Abstract
It is well established that an elevated potassium level (hyperkalemia) is associated with a risk of adverse events including morbidity, mortality and healthcare system cost. Hyperkalemia is commonly encountered in many chronic conditions including kidney disease, diabetes and heart failure. Furthermore, hyperkalemia may result from the use of renin-angiotensin-aldosterone system inhibitors (RAASi), which are disease-modifying treatments for these conditions. Therefore, balancing the benefits of optimizing treatment with RAASi while mitigating hyperkalemia is crucial to ensure patients are optimally treated. In this review, we will briefly discuss the definition, causes, epidemiology and consequences of hyperkalemia. The majority of the review will be focused on management of hyperkalemia in the acute and chronic setting, emphasizing contemporary approaches and evolving data on the relevance of dietary restriction and the use of novel potassium binders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
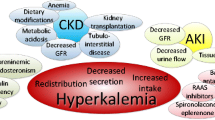
Hyperkalemia is associated with morbidity and mortality, and the risks are even higher in individuals with underlying comorbid conditions such as kidney disease, diabetes and heart failure |
The management of chronic hyperkalemia requires a multimodal approach. This may include the use of loop or thiazide diuretics, potassium binders, dietary modification, correction of acidosis, or adjusting or stopping medications that lead to hyperkalemia when otherwise clinically indicated |
While dietary intake of potassium in those at risk has long been felt to be a cause of hyperkalemia, this paradigm is being challenged, and studies are ongoing to better understand this association |
Care should be taken to avoid permanent discontinuation or suboptimal dosing of goal-directed medical therapy that can cause hyperkalemia (i.e., RAASi). When discontinued because of the risk of hyperkalemia, clinicians should look to restart RAASi when safe to do so; newer potassium binders may allow for maintenance or dose up-titration of RAASi when clinically indicated |
Limited data exist to support the efficacy and safety of long-term use of older potassium binders. Where available, contemporary potassium binders such as sodium zirconium cyclosilicate and patiromer are efficacious and may be safer and better tolerated. Trials are ongoing to further evaluate the efficacy of these binders in facilitating goal-directed therapy for chronic health conditions |
Introduction
Potassium is an essential cation for cellular function, and serum potassium levels are tightly regulated by the body in a narrow range (typically 3.5–5.0 mmol/l). Hyperkalemia is an issue of clinical importance for patients and providers due, in part, to its long-recognized clinical consequences including cardiac arrythmias. More recently, the true epidemiologic impact of hyperkalemia has been even better characterized; an elevated potassium level is associated with hospitalization [1, 2], mortality and large increases in healthcare costs on a population level [1, 3]. These consequences are even more profound in patients with underlying pre-existing comorbid conditions [4, 5].
There is a strong association between renin-angiotensin-aldosterone system inhibitors (RAASi), namely, angiotensin-converting enzyme inhibitors (ACEis), angiotensin receptor blockers (ARBs) and mineralocorticoid receptor antagonists (MRAs), and hyperkalemia [6]. RAASi are a key pillar of therapy for kidney disease, diabetes and heart failure. Therefore, discontinuing these agents in response to hyperkalemia compromises care and may in turn lead to morbidity and mortality. How can providers find the right balance to minimize the risks of hyperkalemia while maintaining patients on therapies that optimize their health? In this review, we will discuss the definition, causes, epidemiology and consequences of hyperkalemia. The focus of the review will be on management, providing clinicians and healthcare professionals with practical suggestions around how best to monitor patients, to prevent the development of hyperkalemia and to lower potassium to support the maintenance or implementation of RAASi when clinically indicated. The article is based on previously conducted studies and does not contain any new studies on human participants or animals performed by any of the authors.
Definition/Pathogenesis
Hyperkalemia is traditionally defined as a serum potassium level > 5.0 mmol/l. More recently, stakeholders participating in the Kidney Disease: Improving Global Outcomes (KDIGO) controversies conference suggested the importance of using clinical information to complement the absolute value of serum potassium. In this regard, the classification of acute hyperkalemia (as mild, moderate or severe) should be based both on the absolute serum level and the presence or absence of electrocardiogram (ECG) changes consistent with hyperkalemia [7]. Using such an approach, hyperkalemia may be classified from mild (potassium concentration of 5.0–5.9 mmol/l with no ECG changes) to severe (potassium concentration of 6.0–6.4 mmol/l with ECG changes or ≥ 6.5 mmol/l [7] irrespective of the ECG findings). This classification emphasizes that some patients may experience the consequences of hyperkalemia at lower serum potassium levels, and if symptoms/signs are present, urgent intervention to avoid morbidity and mortality from cardiac arrythmia is needed [8,9,10]. Somewhat similar thresholds have been used to define hyperkalemia severity in the chronic setting [11, 12].
Notwithstanding the threshold used to define it, the pathogenesis of hyperkalemia is complex and usually involves reduced potassium excretion, movement of intracellular potassium to the extracellular space or, rarely, excess potassium intake. Chronically, hyperkalemia develops primarily because of reduced urinary potassium excretion. Absorption of potassium does occur in the small intestine (and to a lesser extent, colon), and hyperaldosteronism increases fecal loss of potassium through an exchanger. While the contribution of the bowel to potassium absorption is only minimal [13], anephric patients can become hyperkalemic when intestinal potassium excretion is reduced [14]. In the kidney, potassium is freely filtered by the glomerulus, and approximately 90% is reabsorbed proximally (in the proximal convoluted tubule and ascending loop of Henle) [7]. Potassium is more tightly regulated in aldosterone-sensitive distal nephrons under the influence of several factors (including aldosterone, tubular flow and distal sodium delivery), which enhance the secretion of potassium [7]. Alterations in these factors (including loss of nephron function due to kidney disease) can result in hyperkalemia.
Causes
Acknowledging the complex pathophysiology, one must consider a number of potential causes when assessing patients with hyperkalemia. Rapid potassium shift into the extracellular space can result from some forms of metabolic acidosis, medications, insulin deficiency, rhabdomyolysis and tumor lysis syndrome [9]. Any reduction in kidney function (either acutely or chronically) can lead to impaired potassium excretion. In addition, factors that impair distal urinary flow, distal delivery of sodium or electrogenic sodium reabsorption (i.e., due to aldosterone antagonists, aldosterone deficiency or electrogenic sodium channel blockers) can also lead to hyperkalemia. While these conditions need to be thoroughly evaluated in all patients presenting with acute or chronic hyperkalemia, an important condition that should be ruled out is pseudohyperkalemia, which is a rise in potassium level due to excessive release of potassium. Pseudohyperkalemia can occur during or after blood collection and can be caused by hemolysis (e.g., by a faulty sample collection or fist clenching), leukocytosis or thrombocytosis [9, 15]. While an extensive summary of all causes of hyperkalemia is beyond the scope of this review, a brief summary of common causes (including medications and the location of action) is noted in Table 1.
Clinical Consequences, Impact and Epidemiology
The incidence and prevalence of hyperkalemia is quite variable because of factors such as the target population and the potassium cut-off used to define it. In a contemporary meta-analysis, the pooled prevalence of hyperkalemia in the inpatient, outpatient, emergency room, intensive care unit and dialysis unit was 6.3% (95% confidence interval 5.8–6.8%) with an incidence of 2.8 cases/100 person years, but much lower in the general, outpatient population (prevalence 1.3%, incidence 0.4/100 person years) [16]. Expectedly, the prevalence is influenced by the presence or absence of underlying comorbid conditions. In the same meta-analysis, using a potassium threshold of > 5.0 mmol/l and including only studies with a sample size ≥ 1250 participants, Humphrey et al. found that the prevalence was 7.5% in non-dialysis CKD, 8.3% in diabetes, 8.0% in heart failure and 35% in dialysis-dependent CKD [16]. Kidney disease is the single largest predictor of hyperkalemia; in a large meta-analysis by Kovesdy et al., estimated glomerular filtration rate (eGFR) was shown to have a nearly linear relationship with hyperkalemia and those with stage 3 CKD or higher had a nearly threefold increase in moderate to severe hyperkalemia (≥ 5.5 mmol/l) for each 15 ml/min/1.73m2 reduction in eGFR [4].
In addition to comorbid conditions, many medications used to treat kidney disease, heart failure and diabetes are also associated with a higher risk of incident and recurrent hyperkalemia although this may simply represent indication bias. In the above-mentioned meta-analysis, among studies with a larger sample size, RAASi use was associated with a 6.2% prevalence of hyperkalemia (using potassium thresholds of > 5.5 mmol/l) whereas prevalence in the outpatient population was 4.2% [16]. Hyperkalemia is also commonly reported in the treatment arm of large, controlled trials evaluating the use of ACEi or ARBs in heart failure and diabetic nephropathy [17,18,19,20]. Furthermore, the simultaneous use of two RAASi has been shown to confer an even higher risk of hyperkalemia, which suggests that hyperkalemic risk factors are cumulative [21,22,23]. Aldosterone antagonists are also associated with hyperkalemia as demonstrated in the Randomized Aldactone Evaluation Study (RALES) and in post-marketing surveillance [24, 25]. Similar increases in serum potassium were found in hypertension and heart failure trials evaluating the use of eplerenone [26,27,28]. Finally, a post hoc safety analysis of finerenone, a novel, selective nonsteroidal mineralcorticoid receptor antagonist evaluated in two recent clinical trials, also demonstrated a twofold increase in hyperkalemic adverse events, although the risk of serious hyperkalemia-associated morbidity or mortality was low (potentially due to rigorous routine monitoring and management) [29].
Patients with mild hyperkalemia usually present without signs or symptoms, but at higher levels, hyperkalemia can be associated with life-threatening cardiac dysrhythmias [9]. In most, these arrythmias occur with potassium elevations > 6.5 mmol/l; however, some patients can develop arrythmias at lower levels. In contrast, patients with chronic hyperkalemia (e.g., dialysis patients) may remain asymptomatic despite marked elevations in potassium [9, 30, 31]. This is hypothesized to be due to compensatory mechanisms resulting in intracellular potassium storage [11]. In addition to the serum level, the rate of increase also plays a role in determining whether or not a patient will experience symptoms [9]. Other symptoms that can be seen include palpitations, nausea, muscle pain, weakness, paralysis or paresthesia [32, 33]. Chronic hyperkalemia has also been associated with other clinical entities including kidney disease, renal tubular acidosis and peripheral neuropathy [21].
For both the patient and the healthcare system, the burden of hyperkalemia is evident. Patients with hyperkalemia experience a higher frequency of emergency department visits, hospitalizations or even intensive care unit admissions, which in turn amount to a large financial strain [1, 2, 34, 35]. These effects appear to be compounded when hyperkalemia develops in individuals with underlying comorbid conditions [2, 4, 5]. In a large observational study, the predicted risk of all-cause mortality was higher for each 0.5 mmol/l increase in potassium above 4.5 mmol/l. However, the probability of death at each level of potassium was even higher when patients concurrently had diabetes, heart failure or CKD. Specifically, the adjusted all-cause mortality for individuals with hyperkalemia was 7.6-fold higher in those with all three comorbidities and 3.3-, 3.5- and 1.6-fold higher in those with heart failure, chronic kidney disease and diabetes, respectively [5]. While this mortality risk may not all be attributed to hyperkalemia itself, it emphasizes that those with comorbid conditions may require even closer monitoring in the face of hyperkalemia [8].
A Tale of Two Risks
The morbidity and mortality risk associated with hyperkalemia is well reported, even if hyperkalemia results from the initiation of disease-optimizing treatments. For example, in a post-marketing analysis of the RALES Trial there was a substantial increase in spironolactone prescriptions shortly after publication. Unfortunately, this was accompanied by an increase in hospitalizations for hyperkalemia (from 2.4 to 11.0 per 1000 patient years) and mortality (from 0.3 to 2.0 per 1000 patient years) [24, 25]. Many practitioners choose to decrease or discontinue maximal RAASi when faced with hyperkalemia, and it has been shown that this occurs in as high as 47% of cases after moderate-to-severe hyperkalemic events and in 38% of cases after mild hyperkalemic events [36]. While intuitive, this needs to be balanced with the fact that discontinuation of these medications with proven morbidity and mortality benefits may have downstream consequences. One observational study by Epstein et al. following patients with heart failure, CKD and diabetes in whom RAASi was either decreased or discontinued found a significant increase in cardiorenal morbidity and mortality when compared to those who were maintained on maximal RAASi therapy [36]. In fact, looking strictly at mortality, submaximal dosing or discontinuation of RAASi was associated with a twofold increase in death across the total study population [36]. These findings are echoed by two other observational studies, which found a significantly higher mortality when RAASi were discontinued in the advanced CKD population [37, 38]. In another study of incident hyperkalemia during heart failure hospitalizations, a similar risk of discontinuation was observed. In addition, incident hyperkalemia was more commonly observed in patients using MRAs before admission but was not associated with poor outcomes. In contrast, dose reduction of MRAs during hospitalization was associated with a 73% increase in the adjusted relative mortality hazard in the first 180 days post-admission [39].
Hyperkalemia Management
Frequency of Monitoring in the Chronic Setting
As previously mentioned, some patients are at higher risk of developing incident or recurrent hyperkalemia including those with CKD [40], diabetes or heart failure [41] and concomitant use of RAASi [42]. In addition, an initial episode of hyperkalemia (irrespective of the cause) is one of the strongest risk factors for recurrent hyperkalemia [12, 40]. Despite its high prevalence in certain disease states, there is a lack of consensus on how frequently one must monitor serum potassium in the setting of medications that may be associated with hyperkalemia including initiation or up-titration of RAASi or dose reductions in loop or thiazide diuretics (which are in turn associated with a lower risk of incident hyperkalemia) [41]. In these scenarios where patients are identified to be at higher risk, there is a need for more research to guide the frequency of monitoring and need for individualization of care [10, 12]. Generally, comorbidities such as CKD, heart failure and diabetes should prompt more frequent monitoring. Moreover, patients with a history of hyperkalemia should be monitored more closely, especially when increasing doses or reinitiating a previously held RAASi. For patients on RAASi therapy, the serum potassium should be measured prior to the initiation of these medications, within 1–2 weeks of dose initiation and within 1–2 weeks of any dose escalation or discontinuation [10, 43]. While guidelines for monitoring are limited, there are a few suggested approaches that one could consider (Table 2).
Management of Acute Hyperkalemia
At a serum potassium threshold of > 5.5 mmol/l, potassium can disrupt cellular conduction in musculoskeletal and cardiac tissue [5]. Treatment of acute hyperkalemia depends on a number of factors including absolute potassium value, chronicity, concomitant co-morbidity and the presence of hyperkalemia related end organ injury. Most guidelines agree that emergent treatment is warranted for potassium values > 6 mmol/l or > 5.5 mmol/l in the setting of ECG changes, oligoanuria, critical illness or a rapidly rising potassium level [7]. Goals of treatment include reversing conduction abnormalities, shifting potassium intracellularly, promoting potassium elimination and removing contributing factors. Patients with acute hyperkalemia should be examined for otherwise unexplained muscle weakness/paralysis, and an electrocardiogram should be completed to assess for hyperkalemia-related conduction abnormalities. If conduction abnormalities are present, intravenous calcium should be administered to stabilize the myocellular membrane. Calcium chloride and calcium gluconate are two common formulations of intravenous calcium but they should not be used interchangeably. Calcium chloride contains three-fold more elemental calcium than calcium gluconate for a given concentration and should be administered only through central venous access as it carries a higher risk of tissue necrosis should extravasation occur. Calcium gluconate can be given through a peripheral intravenous if needed but may still lead to tissue necrosis with extravasation. Regardless of which calcium formulation is used, ECGs should be repeat after administration to ensure resolution of cardiac conduction abnormalities. Additional doses of calcium may be required given its relatively short duration of effect.
Potassium shifting can rapidly lower serum levels through the movement of potassium from the extracellular to intracellular space. Insulin is the most commonly used shifting agent given its rapid onset, ease of administration and evidence-based effect [44]. Regular insulin is given through the intravenous route, typically as a 10-unit bolus, following administration of 25–50 g of dextrose (50–100 ml of dextrose 50%). The onset of effect occurs within 30–60 min and can last up to 6 h. Serum glucose levels should be trended for up to 6 h after administration of insulin since hypoglycemic episodes have been reported in 13–17% of such treatments [45, 46]. Serum potassium levels should be monitored as repeat insulin doses may be required for refractory hyperkalemia if serum potassium levels remain equal to or greater than the pre-shift value. Beta-2 adrenergic agonist can also be used to shift serum potassium [44, 47]. However, they should be used as an adjunct to intravenous insulin to maximize their potassium-lowering effect. Finally, the ability of sodium bicarbonate to lower potassium (acutely) is minimal and should only be considered as an adjunct to the aforementioned therapies in the setting of a concurrent metabolic acidosis [48].
Endogenous routes of potassium removal primarily involve the gastrointestinal tract and renal excretion. Gastrointestinal excretion can be promoted through the use of potassium-binding resins although the onset of effect is typically delayed; thus, current evidence does not support the use of potassium binders as a treatment for acute hyperkalemia to rapidly lower the potassium level [49]. Renal excretion involves the use of intravenous crystalloid solutions in the setting of hypovolemia and diuretics in the setting of hypervolemia. Eliminating potassium through these mechanisms should be considered sub-acute treatment as time to peak effect is hours. Most episodes of acute hyperkalemia can successfully be treated using a combination of potassium shifting and elimination; however, in situations where hyperkalemia persists despite optimal medical management, hemodialysis should be considered for potassium removal. Most commonly this occurs when hyperkalemia is accompanied by significant and persistent renal impairment.
A final consideration for treating acute hyperkalemia is to ensure exogenous potassium intake is reduced and contributory medications are temporarily discontinued. Exogenous sources of potassium include dietary intake, oral supplements and intravenous fluid additives. Medications related to hyperkalemia include, but are not limited to, renin angiotensin aldosterone system inhibitors, NSAIDs and trimethoprim. Although there is little guiding evidence, treatment algorithms for acute hyperkalemia focused on minimizing harm do exist. We recently proposed such an algorithm to better guide clinicians on how best to manage acute hyperkalemia in the inpatient setting (Fig. 1).
Management of Outpatient Hyperkalemia
Chronic management of hyperkalemia is focused on prevention of adverse hyperkalemic episodes (including those that are recurrent) by attempting to normalize potassium homeostasis [50]. Standard chronic management of hyperkalemia involves removal and/or management of contributors to hyperkalemia, increasing movement of potassium into cells and excretion. To address contributors to hyperkalemia, one must counsel on potassium-restricted diets (appreciating that data supporting this approach is lacking), carefully review any concomitant medications that cause hyperkalemia, potentially dose adjust RAASi (acknowledging that there are risks to doing so) and optimize the management of comorbid conditions, such as CKD and heart failure. To enhance intracellular movement of potassium, oral formulations of sodium bicarbonate can be used, while increasing potassium excretion involves the use of loop or thiazide diuretics and/or potassium binders.
Role of Dietary Restriction
It has been suggested that increased dietary intake of potassium contributes to hyperkalemia, and it may be an important consideration in people with risk factors such as kidney disease [51, 52]. Therefore, patients at risk of hyperkalemia are often recommended to adhere to a potassium restricted diet [53]. The 2004 Kidney Disease Outcomes Quality Initiatives (KDOQI) guidelines [54] recommended limiting potassium to approximately 51–102 mEq/day (2–4 g/day), while the more recent KDIGO 2012 Clinical Practice Guideline [55] recommended that individuals with CKD should receive expert dietary advice on potassium intake.
While it seems intuitive that patients should be counseled on a potassium-restricted diet to help mitigate hyperkalemia, the evidence surrounding potassium restriction is limited, and opinions towards the need for tight dietary restriction have shifted. Dietary liberalization of potassium in someone at risk of hyperkalemia is not universally associated with hyperkalemia or death [56]. In fact, in a prospective observational study, lower dietary potassium intake was associated with higher mortality risk in hemodialysis [57], although this may represent bias (i.e., lower consumption of potassium may be a marker of inadequate nutrition which in turn is associated with a higher mortality risk). While excessive intake of dietary potassium may be associated with hyperkalemia in some situations, there has been recent evidence suggesting that a diet rich in potassium may actually improve blood pressure and lower the risk of cardiovascular disease and stroke [52, 58]. With the introduction of new potassium-binding agents, studies are planned to evaluate whether dietary liberalization of potassium intake (in CKD) may be feasible [59]. In addition to the potential benefits of potassium liberalization, implementation and adherence to potassium-restricted diets is technically challenging and may be overly restrictive and costly. Furthermore, potassium-restricted diets may lead to the exclusion of foods that are otherwise beneficial for health [60]. For example, the Mediterranean diet, which largely focuses on healthy fats, whole grains and plant-based foods, has been associated with reduced cardiovascular disease but is typically associated with a higher potassium intake [61]. The Mediterranean diet may also be associated with a slowing of kidney function decline and improved survival among CKD patients [62]. Plant-based diets in general have shown promising outcomes in CKD [63], and more recent trials evaluating the feasibility of plant-based diets that are high in alkali (which may minimize extracellular shifting of potassium) and strategies to avoid potassium restriction will be important in ascertaining the true risk/benefit ratio of a potassium restricted diet [64]. A recent review emphasized other benefits of plant-based diets that may help with potassium removal including increased fibre content to improve gastrointestinal motility [65]. In addition, it has been shown that the bioavailability of potassium in whole fruits is lower than that of fruit juices. Therefore, a plant-based diet containing whole fruits may lead to relatively less potassium absorption compared with one made of predominantly fruit juice [66]. Finally, some studies have pointed out the importance of potassium kinetics in this conversation; dietary potassium intake does not necessarily associate with plasma potassium concentration [60]. Once again, individualization appears to be the best approach. A recent review highlighted the approach of personalized meal planning and nutritional counseling to account for the fact that some patients experience transient elevations in potassium and post-prandial hyperkalemia [60]. Current literature is limited, and there is a need for more studies, specifically clinical trials, addressing the question of potassium-restricted diets and outcomes.
Metabolic Acidosis
Patients with reduced kidney function are at risk of developing persistent metabolic acidosis, defined as serum bicarbonate < 20–22 mEq/l. Metabolic acidosis causes hyperkalemia through potassium and hydrogen ion exchange. In turn, hyperkalemia can lead to metabolic acidosis through impacts on renal ammonia levels [67]. This cycle leads to deleterious effects which may be treated with therapies such as sodium bicarbonate or sodium citrate [67]. While beneficial in kidney disease for slowing the loss of kidney function, correction of acidosis with oral bicarbonate may require high pill burden and requires careful candidate selection [68]. Furthermore, in patients with heart failure, sodium bicarbonate may precipitate extracellular fluid overload. Most of these patients were excluded from clinical trials of sodium bicarbonate for metabolic acidosis in CKD. Finally, it has been shown that a persistently high bicarbonate level may be associated with death and heart failure events in those with pre-existing kidney disease [69].
In addition to potential risks, the impact on potassium achieved through correcting acidosis with oral sodium bicarbonate may be small. A recent trial [70] that evaluated sodium bicarbonate (0.4 mEq per kg of ideal body weight per day) versus placebo in patients with later stage CKD demonstrated a decrease in serum potassium levels by only 0.1 mEq/l for treated patients, although the risk of a patient having a level > 5.0 mEq/l was lower in treated patients (OR 0.35, 95% CI 0.17–0.74). Nevertheless, other studies have shown that correcting acidosis leads to a lowering of serum potassium, albeit to varying degrees [71, 72]. Thus, in the absence of side effects, and if there may be other benefits that are valued, correction of acidosis may be a therapeutic option.
Medication review
Drug-induced hyperkalemia is considered to account for a large proportion of hyperkalemia [73], and beyond RAASi, many medications may contribute [74, 75]. Therefore, it is critical to conduct a review of a patient’s medications, carefully weighing the risks and benefits and evaluating alternatives where possible.
Loop and Thiazide Diuretics
Diuretics are frequently used in the acute management of hyperkalemia to facilitate potassium excretion. However, they can also be used in the chronic management, especially when already part of the routine care of a patient (for example, heart failure or CKD with fluid overload). The most frequently used diuretics for patients at risk of hyperkalemia are loop and thiazide diuretics as they both cause a relative net lowering in serum potassium [76]. Thiazide diuretics are not recognized as having the same degree of potassium lowering as loop diuretics because of their site of action (namely the distal convoluted tubule at which only a small proportion of filtered sodium is absorbed). However, in a recent trial of chlorthalidone to treat hypertension in patients with stage 4 chronic kidney disease, hypokalemia occurred in 10% versus 0% of those randomized to chlorthalidone [77]. In observational studies, there has been an association between use of non-potassium sparing diuretics and lower potassium levels (on a population level) [41]. While diuretics may be effective at lowering potassium, their efficacy depends on renal function and duration of action as they need to reach the loop of Henle or the distal convoluted tubule to exert their effect, and this may be limited in the setting of reduced GFR [68]. Furthermore, they do have important side effects to consider including volume contraction, worsening of renal function and an association with gout and diabetes (specifically thiazide diuretics) [3].
Sodium Glucose Cotransporter 2 Inhibitors (SGLT2i)
Mechanistically, SGLT2i result in increased tubular flow and overall improved kidney function, so they may protect against hyperkalemia [78]. In the recent FIDELIO-DKD trial, participants in the treatment group who concurrently used SGLT2i had lower serum potassium values; this suggests that SGLT2i may have an independent potassium lowering ability [78]. More recently, two metanalyses of trials evaluating SGLT2i in patients with diabetes mellitus type 2 or CKD found that SGLT2i reduced the risk of serious hyperkalemia [79, 80]. While yet to be explored in a trial with lower potassium as a primary outcome, these drugs may prove to be another tool for the clinician to modestly reduce hyperkalemic events in patients with diabetes, CKD (noting that most studies enrolled patients with an eGFR cutoff of ≥ 20–30 ml/min/1.73 m2) [81, 82] or heart failure, especially when otherwise clinically indicated.
Potassium Binders
Evidence of Efficacy
1. Older potassium binders
Although sodium polystyrene sulfonate (SPS) has been FDA approved since the late 1950s, trials addressing its efficacy as a serum potassium-reducing agent only became available in 2015 [83, 84]. In a single-center double-blind RCT comprising 33 patients with CKD, the use of SPS for an average duration of 6.9 days was associated with a greater serum potassium reduction than placebo (difference of − 1.04 mEq/l; P < 0.001) [84]. Despite this, the difference in the proportion of patients achieving normal serum potassium levels was not statistically significant (P = 0.07) [84]. In this trial, SPS dosing was fixed at 30 g orally daily. However, SPS is suggested to have a dose-dependent potassium-lowering property and can potentially be titrated to achieve normokalemia with doses reaching up to 60 g orally daily [85]. While SPS is effective, there are two main concerns with its widespread use. The first is related to the risk of gastrointestinal complications. In one study, severe gastrointestinal adverse events requiring emergency department visit or hospitalization among SPS users had an incidence rate of 22.97 per 1000 person-years versus 11.01 per 1000 person-years among non-users. However, the risk of colonic necrosis was not as high in another study of inpatients that identified an incidence of 0.14% among SPS users versus 0.07% among non-users (relative risk of 2.10, 95% CI 0.68–6.48) [86, 87]. It was previously thought that these events were related to SPS preparations containing sorbitol, but systematic reviews have shown similar gastrointestinal events in SPS without sorbitol [88]. The second concern is related to the sodium content. One study has shown that the use of SPS has been associated with edema and greater interdialytic weight gain [89]. Calcium polystyrene sulfonate (CPS) is a sodium-free alternative to SPS. Two trials have found no statistical difference in serum potassium reduction between SPS and CPS, and another has shown that CPS significantly decreases serum potassium when compared to placebo in participants undergoing hemodialysis [90,91,92]. While CPS may spare patients from a high sodium load, there is a concern that calcium, the ion replacing sodium in SPS, may increase vascular calcification [91]. While hypercalcemia as a result of CPS has been reported in one case series, none of the aforementioned trials have demonstrated an increase in serum calcium levels with its use [90,91,92,93]. Notably though, CPS has been linked to gastrointestinal ischemia in some case reports and case series [94,95,96]. Despite these concerns and risks, anecdotally, SPS or CPS has been used to lower potassium in the short term (i.e., over a few days) and long term (as chronic potassium lowering therapies in those with a tendency to hyperkalemia), especially in settings where kidney replacement therapies are not immediately available or as a bridge to maintain potassium while kidneys recover from acute kidney injury. In addition, novel potassium binders are not readily available in many countries. Nonetheless, considering the potential for risk and lack of robust studies demonstrating benefit, chronic use of SPS or CPS to support RAASi initiation or up titration is generally not advised and newer potassium binders (if available) are usually recommended instead [12, 97].
2. New potassium binders
Patiromer and sodium zirconium cyclosilicate (SZC) are newer potassium binders with different mechanisms and adverse events compared with SPS (Table 3) although no randomized trials have compared SPS to patiromer or SZC. These two agents have only recently been approved for use in Canada. Both agents have evidence supporting their dose-dependent potassium-lowering activity in various populations, specifically in those with CKD, diabetes, hypertension and taking RAASi [98,99,100,101,102,103,104]. Both agents also have safety data from trials lasting up to 1 year and are generally well tolerated [103, 105,106,107]. A summary of recent major trials evaluating the efficacy of these agents is noted in Table 4.
Patiromer was found to be effective when compared to placebo in the OPAL-HK trial [99]. In this trial, 76% of the 237 hyperkalemic participants (with a serum potassium at a day 3 scheduled visit) who were started on patiromer achieved normokalemia after 4 weeks. At the end of the 4th week, mean serum potassium values had decreased by 1.0 mmol/l from a mean baseline of 5.6 mmol/l. Of those who achieved normokalemia, 107 were then randomized to either maintenance with patiromer or placebo. Overall, 60% of the patients in the placebo group versus 15% in the patiromer group had at least one potassium of ≥ 5.5 after 8 weeks of follow-up, and the median change in potassium was 0.72 mmol/l in the placebo group versus 0 in the patiromer group [99]. As for SZC, the HARMONIZE trial [108] was similarly constructed with a 2-day open-label phase with a 28-day randomized phased. Of the participants in the first phase, 98% achieved normokalemia after 48 h. The baseline mean serum potassium was 5.6 mmol/l, and the absolute reduction was − 1.1 mmol/l at 48 h. Two hundred thirty-seven participants were then randomized to placebo or SZC at doses of 5 g, 10 g and 15 g per day. The mean baseline serum potassium values were 4.6 mmol/l, 4.5 mmol/l, 4.4 mmol/l and 4.5 mmol/l, respectively, and the mean serum potassium values from day 8 to 29 were 5.1 mmol/l, 4.8 mmol/l, 4.5 mmol/l and 4.4. mmol/l, respectively (P < 0.01) [108].
Studies evaluating the use of binders with RAASi
Appreciating the benefits of maintaining patients on RAASi while avoiding the morbidity and mortality of hyperkalemia, there have been a number of studies evaluating combination therapy with RAASi and novel potassium binders. The four largest trials studying patiromer, (AMETHYST-DN [105], AMBER [100], DIAMOND [104] and the aforementioned OPAL-HK [99]) were designed to exclusively include participants who were taking a RAASi. The first, AMETHYST-DN, was an open-label trial of 306 patients evaluating the long-term use of patiromer in those with diabetes and CKD taking one or more RAASi agent. Mean serum potassium values at baseline were 5.3 mmol/l, and the mean reduction from baseline after initiation of patiromer was between 0.35 and 0.97 mmol/l (depending on the dose of patiromer and baseline hyperkalemia severity). AMETHYST-DN showed a significant reduction in baseline serum potassium at every monthly time point with serum potassium values within the target range in 83.1% to 92.7% of those with mild hyperkalemia (> 5.0–5.5 mmol/l) at baseline and 77.4–95.1% of those with moderate hyperkalemia (> 5.5–6.0 mmol/l) at baseline [105].
In the ZS-002 trial, 90 participants with CKD and hyperkalemia were randomized to placebo or to SZC at doses of 0.3 g, 3 g or 10 g three times daily. Two days later, serum potassium in the 10 g RAASi subgroup (n = 20) had dropped by 1.1 mmol/l from a baseline of 5.1 mmol/l (P < 0.001) [102]. In the larger, open-label trial, ZS-005, hyperkalemic individuals (mean baseline serum potassium 5.6 mmol/l) were subject to a treatment phase followed by a maintenance phase (baseline serum potassium 4.8 mmol/l). Fourteen percent of the 263 RAASi-naïve participants who were hyperkalemic at baseline were able to, over a 12-month period, initiate RAASi therapy after starting SZC. As for the 483 participants who were already on RAASi therapy, 74% maintained their dose [103].
Studies evaluating the use of binders with potassium sparing diuretics
The use of patiromer as an enabler for potassium-sparing diuretics has been studied in three large trials. In the AMBER trial (which sought to evaluate the efficacy of patiromer as an enabler for K-sparing diuretics in those with resistant hypertension) [100], use of patiromer allowed for 86% of participants taking an ACEi or an ARB to remain on spironolactone without a hyperkalemic event compared to 66% in the placebo arm for the duration of the 12-week trial [100]. In one of the first patiromer trials, PEARL-HF, after starting spironolactone, serum potassium values increased by a mean of 0.23 mmol/l in the placebo group and decreased by 0.22 mmol/l in the patiromer group. In the patiromer group, spironolactone doses could be increased from 25 mg daily to 50 mg daily without hyperkalemic event in 91% of participants with heart failure compared to 74% in the placebo arm [98, 137]. The recently published DIAMOND study revealed similar findings in the heart failure population. Patiromer was shown to reduce the occurrence of hyperkalemia (> 5.5 mmol/l) and MRA dose reductions by slightly more than one third over a median follow-up period of 27 weeks [104].
Although SZC has had fewer trials evaluating its use specifically in populations taking potassium-sparing diuretics, it appears the use of SZC for optimizing RAASi is intended for future study [109].
Other clinical scenarios
In maintenance hemodialysis patients, 51.5% of participants in the SZC arm of the DIALIZE study had pre-dialysis serum potassium levels between 3.5 and 5.5 mmol/l in at least four long interdialytic intervals compared with only 5.1% of those in the placebo arm [110]. Despite this, comparable proportions of patients in both groups required potassium-lowering rescue therapies (2.1% and 5.1% in the SZC and placebo groups, respectively) [110]. A small study suggested that patiromer may decrease serum potassium levels in hemodialysis patients, but these findings were limited by a sample size of six and the absence of a placebo arm [111]. Finally, both patiromer and SZC have had trials evaluating their use in the emergency department for the acute treatment of hyperkalemia; while potentially beneficial in a short-term setting (the patiromer group had a significant mean reduction of serum K by 0.52 mmol/l compared to 0.17 mmol/l with placebo at the 2-h time point and the SZC group had a 0.72 mmol/l reduction compared to 0.36 mmol/l in the placebo group), neither showed a significant reduction in serum potassium values at their study primary endpoints of 6 h and 4 h for patiromer and SZC, respectively [112, 113].
Side Effects
As with SPS, SZC is a sodium-based potassium binder and thus has the potential of causing peripheral edema. Two studies found that edema was reported by participants in a dose-dependent fashion and became more significant at SZC doses > 10 g/day [103, 108]. Patiromer is not a sodium-based binder and therefore not believed to be associated with this same side effect. However, patiromer is less specific regarding its affinity and also binds magnesium. Serum magnesium levels were significantly lower in one study; however, the mean magnesium levels for both arms remained within normal range, and there were no clinically significant arrhythmias associated with hypomagnesemia [98].
Upcoming Trials
1. Novel potassium binders
While the new potassium binders have robust data supporting their potassium-lowering ability, the major limitation has been a lack of data on the impact of novel potassium binders on hard outcomes including death or hospitalization. In fact, out of all the placebo-controlled trials reported above, only three deaths and three hospitalizations were reported [114]. It is hoped that newer trials will help to better address this limitation. The recently published DIAMOND trial evaluating patiromer-enabled optimization of RAASi therapy was initially designed with cardiovascular death or cardiovascular hospitalization as primary outcomes. Unfortunately, due to logistic challenges associated with the COVID-19 pandemic, the primary outcome was changed to the difference in serum potassium compared to baseline [115]. Specific to SZC, DIALIZE-Outcome, an upcoming placebo-controlled trial, is currently recruiting participants on maintenance hemodialysis to evaluate whether SZC decreases the risk of sudden cardiac death, strokes and arrhythmia-related hospitalizations [115]. It will be the first trial evaluating mortality and patient-centered outcomes with the use of the novel potassium binders. In addition to hard outcomes, evaluating the role of novel binders in optimization of therapy is another area of focus. In this regard, the LIFT study is aimed at evaluating the effect of SZC on facilitating up titration of both RAASi and MRA while maintaining normokalemia in participants with heart failure [117].
In addition to future studies evaluating the role of binders on different outcomes, upcoming trials for both drugs are looking at broader populations including pediatric patients and participants on calcineurin inhibitors for dose selection and safety studies [118,119,120,121,122]. Both drugs will also be studied as enablers for potassium-liberalized diets [59, 123]. Trials evaluating patiromer use in end-stage kidney disease are currently recruiting as are others looking at use of SZC after hyperkalemia hospitalization and in participants who have undergone a parathyroidectomy [124,125,126]. It is hoped that the results from these studies may support the use of novel potassium-binding agents more broadly.
2. Thiazide-like Diuretics
While not typically used for the purposes of treating hyperkalemia, the CLICK trial published in 2021 that evaluated the use of thiazide-like diuretics in CKD demonstrated a higher incidence of hypokalemic events compared to placebo (10% vs 0%) [77]. The SPICE-PILOT trial, another study by Agarwal, is currently recruiting participants to assess whether chlorthalidone’s hypokalemic properties will enable higher doses of spironolactone to be used in participants with resistant hypertension [127].
3. Dietary intake of potassium and safety
The extent to which dietary potassium plays a role in serum potassium levels remains to be fully understood. One upcoming trial, DK-LIB, a randomized-crossover study, will randomize participants with CKD to either restricted (< 2000 mg) or liberalized (3500 mg) potassium diets and follow changes in serum potassium [128]. This is one of only few trials exploring this question. This study may provide the groundwork for the upcoming studies involving the use of new potassium binders to enable liberalized dietary potassium diets.
Putting It All Together—a Management Approach for Chronic Hyperkalemia in the Setting of RAASi Use
How might one incorporate the principles of diagnosis, monitoring and therapy into clinical practice—especially when treating a patient with RAASi? Generally speaking, RAASi should not be initiated unless the serum potassium is ≤ 5.0 mmol/l. For those already receiving a RAASi, drawing on existing recommendations, we propose a simple approach that may be helpful to consider when faced with an individual with hyperkalemia (Fig. 2). Such an approach considers the risks and benefits of treating hyperkalemia, balanced with the potential risks of discontinuing disease-modifying therapy. This approach also considers the use of novel medications (i.e., potassium binders), recognizing that these therapies may not be available in all countries. The principles of this approach includes the following:
-
1.
Recognize the potential morbidity and mortality associated with hyperkalemia, especially for those with pre-existing risk factors.
-
2.
Be aware of conditions that place patients at a higher risk of developing hyperkalemia
-
3.
Classify hyperkalemia into risk thresholds to be better prepared for the potential consequences and to help guide when to intervene with therapy.
-
4.
Monitor patients who are at risk with serial blood work, especially those who are initiating or up-titrating RAASi.
-
5.
Address reversible causes of hyperkalemia (medications, dietary, correcting metabolic acidosis, non-potassium sparing diuretics when accompanied by volume overload) in all patients with hyperkalemia.
-
6.
Consider the use of novel potassium binders (if available, affordable, and tolerated) in eligible patients if hyperkalemia is moderate to severe; especially in those individuals who are candidates for initiation of a RAASi or who have not yet reached target dose.
-
7.
If hyperkalemia is moderate to severe, discontinue RAASi, but look to re-trialing them when clinically indicated and the potassium improves to safe range.
Conclusion
Hyperkalemia places patients at risk of morbidity and mortality. The principles of management include identifying those at risk, close monitoring and instituting therapy to lower potassium into a safe range while trying to ensure disease-modifying treatments that cause hyperkalemia (i.e., RAASi) can be safely utilized. While there is much to be learned, a number of future studies will help to better understand the consequences of hyperkalemia and how best to treat patients using evidence-based approaches.
Data availability
This narrative review utilized publically available data that has already been published and can be accessed through the reference list.
References
Kanda E, Kashihara N, Kohsaka S, Okami S, Yajima T. Clinical and economic burden of hyperkalemia: a nationwide hospital-based cohort study in Japan. Kidney Med Nov-Dec. 2020;2(6):742-752.e1. https://doi.org/10.1016/j.xkme.2020.09.003.
Hougen I, Leon SJ, Whitlock R, et al. Hyperkalemia and its association with mortality, cardiovascular events, hospitalizations, and intensive care unit admissions in a population-based retrospective cohort. Kidney Int Rep. 2021;6(5):1309–16. https://doi.org/10.1016/j.ekir.2021.02.038.
Fitch K, Woolley JM, Engel T, Blumen H. The clinical and economic burden of hyperkalemia on medicare and commercial payers. Am Health Drug Benefits. 2017;10(4):202–10.
Kovesdy CP, Matsushita K, Sang Y, et al. Serum potassium and adverse outcomes across the range of kidney function: a CKD Prognosis Consortium meta-analysis. Eur Heart J. 2018;39(17):1535–42. https://doi.org/10.1093/eurheartj/ehy100.
Collins AJ, Pitt B, Reaven N, et al. Association of serum potassium with all-cause mortality in patients with and without heart failure, chronic kidney disease, and/or diabetes. Am J Nephrol. 2017;46(3):213–21. https://doi.org/10.1159/000479802.
Trevisan M, de Deco P, Xu H, et al. Incidence, predictors and clinical management of hyperkalaemia in new users of mineralocorticoid receptor antagonists. Eur J Heart Fail. 2018;20(8):1217–26. https://doi.org/10.1002/ejhf.1199.
Clase CM, Carrero JJ, Ellison DH, et al. Potassium homeostasis and management of dyskalemia in kidney diseases: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2020;97(1):42–61. https://doi.org/10.1016/j.kint.2019.09.018.
Einhorn LM, Zhan M, Hsu VD, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009;169(12):1156–62. https://doi.org/10.1001/archinternmed.2009.132.
Simon LV, Hashmi MF, Farrell MW. Hyperkalemia. StatPearls. StatPearls Publishing. Copyright © 2022, StatPearls Publishing LLC.; 2022.
Palmer BF, Carrero JJ, Clegg DJ, et al. Clinical Management of Hyperkalemia. Mayo Clin Proc. 2021;96(3):744–62. https://doi.org/10.1016/j.mayocp.2020.06.014.
Morales E, Cravedi P, Manrique J. Management of chronic hyperkalemia in patients with chronic kidney disease: an old problem with news options. Front Med (Lausanne). 2021;8: 653634. https://doi.org/10.3389/fmed.2021.653634.
Weinstein J, Girard L-P, Lepage S, McKelvie RS, Tennankore K. Prevention and management of hyperkalemia in patients treated with renin–angiotensin–aldosterone system inhibitors. Can Med Assoc J. 2021;193(48):E1836–41. https://doi.org/10.1503/cmaj.210831.
Agarwal R, Afzalpurkar R, Fordtran JS. Pathophysiology of potassium absorption and secretion by the human intestine. Gastroenterology. 1994;107(2):548–71. https://doi.org/10.1016/0016-5085(94)90184-8.
Kononowa N, Dickenmann MJ, Kim MJ. Severe hyperkalemia following colon diversion surgery in a patient undergoing chronic hemodialysis: a case report. J Med Case Rep. 2013;7:207. https://doi.org/10.1186/1752-1947-7-207.
Saleh-Anaraki K, Jain A, Wilcox CS, Pourafshar N. Pseudohyperkalemia: three cases and a review of literature. Am J Med. 2022;135(7):e150–4. https://doi.org/10.1016/j.amjmed.2022.01.036.
Humphrey T, Davids MR, Chothia MY, Pecoits-Filho R, Pollock C, James G. How common is hyperkalaemia? A systematic review and meta-analysis of the prevalence and incidence of hyperkalaemia reported in observational studies. Clin Kidney J. 2022;15(4):727–37. https://doi.org/10.1093/ckj/sfab243.
Young JB, Dunlap ME, Pfeffer MA, et al. Mortality and morbidity reduction with Candesartan in patients with chronic heart failure and left ventricular systolic dysfunction: results of the CHARM low-left ventricular ejection fraction trials. Circulation. 2004;110(17):2618–26. https://doi.org/10.1161/01.Cir.0000146819.43235.A9.
Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325(5):293–302. https://doi.org/10.1056/nejm199108013250501.
Miao Y, Dobre D, Heerspink HJ, et al. Increased serum potassium affects renal outcomes: a post hoc analysis of the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) trial. Diabetologia. 2011;54(1):44–50. https://doi.org/10.1007/s00125-010-1922-6.
Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851–60. https://doi.org/10.1056/NEJMoa011303.
Hunter RW, Bailey MA. Hyperkalemia: pathophysiology, risk factors and consequences. Nephrol Dial Transplant. 2019;34(Suppl 3):iii2–11. https://doi.org/10.1093/ndt/gfz206.
Fried LF, Emanuele N, Zhang JH, et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369(20):1892–903. https://doi.org/10.1056/NEJMoa1303154.
Wetmore JB, Yan H, Horne L, Peng Y, Gilbertson DT. Risk of hyperkalemia from renin-angiotensin-aldosterone system inhibitors and factors associated with treatment discontinuities in a real-world population. Nephrol Dial Transplant. 2021;36(5):826–39. https://doi.org/10.1093/ndt/gfz263.
Juurlink DN, Mamdani MM, Lee DS, et al. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med. 2004;351(6):543–51. https://doi.org/10.1056/NEJMoa040135.
Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized aldactone evaluation study investigators. N Engl J Med. 1999;341(10):709–17. https://doi.org/10.1056/nejm199909023411001.
Craft J. Eplerenone (Inspra), a new aldosterone antagonist for the treatment of systemic hypertension and heart failure. Proc (Bayl Univ Med Cent). 2004;17(2):217–20. https://doi.org/10.1080/08998280.2004.11927973.
White WB, Carr AA, Krause S, Jordan R, Roniker B, Oigman W. Assessment of the novel selective aldosterone blocker eplerenone using ambulatory and clinical blood pressure in patients with systemic hypertension. Am J Cardiol. 2003;92(1):38–42. https://doi.org/10.1016/s0002-9149(03)00461-2.
Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348(14):1309–21. https://doi.org/10.1056/NEJMoa030207.
Agarwal R, Joseph A, Anker SD, et al. Hyperkalemia risk with finerenone: results from the FIDELIO-DKD Trial. J Am Soc Nephrol. 2022;33(1):225–37. https://doi.org/10.1681/asn.2021070942.
Karaboyas A, Zee J, Brunelli SM, et al. Dialysate potassium, serum potassium, mortality, and arrhythmia events in hemodialysis: results from the dialysis outcomes and practice patterns study (DOPPS). Am J Kidney Dis. 2017;69(2):266–77. https://doi.org/10.1053/j.ajkd.2016.09.015.
Brunelli SM, Du Mond C, Oestreicher N, Rakov V, Spiegel DM. Serum potassium and short-term clinical outcomes among hemodialysis patients: impact of the long interdialytic interval. Am J Kidney Dis. 2017;70(1):21–9. https://doi.org/10.1053/j.ajkd.2016.10.024.
Viera AJ, Wouk N. Potassium disorders: hypokalemia and hyperkalemia. Am Fam Physician. 2015;92(6):487–95.
Te Dorsthorst RPM, Hendrikse J, Vervoorn MT, van Weperen VYH, van der Heyden MAG. Review of case reports on hyperkalemia induced by dietary intake: not restricted to chronic kidney disease patients. Eur J Clin Nutr. 2019;73(1):38–45. https://doi.org/10.1038/s41430-018-0154-6.
Mu F, Betts KA, Woolley JM, et al. Prevalence and economic burden of hyperkalemia in the United States Medicare population. Curr Med Res Opin. 2020;36(8):1333–41. https://doi.org/10.1080/03007995.2020.1775072.
Betts KA, Woolley JM, Mu F, Xiang C, Tang W, Wu EQ. The cost of hyperkalemia in the United States. Kidney Int Rep. 2018;3(2):385–93. https://doi.org/10.1016/j.ekir.2017.11.003.
Epstein M, Reaven NL, Funk SE, McGaughey KJ, Oestreicher N, Knispel J. Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin-aldosterone system inhibitors. Am J Manag Care. 2015;21(11 Suppl):S212–20.
Fu EL, Evans M, Clase CM, et al. Stopping renin-angiotensin system inhibitors in patients with advanced CKD and risk of adverse outcomes: a nationwide study. J Am Soc Nephrol. 2021;32(2):424–35. https://doi.org/10.1681/asn.2020050682.
Walther CP, Winkelmayer WC, Richardson PA, Virani SS, Navaneethan SD. Renin-angiotensin system blocker discontinuation and adverse outcomes in chronic kidney disease. Nephrol Dial Transplant. 2021;36(10):1893–9. https://doi.org/10.1093/ndt/gfaa300.
Beusekamp JC, Tromp J, Cleland JGF, et al. Hyperkalemia and treatment with RAAS inhibitors during acute heart failure hospitalizations and their association with mortality. JACC Heart Fail. 2019;7(11):970–9. https://doi.org/10.1016/j.jchf.2019.07.010.
Adelborg K, Nicolaisen SK, Hasvold P, Palaka E, Pedersen L, Thomsen RW. Predictors for repeated hyperkalemia and potassium trajectories in high-risk patients—a population-based cohort study. PLoS ONE. 2019;14(6): e0218739. https://doi.org/10.1371/journal.pone.0218739.
Sriperumbuduri S, McArthur E, Hundemer GL, et al. Initial and recurrent hyperkalemia events in patients with CKD in Older adults: a population-based cohort study. Can J Kidney Health Dis. 2021;8:20543581211017410. https://doi.org/10.1177/20543581211017408.
Silva-Cardoso J, Brito D, Frazão JM, et al. Management of RAASi-associated hyperkalemia in patients with cardiovascular disease. Heart Fail Rev. 2021;26(4):891–6. https://doi.org/10.1007/s10741-020-10069-3.
Group KDIGOW. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Off J Int Soc Nephrol. 2013;3(1):1–150.
Allon M, Copkney C. Albuterol and insulin for treatment of hyperkalemia in hemodialysis patients. Kidney Int. 1990;38(5):869–72. https://doi.org/10.1038/ki.1990.284.
Apel J, Reutrakul S, Baldwin D. Hypoglycemia in the treatment of hyperkalemia with insulin in patients with end-stage renal disease. Clin Kidney J. 2014;7(3):248–50. https://doi.org/10.1093/ckj/sfu026.
Chothia MY, Humphrey T, Schoonees A, Chikte UME, Davids MR. Hypoglycaemia due to insulin therapy for the management of hyperkalaemia in hospitalised adults: a scoping review. PLoS ONE. 2022;17(5): e0268395. https://doi.org/10.1371/journal.pone.0268395.
Liou HH, Chiang SS, Wu SC, Yang WC, Huang TP. Intravenous infusion or nebulization of salbutamol for treatment of hyperkalemia in patients with chronic renal failure. Zhonghua Yi Xue Za Zhi (Taipei). 1994;53(5):276–81.
Blumberg A, Weidmann P, Ferrari P. Effect of prolonged bicarbonate administration on plasma potassium in terminal renal failure. Kidney Int. 1992;41(2):369–74. https://doi.org/10.1038/ki.1992.51.
Wong SWS, Zhang G, Norman P, Welihinda H, Wijeratne DT. Polysulfonate resins in hyperkalemia: a systematic review. Can J Kidney Health Dis. 2020;7:2054358120965838. https://doi.org/10.1177/2054358120965838.
Kovesdy CP. Management of hyperkalaemia in chronic kidney disease. Nat Rev Nephrol. 2014;10(11):653–62. https://doi.org/10.1038/nrneph.2014.168.
Simon LV, Hashmi MF, Farrell MW. Hyperkalemia. StatPearls Publishing LLC; 2022.
Clegg DJ, Headley SA, Germain MJ. Impact of dietary potassium restrictions in CKD on clinical outcomes: benefits of a plant-based diet. Kidney Med. 2020;2(4):476–87. https://doi.org/10.1016/j.xkme.2020.04.007.
Cupisti A, Kovesdy CP, D’Alessandro C, Kalantar-Zadeh K. Dietary approach to recurrent or chronic hyperkalaemia in patients with decreased kidney function. Nutrients. 2018. https://doi.org/10.3390/nu10030261.
Levey AS, Rocco MV, Anderson S, et al. K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43(5 SUPPL. 1):i-S290.
Levin A, Stevens PE, Bilous RW, et al. Kidney disease: improving global outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1):1–150.
Bernier-Jean A, Wong G, Saglimbene V, et al. Dietary potassium intake and all-cause mortality in adults treated with hemodialysis. Clin J Am Soc Nephrol. 2021;16(12):1851–61. https://doi.org/10.2215/cjn.08360621.
Narasaki Y, Okuda Y, Kalantar SS, et al. Dietary potassium intake and mortality in a prospective hemodialysis cohort. J Ren Nutr. 2021;31(4):411–20. https://doi.org/10.1053/j.jrn.2020.05.008.
Chiavaroli L, Viguiliouk E, Nishi SK, et al. DASH dietary pattern and cardiometabolic outcomes: an umbrella review of systematic reviews and meta-analyses. Nutrients. 2019. https://doi.org/10.3390/nu11020338.
Institutet K, AstraZeneca. Healthy Diet Rich in Potassium to Chronic Kidney Disease With Sodium Zirconium Cyclosilicate: A Feasibility Study. https://ClinicalTrials.gov/show/NCT04207203; 2020.
St-Jules DE, Fouque D. Etiology-based dietary approach for managing hyperkalemia in people with chronic kidney disease. Nutr Rev. 2022. https://doi.org/10.1093/nutrit/nuac026.
Chauveau P, Aparicio M, Bellizzi V, et al. Mediterranean diet as the diet of choice for patients with chronic kidney disease. Nephrol Dial Transplant. 2018;33(5):725–35. https://doi.org/10.1093/ndt/gfx085.
Smyth A, Griffin M, Yusuf S, et al. Diet and major renal outcomes: a prospective cohort study. The NIH-AARP diet and health study. J Ren Nutr. 2016;26(5):288–98. https://doi.org/10.1053/j.jrn.2016.01.016.
Joshi S, McMacken M, Kalantar-Zadeh K. Plant-based diets for kidney disease: a guide for clinicians. Am J Kidney Dis. 2021;77(2):287–96. https://doi.org/10.1053/j.ajkd.2020.10.003.
Dietary Potassium Liberalization in Pre-Dialysis Patients (DK-LIB). ClinicalTrialsgov Identifier: NCT05090865.
Babich JS, Kalantar-Zadeh K, Joshi S. Taking the kale out of hyperkalemia: plant foods and serum potassium in patients with kidney disease. J Ren Nutr. 2022. https://doi.org/10.1053/j.jrn.2022.01.013.
Naismith DJ, Braschi A. An investigation into the bioaccessibility of potassium in unprocessed fruits and vegetables. Int J Food Sci Nutr. 2008;59(5):438–50. https://doi.org/10.1080/09637480701690519.
Cook EE, Davis J, Israni R, et al. Prevalence of metabolic acidosis among patients with chronic kidney disease and hyperkalemia. Adv Ther. 2021;38(10):5238–52. https://doi.org/10.1007/s12325-021-01886-5.
Dunn JD, Benton WW, Orozco-Torrentera E, Adamson RT. The burden of hyperkalemia in patients with cardiovascular and renal disease. Am J Manag Care. 2015;21(15 Suppl):s307–15.
Dobre M, Yang W, Pan Q, et al. Persistent high serum bicarbonate and the risk of heart failure in patients with chronic kidney disease (CKD): a report from the Chronic Renal Insufficiency Cohort (CRIC) study. J Am Heart Assoc. 2015. https://doi.org/10.1161/jaha.114.001599.
Melamed ML, Horwitz EJ, Dobre MA, et al. Effects of sodium bicarbonate in CKD stages 3 and 4: a randomized, placebo-controlled, multicenter clinical trial. Am J Kidney Dis. 2020;75(2):225–34. https://doi.org/10.1053/j.ajkd.2019.07.016.
Abramowitz MK, Melamed ML, Bauer C, Raff AC, Hostetter TH. Effects of oral sodium bicarbonate in patients with CKD. Clin J Am Soc Nephrol. 2013;8(5):714–20. https://doi.org/10.2215/cjn.08340812.
de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol. 2009;20(9):2075–84. https://doi.org/10.1681/asn.2008111205.
Ben Salem C, Badreddine A, Fathallah N, Slim R, Hmouda H. Drug-induced hyperkalemia. Drug Saf. 2014;37(9):677–92. https://doi.org/10.1007/s40264-014-0196-1.
Rimmer JM, Horn JF, Gennari FJ. Hyperkalemia as a complication of drug therapy. Arch Intern Med. 1987;147(5):867–9. https://doi.org/10.1001/archinte.1987.00370050063011.
Perazella MA. Drug-induced hyperkalemia: old culprits and new offenders. The Am J Med. 2000;109(4):307–14. https://doi.org/10.1016/S0002-9343(00)00496-4.
Sica DA. Diuretic-related side effects: development and treatment. J Clin Hypertens (Greenwich). 2004;6(9):532–40. https://doi.org/10.1111/j.1524-6175.2004.03789.x.
Agarwal R, Sinha AD, Cramer AE, et al. Chlorthalidone for hypertension in advanced chronic kidney disease. N Engl J Med. 2021;385(27):2507–19. https://doi.org/10.1056/NEJMoa2110730.
Rossing P, Filippatos G, Agarwal R, et al. Finerenone in predominantly advanced CKD and type 2 diabetes with or without sodium-glucose cotransporter-2 inhibitor therapy. Kidney Int Rep. 2022;7(1):36–45. https://doi.org/10.1016/j.ekir.2021.10.008.
Neuen BL, Oshima M, Agarwal R, et al. Sodium-glucose cotransporter 2 inhibitors and risk of hyperkalemia in people with type 2 diabetes: a meta-analysis of individual participant data from randomized, controlled trials. Circulation. 2022;145(19):1460–70. https://doi.org/10.1161/circulationaha.121.057736.
Lo KB, Gul F, Ram P, et al. The effects of SGLT2 inhibitors on cardiovascular and renal outcomes in diabetic patients: a systematic review and meta-analysis. Cardiorenal Med. 2020;10(1):1–10. https://doi.org/10.1159/000503919.
Bailey CJ, Day C, Bellary S. Renal protection with SGLT2 inhibitors: effects in acute and chronic kidney disease. Curr Diab Rep. 2022;22(1):39–52. https://doi.org/10.1007/s11892-021-01442-z.
Herrington W, Wanner C, Green JB. Design, recruitment, and baseline characteristics of the EMPA-KIDNEY trial. Nephrol Dial Transplant. 2022;37(7):1317–29. https://doi.org/10.1093/ndt/gfac040.
Evans BM, Milne MD, Jones NC, Yellowlees H. Ion-exchange resins in the treatment of anuria. Lancet. 1953;265(6790):791–5. https://doi.org/10.1016/s0140-6736(53)90465-6.
Lepage L, Dufour AC, Doiron J, et al. Randomized clinical trial of sodium polystyrene sulfonate for the treatment of mild hyperkalemia in CKD. Clin J Am Soc Nephrol. 2015;10(12):2136–42. https://doi.org/10.2215/cjn.03640415.
Fordjour KN, Walton T, Doran JJ. Management of hyperkalemia in hospitalized patients. Am J Med Sci. 2014;347(2):93–100. https://doi.org/10.1097/MAJ.0b013e318279b105.
Noel JA, Bota SE, Petrcich W, et al. Risk of hospitalization for serious adverse gastrointestinal events associated with sodium polystyrene sulfonate use in patients of advanced age. JAMA Intern Med. 2019;179(8):1025–33. https://doi.org/10.1001/jamainternmed.2019.0631.
Watson MA, Baker TP, Nguyen A, et al. Association of prescription of oral sodium polystyrene sulfonate with sorbitol in an inpatient setting with colonic necrosis: a retrospective cohort study. Am J Kidney Dis. 2012;60(3):409–16. https://doi.org/10.1053/j.ajkd.2012.04.023.
Harel Z, Harel S, Shah PS, Wald R, Perl J, Bell CM. Gastrointestinal adverse events with sodium polystyrene sulfonate (Kayexalate) use: a systematic review. Am J Med. 2013;126(3):264.e9-24. https://doi.org/10.1016/j.amjmed.2012.08.016.
Jadoul M, Karaboyas A, Goodkin DA, et al. Potassium-binding resins: associations with serum chemistries and interdialytic weight gain in hemodialysis patients. Am J Nephrol. 2014;39(3):252–9. https://doi.org/10.1159/000360094.
Nasir K, Ahmad A. Treatment of hyperkalemia in patients with chronic kidney disease: a comparison of calcium polystyrene sulphonate and sodium polystyrene sulphonate. J Ayub Med Coll Abbottabad. 2014;26(4):455–8.
Nakayama Y, Ueda K, Yamagishi SI, et al. Compared effects of calcium and sodium polystyrene sulfonate on mineral and bone metabolism and volume overload in pre-dialysis patients with hyperkalemia. Clin Exp Nephrol. 2018;22(1):35–44. https://doi.org/10.1007/s10157-017-1412-y.
Wang J, Lv MM, Zach O, et al. Calcium-polystyrene sulfonate decreases inter-dialytic hyperkalemia in patients undergoing maintenance hemodialysis: a prospective, randomized, crossover study. Ther Apher Dial. 2018;22(6):609–16. https://doi.org/10.1111/1744-9987.12723.
Arroyo D, Panizo N, García de Vinuesa S, Goicoechea M, Verdalles U, Luño J. Hypercalcemia as a side effect of potassium binding agents. Nefrologia. 2012;32(5):655–8. https://doi.org/10.3265/Nefrologia.pre2012.Jun.11500.
Buraphat P, Niyomnaitham S, Pongpaibul A, Maneerattanaporn M. Calcium polystyrene sulfonate-induced gastrointestinal tract necrosis and perforation. Acta Gastroenterol Belg. 2019;82(4):542–3.
Ribeiro H, Pereira E, Banhudo A. Colonic Necrosis Induced by Calcium Polystyrene Sulfonate. GE Port J Gastroenterol. 2018;25(4):205–7. https://doi.org/10.1159/000481288.
Castillo-Cejas MD, de-Torres-Ramírez I, Alonso-Cotoner C. Colonic necrosis due to calcium polystyrene sulfonate (Kalimate) not suspended in sorbitol. Rev Esp Enferm Dig. 2013;105(4):232–4. https://doi.org/10.4321/s1130-01082013000400010.
Rosano GMC, Tamargo J, Kjeldsen KP, et al. Expert consensus document on the management of hyperkalaemia in patients with cardiovascular disease treated with renin angiotensin aldosterone system inhibitors: coordinated by the Working Group on Cardiovascular Pharmacotherapy of the European Society of Cardiology. Eu Heart J Cardiovasc Pharmacother. 2018;4(3):180–8. https://doi.org/10.1093/ehjcvp/pvy015.
Pitt B, Bakris GL, Bushinsky DA, et al. Effect of patiromer on reducing serum potassium and preventing recurrent hyperkalaemia in patients with heart failure and chronic kidney disease on RAAS inhibitors. Eur J Heart Fail. 2015;17(10):1057–65. https://doi.org/10.1002/ejhf.402.
Weir MR, Bakris GL, Bushinsky DA, et al. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med. 2015;372(3):211–21. https://doi.org/10.1056/NEJMoa1410853.
Agarwal R, Rossignol P, Romero A, et al. Patiromer versus placebo to enable spironolactone use in patients with resistant hypertension and chronic kidney disease (AMBER): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2019;394(10208):1540–50. https://doi.org/10.1016/s0140-6736(19)32135-x.
Packham DK, Rasmussen HS, Lavin PT, et al. Sodium zirconium cyclosilicate in hyperkalemia. N Engl J Med. 2015;372(3):222–31. https://doi.org/10.1056/NEJMoa1411487.
Ash SR, Singh B, Lavin PT, Stavros F, Rasmussen HS. A phase 2 study on the treatment of hyperkalemia in patients with chronic kidney disease suggests that the selective potassium trap, ZS-9, is safe and efficient. Kidney Int. 2015;88(2):404–11. https://doi.org/10.1038/ki.2014.382.
Spinowitz BS, Fishbane S, Pergola PE, et al. Sodium zirconium cyclosilicate among individuals with hyperkalemia: a 12-month phase 3 study. Clin J Am Soc Nephrol. 2019;14(6):798–809. https://doi.org/10.2215/cjn.12651018.
Butler J, Anker SD, Lund LH, et al. Patiromer for the management of hyperkalemia in heart failure with reduced ejection fraction: the DIAMOND trial. Eur Heart J. 2022. https://doi.org/10.1093/eurheartj/ehac401.
Bakris GL, Pitt B, Weir MR, et al. Effect of patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease: the AMETHYST-DN randomized clinical trial. JAMA. 2015;314(2):151–61. https://doi.org/10.1001/jama.2015.7446.
Roger SD, Spinowitz BS, Lerma EV, et al. Efficacy and safety of sodium zirconium cyclosilicate for treatment of hyperkalemia: an 11-month open-label extension of HARMONIZE. Am J Nephrol. 2019;50(6):473–80. https://doi.org/10.1159/000504078.
Kashihara N, Nishio T, Osonoi T, et al. Correction of serum potassium with sodium zirconium cyclosilicate in Japanese patients with hyperkalemia: a randomized, dose-response, phase 2/3 study. Clin Exp Nephrol. 2020;24(12):1144–53. https://doi.org/10.1007/s10157-020-01937-1.
Kosiborod M, Rasmussen HS, Lavin P, et al. Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days among outpatients with hyperkalemia: the HARMONIZE randomized clinical trial. JAMA. 2014;312(21):2223–33. https://doi.org/10.1001/jama.2014.15688.
CADTH Common Drug Reviews. CADTH Canadian Drug Expert Committee Recommendation: Sodium Zirconium Cyclosilicate (Lokelma—Astrazeneca Canada Inc): Indication: Hyperkalemia. Canadian Agency for Drugs and Technologies in Health Copyright © 2020 Canadian Agency for Drugs and Technologies in Health.; 2020.
Fishbane S, Ford M, Fukagawa M, et al. A phase 3b, randomized, double-blind, placebo-controlled study of sodium zirconium cyclosilicate for reducing the incidence of predialysis hyperkalemia. J Am Soc Nephrol. 2019;30(9):1723–33. https://doi.org/10.1681/asn.2019050450.
Bushinsky DA, Rossignol P, Spiegel DM, et al. Patiromer decreases serum potassium and phosphate levels in patients on hemodialysis. Am J Nephrol. 2016;44(5):404–10. https://doi.org/10.1159/000451067.
Rafique Z, Liu M, Staggers KA, Minard CG, Peacock WF. Patiromer for treatment of hyperkalemia in the emergency department: a pilot study. Acad Emerg Med. 2020;27(1):54–60. https://doi.org/10.1111/acem.13868.
Peacock WF, Rafique Z, Vishnevskiy K, et al. Emergency potassium normalization treatment including sodium zirconium cyclosilicate: a phase II, randomized, double-blind, placebo-controlled study (ENERGIZE). Acad Emerg Med. 2020;27(6):475–86. https://doi.org/10.1111/acem.13954.
Natale P, Palmer SC, Ruospo M, Saglimbene VM, Strippoli GF. Potassium binders for chronic hyperkalaemia in people with chronic kidney disease. Cochrane Database Syst Rev. 2020;6(6):Cd013165. https://doi.org/10.1002/14651858.CD013165.pub2.
Butler J, Anker SD, Siddiqi TJ, et al. Patiromer for the management of hyperkalaemia in patients receiving renin-angiotensin-aldosterone system inhibitors for heart failure: design and rationale of the DIAMOND trial. Eur J Heart Fail. 2022;24(1):230–8. https://doi.org/10.1002/ejhf.2386.
Effect of Sodium Zirconium Cyclosilicate on Arrythmia-related Cardiovascular Outcomes in Participants on Chronic Hemodialysis With Recurrent Hyperkalemia (DIALIZE-Outcomes). https://ClinicalTrials.gov/show/NCT04847232.
Murphy D, Ster IC, Kaski JC, Anderson L, Banerjee D. The LIFT trial: study protocol for a double-blind, randomised, placebo-controlled trial of K(+)-binder Lokelma for maximisation of RAAS inhibition in CKD patients with heart failure. BMC Nephrol. 2021;22(1):254. https://doi.org/10.1186/s12882-021-02439-2.
Hospital OU. Effects of patiromer on pharmacokinetics of immunosuppresive drugs in renal transplant recipients. https://ClinicalTrials.gov/show/NCT05029310; 2021.
AstraZeneca. An open-label study to assess safety and efficacy of szc in paediatric patients with hyperkalaemia. https://ClinicalTrials.gov/show/NCT03813407; 2019.
AstraZeneca, Parexel. Study to assess the effect of sodium zirconium cyclosilicate on the pharmacokinetics of tacrolimus and cyclosporin in healthy subjects. https://ClinicalTrials.gov/show/NCT04788641; 2021.
Vifor Pharma I, Pharma V. Pharmacodynamic & safety of Patiromer in children & adolescents (2-<18 Yrs) with chronic kidney disease and hyperkalemia. https://ClinicalTrials.gov/show/NCT03087058; 2017.
Institute TR, University WMCoC. Pharmacokinetic study of tacrolimus and mycophenolate mofetil in kidney transplant recipients with hyperkalemia receiving patiromer. https://ClinicalTrials.gov/show/NCT03229265; 2017.
Wadhwa N, Cancer NHdbaNY, Specialists B. Evaluation of increased fruits and vegetables consumption in chronic kidney disease. https://ClinicalTrials.gov/show/NCT05050110; 2020.
University D, Pharma V. Patiromer Efficacy to reduce episodic hyperkalemia in end stage renal disease patients. https://ClinicalTrials.gov/show/NCT03781089; 2019.
AstraZeneca. Continuing sodium zirconium cyclosilicate (SZC) after discharge study. https://ClinicalTrials.gov/show/NCT05347693; 2022.
Hospital GPPs. Sodium zirconium cyclosilicate lowers hyperkalemia after parathyroidectomy. https://ClinicalTrials.gov/show/NCT05382988; 2016.
Research IIfM. Spironolactone in CKD enabled by chlorthalidone: PILOT. https://ClinicalTrials.gov/show/NCT05222191; 2022.
Manitoba Uo, University D. Dietary Potassium Liberalization in Pre-Dialysis Patients. https://ClinicalTrials.gov/show/NCT05090865; 2022.
KDIGO. Clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2020;98(4s):S1-s115. https://doi.org/10.1016/j.kint.2020.06.019.
Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–200. https://doi.org/10.1093/eurheartj/ehw128.
Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):1810–52. https://doi.org/10.1161/CIR.0b013e31829e8807.
Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825–30. https://doi.org/10.7326/0003-4819-158-11-201306040-00007.
National Institute for Health and Care Excellence: Guidelines. Chronic kidney disease: assessment and management. National Institute for Health and Care Excellence (NICE). Copyright © NICE 2021.; 2021.
sanofi-aventis Canada Inc. Prescribing Information: Kayexalate. Sodium Polystyrene Sulfonate. Cation - Exchange Resin. sanofi-aventis Canada Inc. https://products.sanofi.ca/en/kayexalate.pdf. Accessed 10 May 2022.
Ltd. VFMCRP. Product Monograph: Veltassa. Patiromer Powder for Oral Suspension (Potassium Binder (ATC Code: V03AE09)). Vifor Fresenius Medical Care Renal Pharma Ltd. https://pdf.hres.ca/dpd_pm/00053458.PDF. Accessed 10 May 2022.
Inc. AC. Product Monograph: Lokelma. Sodium zirconium cyclosilicate powder for oral suspension. (Potassium Binder (ATC Code V03AE10)). ZS Pharma, Inc. https://www.astrazeneca.ca/content/dam/az-ca/downloads/productinformation/lokelma-product-monograph-en.pdf. Accessed 10 May 2022.
Pitt B, Anker SD, Bushinsky DA, Kitzman DW, Zannad F, Huang IZ. Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebo-controlled study in patients with chronic heart failure (the PEARL-HF) trial. Eur Heart J. 2011;32(7):820–8. https://doi.org/10.1093/eurheartj/ehq502.
Rossignol P, Williams B, Mayo MR, et al. Patiromer versus placebo to enable spironolactone use in patients with resistant hypertension and chronic kidney disease (AMBER): results in the pre-specified subgroup with heart failure. Eur J Heart Fail. 2020;22(8):1462–71. https://doi.org/10.1002/ejhf.1860.
Agarwal R, Rossignol P, Budden J, et al. Patiromer and spironolactone in resistant hypertension and advanced CKD: analysis of the randomized AMBER trial. Kidney360. 2021;2(3):425–34. https://doi.org/10.34067/kid.0006782020.
Anker SD, Kosiborod M, Zannad F, et al. Maintenance of serum potassium with sodium zirconium cyclosilicate (ZS-9) in heart failure patients: results from a phase 3 randomized, double-blind, placebo-controlled trial. Eur J Heart Fail. 2015;17(10):1050–6. https://doi.org/10.1002/ejhf.300.
Kashihara N, Yamasaki Y, Osonoi T, et al. A phase 3 multicenter open-label maintenance study to investigate the long-term safety of sodium zirconium cyclosilicate in Japanese subjects with hyperkalemia. Clin Exp Nephrol. 2021;25(2):140–9. https://doi.org/10.1007/s10157-020-01972-y.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Author Contributions
Karthik K. Tennankore conceived and designed the review. Jacob B. Michaud, Natasha L. Larivée, Keigan M. More, Jo-Anne Wilson, and Karthik K. Tennankore participated in data collection, draft manuscript preparation, and draft manuscript revision. The final manuscript has been approved by all authors.
Disclosures
Jacob B. Michaud, Natasha L. Larivée, Keigan M. More and Jo-Anne Wilson have nothing to disclose. Karthik K. Tennankore has done consultancy, advisory board and CME work for Otsuka, AstraZeneca, GSK, Bayer and Vifor and CME. He has received unrestricted investigator initiated grant funding from Otsuka for an unrelated project.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Larivée, N.L., Michaud, J.B., More, K.M. et al. Hyperkalemia: Prevalence, Predictors and Emerging Treatments. Cardiol Ther 12, 35–63 (2023). https://doi.org/10.1007/s40119-022-00289-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40119-022-00289-z