epileptiform activity after cardiac arrest. Resuscitation. 2019;09(142):82–90. https://doi.org/10.1016/j.resuscitation.2019.07.007.
Elmer J, Coppler PJ, Solanki P, et al. Sensitivity of continuous electroencephalography to detect ictal activity after cardiac arrest. JAMA Netw Open. 2020;3(4): e203751. https://doi.org/10.1001/jamanetworkopen.2020.3751.
Callaway CW, Donnino MW, Fink EL, et al. Part 8: post-cardiac arrest care: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(18 Suppl 2):S465–82. https://doi.org/10.1161/CIR.0000000000000262.
Roberts BW, Kilgannon JH, Chansky ME, Mittal N, Wooden J, Trzeciak S. Association between postresuscitation partial pressure of arterial carbon dioxide and neurological outcome in patients with post-cardiac arrest syndrome. Circulation. 2013;127(21):2107–13. https://doi.org/10.1161/CIRCULATIONAHA.112.000168.
Bouzat P, Suys T, Sala N, Oddo M. Effect of moderate hyperventilation and induced hypertension on cerebral tissue oxygenation after cardiac arrest and therapeutic hypothermia. Resuscitation. 2013;84(11):1540–5. https://doi.org/10.1016/j.resuscitation.2013.05.014.
Silverman MG, Scirica BM. Cardiac arrest and therapeutic hypothermia. Trends Cardiovasc Med. 2016;26(4):337–44. https://doi.org/10.1016/j.tcm.2015.10.002.
Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–97. https://doi.org/10.1056/NEJMoa0810625.
Kim YM, Youn CS, Kim SH, et al. Adverse events associated with poor neurological outcome during targeted temperature management and advanced critical care after out-of-hospital cardiac arrest. Crit Care. 2015;19:283. https://doi.org/10.1186/s13054-015-0991-9.
Nayeri A, Gluck H, Farber-Eger E, et al. Temporal pattern and prognostic significance of hypokalemia in patients undergoing targeted temperature management following cardiac arrest. Am J Cardiol. 2017;120(7):1110–3. https://doi.org/10.1016/j.amjcard.2017.06.051.
Harmon MBA, Hodiamont CJ, Dankiewicz J, et al. Microbiological profile of nosocomial infections following cardiac arrest: insights from the targeted temperature management (TTM) trial. Resuscitation. 2020;148:227–33. https://doi.org/10.1016/j.resuscitation.2019.11.033.
François B, Cariou A, Clere-Jehl R, et al. Prevention of early ventilator-associated pneumonia after cardiac arrest. N Engl J Med. 2019;381(19):1831–42. https://doi.org/10.1056/NEJMoa1812379.
Salinas P, Lopez-de-Sa E, Pena-Conde L, et al. Electrocardiographic changes during induced therapeutic hypothermia in comatose survivors after cardiac arrest. World J Cardiol. 2015;7(7):423–30. https://doi.org/10.4330/wjc.v7.i7.423.
Bro-Jeppesen J, Hassager C, Wanscher M, et al. Targeted temperature management at 33 °C versus 36 °C and impact on systemic vascular resistance and myocardial function after out-of-hospital cardiac arrest: a sub-study of the Target Temperature Management Trial. Circ Cardiovasc Interv. 2014;7(5):663–72. https://doi.org/10.1161/CIRCINTERVENTIONS.114.001556.
Granfeldt A, Holmberg MJ, Nolan JP, Soar J, Andersen LW, International Liaison Committee on Resuscitation (ILCOR) Advanced Life Support Task Force. Targeted temperature management in adult cardiac arrest: systematic review and meta-analysis. Resuscitation. 2021;167:160–72. https://doi.org/10.1016/j.resuscitation.2021.08.040.
Callaway CW, Coppler PJ, Faro J, et al. Association of initial illness severity and outcomes after cardiac arrest with targeted temperature management at 36 °C or 33 °C. JAMA Netw Open. 2020;3(7):e208215. https://doi.org/10.1001/jamanetworkopen.2020.8215.
Bradley SM, Liu W, McNally B, et al. Temporal trends in the use of therapeutic hypothermia for out-of-hospital cardiac arrest. JAMA Netw Open. 2018;1(7):e184511. https://doi.org/10.1001/jamanetworkopen.2018.4511.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Author Contributions
Study design, literature review, statistical analysis: ADB, YRS, SV; Data management, data analysis, drafting manuscript: ADB, YRS, SV; Access to data: ADB, YRS, AGT, AKK, JDM, PMB, DXZ, SV; Manuscript revision, intellectual revisions, mentorship: AGT, AKK, JDM, PMB, DXZ, SV; Final approval: ADB, YRS, AGT, AKK, JDM, PMB, DXZ, SV.
Disclosures
Agastya D Belur, Yub Raj Sedhai, Alexander G Truesdell, Ashish K Khanna, Joseph D Mishkin, P Matthew Belford, David X Zhao and Saraschandra Vallabhajosyula have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Belur, A.D., Sedhai, Y.R., Truesdell, A.G. et al. Targeted Temperature Management in Cardiac Arrest: An Updated Narrative Review. Cardiol Ther 12, 65–84 (2023). https://doi.org/10.1007/s40119-022-00292-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40119-022-00292-4