Abstract
Advances in coronary plaque imaging over the last few decades have led to an increased interest in the identification of novel high-risk plaque features that are associated with cardiovascular events. Existing practices focus on risk stratification and lipid monitoring for primary and secondary prevention of cardiac events, which is limited by a lack of assessment and treatment of vulnerable plaque. In this review, we summarize the multitude of studies that have identified plaque, haemodynamic and patient factors associated with risk of acute coronary syndrome. Future progress in multi-modal imaging strategies and in our understanding of high-risk plaque features could expand treatment options for coronary disease and improve patient outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Existing practices focus on risk stratification and lipid monitoring for primary and secondary prevention of cardiac events. |
Advances in coronary plaque imaging have led to an increased interest in the identification of high-risk plaque features that are associated with cardiovascular events. |
There is now a broad evidence base identifying plaque, haemodynamic and patient factors associated with coronary event risk. |
Future progress in multi-modal imaging strategies and in our understanding of high-risk plaque features could expand treatment options for coronary disease and improve patient outcomes. |
Introduction
Over the last three decades, there have been substantial improvements in the assessment and management of atherosclerotic coronary disease, resulting in a reduction in the incidence of cardiovascular (CV) events and improved outcomes. However, despite these improvements, CV events relating to coronary plaque remain the most common cause of morbidity and mortality, with an estimated 9 million annual deaths relating to ischaemic heart disease globally [1]. CV events largely relate to plaque rupture or erosion, and numerous high-risk plaque features associated with plaque rupture risk and the risk of CV events have been identified in an expanding body of evidence. Many of these advances have been driven by advances in non-invasive and invasive coronary imaging that have enabled improved assessment of plaque composition and rupture risk in large outcome-based studies. In this review, we detail recent advances in the understanding of high-risk plaque characteristics and causes of plaque rupture, in addition to summarizing existing and developing imaging approaches to assess coronary plaque rupture risk.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Definitions of High-Risk Plaque
High-risk plaque, also termed vulnerable plaque, can be defined according to risk of causing a subsequent CV event, usually by causing plaque rupture or erosion [2, 3]. High-risk plaque features therefore include any identifiable plaque feature that are associated with a higher risk of CV events in comparison to plaque without such features. Initial studies determining plaque risk were generally post-mortem assessments of patients suffering CV events that resulted in death, demonstrating several plaque morphologies, including thin-cap fibroatheromas, large lipid pools, microcalcifications and intraplaque haemorrhage [4]. In these studies, approximately 60% of CV events appeared to be caused by plaque rupture while 40% were caused by plaque erosion [5, 6]. In more recent years, studies assessing high-risk plaque have more commonly used either invasive or non-invasive plaque imaging to assess plaque characteristics and correlate these features with rates of lesion-specific CV events over 5–10 years. Post-mortem studies demonstrate that thin-cap fibroatheromas detected by intravascular ultrasound (IVUS) and optical coherence tomography (OCT) are surrogates for histopathological thin-cap fibroatheromas [7,8,9].
While the vulnerable, or high-risk, plaque is a logical paradigm, an emerging notion is that of the high-risk patient (or vulnerable patient), which does not limit the risk assessment to lesion-specific, high-risk features [3]. Plaque rupture or erosion risk, and therefore CV event risk, results from a combination of plaque morphology characteristics in addition to biomechanics, such as shear stress, and patient factors, such as inflammatory states [2]. Importantly, plaque vulnerability is not a static process, and up to three quarters of vulnerable plaques can lose features of vulnerability over time with appropriate optimal medical therapy [10]. Conversely, stable plaques may progress towards a morphologically more vulnerable plaque in a proportion of patients [11,12,13].
Mechanisms of High-Risk Plaque Formation
Atherosclerotic plaque formation is a progressive progress, beginning with endothelial dysfunction related to a range of pathogenic precipitants [14, 15]. This in turn allows lipoproteins to move into the increasingly permeable endothelium with the recruitment of inflammatory cells that ingest lipoprotein-cholesterol to form foam cells [16, 17]. Subsequent smooth muscle proliferation results in fibrous cap formation and the development of an established atherosclerotic plaque [16, 17]. These initial stages (intimal thickening, intimal xanthoma, thick-cap fibroatheroma) represent asymptomatic disease, but ongoing plaque stressors, such as local and systemic inflammation and shear stress, can lead to plaque instability [15, 18]. Unstable plaque includes the formation of thin-fibrous cap atheroma and calcified nodule formation, both of which are associated with rupture or erosion risk and coronary events, resulting in symptomatic disease [15, 18]. The final stage of atherosclerotic plaque progression is fibrocalcific plaque, or a stable stenosis [15].
Identifying High-Risk Plaque with Imaging
Imaging strategies that can assess the coronary vasculature and coronary plaque morphologies have improved substantially over the last two decades and can be categorized as non-invasive or invasive. The advantages and disadvantages of the various non-invasive and invasive coronary imaging modalities are shown in Table 1.
Non-invasive Imaging
Coronary computed tomography angiography (CCTA) is the most commonly used non-invasive anatomical coronary imaging modality and can quantify plaque burden and morphology. The advantages of CCTA include assessment of the entire coronary tree and moderate resolution to assess plaque composition, including differentiation of calcified, mixed and non-calcified plaque [18]. High-risk plaque features that can be reliably assessed with CCTA include the napkin ring sign, positive remodelling, spotty microcalcifications and low-attenuation plaque [19, 20]. CCTA can also be used to image peri-coronary adipose tissue and epicardial adipose tissue, both of which are associated with increased risk of coronary disease and CV events [21].
Positron emission tomography (PET), which uses gamma cameras to detect intravenously administered radiotracers such as 18F-fluorodeoxyglucose (18F-FDG), has not yet seen widespread use for plaque assessment outside of research studies so far. However, several studies have shown that plaque inflammation and calcification can be identified using a variety of positron-emitting radioligands, which are taken up by regions of high metabolic activity such as inflammation, including high-risk coronary plaque [22]. Of these radiotracers, the most evidence obtained to date is with 18F-sodium fluoride, which binds to coronary microcalcifications; the results indicate that this radiotracer may be useful in the future for identifying high-risk plaque [23, 24].
Magnetic resonance imaging (MRI) is currently limited by difficulties in reproducibility that are mostly related to limitations in spatial resolution and long-scan times [25]. MRI has been studied in other vascular territories, especially the carotid arteries, with the results demonstrating an increased risk of CV events and stroke with carotid thickening shown on the MRI scan [18]. Coronary MRI studies have demonstrated that thicker coronary walls are associated with increased CV risk [6]. However, improvements in the technical requirements for coronary MRI are required before this modality is likely to see greater application. Several molecular probes also show promise with MRI imaging, including elastin-specific gadolinium-based probes that target features of remodelling and plaque burden [26].
Invasive Imaging
Intravascular ultrasound (IVUS), which derives images from a piezoelectric transducer that produces sound waves, has formed the backbone of invasive coronary imaging since the late 1990s and has been used in numerous trials of treatments aimed at reducing CV events through plaque modification [14]. The advantages of IVUS include being the gold-standard for quantifying coronary plaque volumes and the availability of post-processing methods, such as virtual histology IVUS and integrated backscatter IVUS, both of which can characterize plaque as fibrofatty, fibrous, necrotic or calcium [27, 28].
OCT, which measures echo time delay of reflected low-coherence light, is the main invasive alternative to IVUS and has greater spatial resolution, making it the preferred invasive imaging modality to assess fibroatheroma cap thickness and quantify lipid content [29]. However, overall plaque volume quantification is limited by the reduced tissue penetrance of this imaging modality.
Near-infrared spectroscopy (NIRS) is used to measure plaque lipid content using near-infrared spectrum light absorbance [30]. Hybrid catheters using NIRS and IVUS or NIRS and OCT have been developed, but are currently limited largely to research settings [4]. Intracoronary imaging techniques in development include near-infrared fluorescence molecular imaging, which can detect inflammation, and intravascular photo-acoustics, which uses similarly technology to IVUS but provides more information on plaque composition [2].
High-Risk Plaque Features
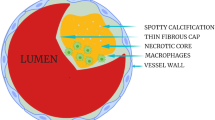
High-risk-atherosclerotic coronary plaque features leading to increased risk of CV events can broadly be categorized into (1) plaque characteristics, (2) flow dynamics and shear stress and (3) patient and systemic factors, such as inflammatory states (Fig. 1). These features and the best imaging modalities to assess them are summarized in the following section. High-risk plaque features assessed with CCTA are shown in Fig. 2. A comparison of estimated increase in CV event risk according to plaque feature is shown in Table 2.

Adapted from Yan et al. under the terms of the Creative Commons Attribution 4.0 licence [31]
High-risk plaque features on coronary computed tomography angiography.
Plaque Characteristics
Total Plaque Volumes
Total plaque volumes are representative of overall disease activity and have been associated with CV events in multiple outcome studies. Moreover, plaque may expand rapidly in the days and weeks before myocardial infarction [37]. Extensive plaque volume, as assessed by CCTA, without obstructive disease has been shown to increase the risk of CV events to a similar degree to obstructive but less extensive disease [38]. CCTA studies assessing the association between total plaque volumes have demonstrated that lesions causing acute coronary syndrome (ACS) have significantly higher total plaque volumes in comparison to non-culprit lesions within the same patient, and in comparison to patients with coronary disease without ACS [39, 40]. At the lesion level, lesion plaque volume is thought to rapidly expand in the 1- to 3-month period prior to a CV event, and several studies measuring plaque volume have demonstrated that plaque volume approximately doubles when measured more than 3 months prior to in comparison to at the time of the event [41]. In the PROSPECT study, which measured plaque volumes of 106 lesions prior to ACS using IVUS, interim plaque enlargement was associated with a fourfold higher risk of subsequent lesion-specific ACS [42]. Similarly, in the larger PROSPECT 2 study, which used IVUS and NIRS to assess non-culprit lesions in 898 patients following ACS presentation, large plaque burden (≥ 70% plaque burden) was associated with an unadjusted odds ratio (OR) of 11.4 for subsequent major adverse CV events (MACE) at 4 years [43]. In one of the longest studies to date, Halon et al. demonstrated plaque burden was associated with increased risk of MACE in 499 patients with diabetes mellitus over 9.2 years of follow-up (plaque volume upper vs lower quartile hazard ratio [HR] 6.9, 95% confidence interval [CI] 1.6–30.8) [44]. Lower mean luminal area measured by IVUS or OCT has also been used in some studies and is associated with increased risk of MACE [45].
Plaque Lipid Volume
Plaque lipid pool volume has been associated with CV event risk in multiple studies, likely being related to a high content of tissue factor in the lipid core increasing thrombogenic potential [46]. IVUS and NIRS have been the main imaging modalities used in these studies; however, CCTA quantification of low-attenuation plaque regions (defined as Hounsfield units [HU] < 30) is improving and is correlated with echo-attenuation on IVUS and with greater CV event risk [40, 47, 48]. In the CT arm of the ROMICAT-II trial of patients presenting with suspected ACS, low-attenuation plaque on CT was associated with index ACS (Relative risk 8.2, 95% CI 4.7–14.5) [49]. Motomoya et al. demonstrated that low-attenuation plaque measured by CCTA was associated with a higher risk of ACS during 27 months of follow-up in a cohort of 1059 patients [50]. In the ICONIC study, low-attenuation plaque was associated with increased risk of MACE during 3.4 years of follow-up (HR 1.38, 95% CI 1.05–1.81) [39]. Multiple studies using NIRS to assess lipid core content have demonstrated a clear correlation between lipid content and CV event risk, and a higher lipid content in ruptured plaques causing ST-elevation myocardial infarction (STEMI) [51,52,53,54]. In a study of 1271 patients with suspected ACS, non-culprit lesions with a 4-mm maximum lipid content burden index of > 400 HU were associated with subsequent ACS at 2 years with approximately double the risk at the patient level and fourfold the risk at the lesion level [55]. The larger PROSPECT 2 study used IVUS and NIRS in 898 patients (3629 non-culprit lesions) within 4 weeks of ACS, and demonstrated high lipid content (defined as 4 mm maximum lipid content burden index > 324.7 HU) was associated with increased risk of MACE over 4 years with an OR of 2.27 at the patient level and of 7.83 at the lesion level [43]. Echo-attenuated plaques detected by IVUS correlate relatively well with fibroatheroma or necrotic core detected on NIRS and are also a marker of plaque vulnerability, but limitations in quantification and distribution have led to using IVUS in combination with NIRS in research studies where lipid quantification is needed [56]. In the CLIMA study, an OCT study enrolling 1003 patients with untreated proximal left anterior descending artery lesions, lipid arc circumferential extension > 180° measured by OCT was associated with increased risk of MACE at 1 year (HR 2.4, 95% CI 1.2–4.8)[45]
Calcification and Microcalcifications
The presence and quantity of coronary calcification is commonly used as a high-risk plaque feature to guide clinical practice, and may influence rupture risk through modulation of fibrous cap thickness, necrotic core volume or inflammatory state [37]. The ‘power of zero’ had been proposed as a clinical rule to guide decisions regarding primary prevention for coronary disease, but recent data have questioned this approach to aspirin and statin management decisions [57]. Microcalcifications identified on CCTA, also termed spotty calcification, are an early sign of atherosclerosis and can be representative of active inflammation. Spotty calcification on CCTA was found to be predictive of ACS in the CT arm of the ROMICAT-II trial of patients presenting with chest pain (Relative risk [RR] 37.2, 95% CI 9.1–152.7) [49]. Outcome-based studies have demonstrated an association between microcalcification and risk of CV events; therefore, microcalcifications can be considered a high-risk plaque feature [40, 58]. In the ICONIC study, the presence of spotty calcification was associated with greater risk of ACS at 3.4 years (HR 1.54, 95% CI 1.17–2.04) [39]
Conversely, plaque calcification can increase following treatment with cholesterol lowering agents, and an inverse relationship between low-density lipoprotein-cholesterol (LDL-C) and plaque calcification has been observed, suggesting this process represents a more stable plaque phenotype [59, 60]. Similarly, in a substudy of the PARADIGM trial, although calcified plaque volume was associated with increased risk of MACE over 4 years (HR 3.01, 95% CI 1.58–5.72), percent calcified plaque volume (calcified plaque volume divided by total plaque volume) was inversely associated with subsequent MACE (HR 0.53, 95% CI 0.29–0.97) [61].
Thin-cap Fibroatheroma
Thin-cap fibroatheroma, which can lead to defects in the fibrous cap that result in exposure of the thrombogenic lipid core to circulating blood, is associated with an increased risk of CV events [37]. Fibroatheroma cap thickness can be assessed using several imaging modalities, but given its higher resolution, OCT is the optimal imaging modality to assess cap thickness. The COMBINE OCT-FFR trial included diabetic patients with fractional flow reserve (FFR)-negative lesions. At 18 months, substantially higher rates of MACE were demonstrated in patients with lesions with thin-cap fibroatheroma compared to those without (HR 5.12, 95% CI 2.12–12.34) [62]. Similarly, in the CLIMA study, fibrous cap thickness of < 75 µm for untreated left anterior descending artery lesions was associated with increased risk of MACE at 1 year (HR 4.7, 95% CI 2.4–9.0) [45].
An extension of this is the CCTA napkin-ring sign, which is a low attenuation plaque core near the lumen with a high-enhancing rim, a feature representative of lipid content and associated with thin-cap fibroatheroma on OCT. Several prospective studies have identified a strong association between the presence of the napkin-ring sign on CCTA and subsequent lesion-specific MACE [20, 63,64,65]. A meta-analysis assessing the association between high-risk plaque features on CCTA and future MACE estimated that the presence of the napkin-ring sign was associated with a HR of 5.06 (95% CI 3.23–7.94) [48].
Other Features
Several other plaque features are associated with high plaque risk. In the CLIMA OCT study, OCT-defined macrophages were associated with an increased risk of MACE at 1 year (HR 2.7, 95% CI 1.2–6.1) [45]. In a PET study of patients with and without ACS, 18F-NaF uptake was significantly higher in culprit coronary lesions compared to non-culprit lesions, and correlated with active calcification and macrophage infiltration [66]. Positive remodelling, defined as a remodelling index > 1.1, as determined by CCTA, is associated with an increased risk of subsequent coronary events [40, 49].
Flow Dynamics and Shear Stress
Endothelial dysfunction relating to low shear stress is thought to be the initiating event in plaque development [67, 68]. Shear stress leads to a number of cellular signalling pathways, including reduced synthesis of nitric oxide, a molecule which has a protective role for atherosclerosis through preventing inflammation, apoptosis, thrombosis and endothelial permeability [67]. Low endothelial shear stress is associated with plaque oxidative stress, extracellular matrix turnover, arterial remodelling, intra-plaque bleeding and endothelial erosion [67]. Several studies have demonstrated more rapid plaque development in the setting of low endothelial shear stress [69], which subsequently is associated with CV events. In the PROSPECT study, local low endothelial shear stress (< 1.3 Pa) was associated with subsequent MACE over 3.4 years of follow-up (HR 4.34, 95% CI 1.89–10.0) [70].
Several haemodynamic factors are thought to play a role in plaque erosion as a separate process to plaque rupture. Plaque erosion has been demonstrated to occur more commonly near bifurcations, particularly in the left anterior descending artery in a large IVUS study of patients with STEMI [71]. This is thought to relate to the impacts of bifurcations on shear stress within the main vessel, and disturbed blood flow is associated with chronic endothelial activation and endothelial denudation and subsequent thrombosis [72, 73].
Inflammation and Systemic Features
Both local and systemic inflammation are associated with increased CV event risk [40, 74]. Locally, lipid accumulation leads to free radical formation, endothelial activation and inflammation, all resulting in an imbalance in reactive oxygen species, which transform monocytes into foam cells [75]. This process involves multiple cell types, both intracellular and extracellular reactive oxygen and nitrogen species and complex signalling pathways, including the NLRP3 inflammasome pathway, the Wnt and Notch pathways, Toll-like receptors and proprotein convertase subtilisin/kexin type 9 [75, 76]. Systemic biomarkers of inflammation, such as high-sensitivity C-reactive protein and interleukin-6, are both associated with risk of ACS [77]. Acute inflammatory conditions in addition to acute infections that trigger acute inflammation are both associated with increased risk of MACE [74, 78]. Other stressors, such as emotional distress and sleep deprivation, are associated with increased CV event risk, possibly via inflammatory processes. Similarly, ACS occurs more commonly in colder months and in the morning, findings which may related to a multitude of factors, including temperature changes, inflammatory state, air pollution and metabolic changes throughout the day, such as the cortisol circadian rhythm [79].
Pericardial fat volume and inflammation within pericardial fat, measured using the fat attenuation index, has been associated with CV and increased mortality [80,81,82]. Right coronary artery peri-coronary adipose tissue attenuation is associated with CV risk factors and significant coronary stenoses [83]. Epicardial adipose tissue secretes adipocytokines, leading to a pro-inflammatory state and progression of atherosclerotic disease [21, 84, 85]. Therefore, the presence of peri-coronary adipose tissue especially around the right coronary artery is suggested as a promising imaging biomarker to allow non-invasive assessment of coronary inflammation.
Treatments Targeting Plaque Risk
Pharmacological treatments aimed at reducing the risk of plaque rupture and CV events have improved substantially over the last few decades. First, statins, which inhibit HMG-CoA reductase, are the most widely used pharmacological treatment for atherosclerotic plaque, and their use results in LDL-C reductions of 30–50% [86]. Multiple studies have demonstrated consistent reductions in CV events with statin treatment; consequently, moderate- or high-dose statins are recommended for established coronary artery disease [14]. For high-risk plaque, multiple studies have demonstrated that statins stabilize plaque by reducing lipid core volumes and resulting thickening of the fibroatheroma cap. Second, PCSK-9 inhibitors have demonstrated similar improvements in reducing CV events and also improve plaque morphology, but are limited by higher costs [59, 87]. In the HUYGENS study, the addition of evolocumab to statin therapy following non-STEMI resulted in greater increases in minimum fibrous cap thickness, decreases in maximum lipid arc and macrophage index, and greater regression of plaque volumes over 12 months, as assessed by OCT [88]. Third, ezetimibe is similarly associated with improvements in lesion risk profile and, to a lesser degree, reductions in MACE [89].
Other treatments target inflammation to reduce CV events and include colchicine, canakinumab and aspirin. Aspirin, which irreversibly inhibits cyclooxygenase and suppresses prostaglandin production, thereby suppressing platelet aggregation, has previously been a mainstay of primary prevention for CV. However, recent data from randomized controlled trials suggest increased bleeding risks with minimal benefits, which has led to reconsideration of this approach [90]. In the CANTOS trial, anti-inflammatory therapy with canakinumab, a monoclonal antibody targeting interleukin-1B, led to significantly lower MACE compared to placebo [91]. Several trials have demonstrated reduced CV events with colchicine treatment following ACS [92, 93], and further studies assessing the impact of colchicine on coronary plaque features are underway.
Little data are available on the assessment of shear stress as a target of coronary treatments, but one study demonstrated greater rates of low endothelial shear stress with nebivolol versus atenolol treatment, which was associated with greater plaque progression [94]. Further studies into whether treatments targeting localized haemodynamics can reduce plaque vulnerability are warranted.
The identification of high-risk plaque has led to interest in whether prophylactic percutaneous coronary intervention, especially with bioresorbable scaffolds, may reduce subsequent MACE. The PROSPECT ABSORB trial randomized 182 patients with lesion plaque burden ≥ 65% on IVUS to bioresorbable scaffold or medical management, demonstrating improvements in the powered outcome of minimum lumen area at 4 years of follow-up, as well as favourable long-term clinical outcomes for the unpowered endpoint of lesion-related MACE (4.3% for bioresorbable scaffold patients vs 10.7% for medically managed patients; P = 0.12) [95]. The larger PREVENT trial, which is powered for outcomes, is ongoing.
Individualized Patient-Specific Approach for High-Risk Plaque
Given that high-risk plaque assessment and identification have been largely limited to research settings to date, it can be difficult to decide the best management approach for patients in whom high-risk plaque features are identified on coronary imaging. A major limitation for the clinical use of high-risk plaque features is the low positive predictive value of individual features on subsequent MACE risk, which are mostly in the vicinity of 5–30% [2]. Development of combined measures of risk using multiple high-risk plaque features, and potentially incorporating machine learning processes, might be useful to overcome this limitation.
High-risk features identified through CCTA are the most likely features to occur in routine clinical practice, and their presence should prompt a cautious approach to management. In the setting of high-risk plaque, patients should commence or continue on optimal medical therapy in addition to lifestyle changes, such as improved diet, exercise and smoking cessation. The potential benefits of up-titrating plaque-modifying treatments should be considered in the context of the clinical setting, including measures such as LDL-C levels. Similarly, invasive assessment should be considered in certain settings in line with existing ACS and stable coronary syndrome guidelines.
Future Directions
Advances over the last 2 decades in the ability to image coronary plaque and define high-risk features have been rapid and expansive, and this trend might be expected to continue. Improvements in spatial and temporal resolution and radiation doses for non-invasive imaging modalities are likely, leading to greater applicability of these technologies in coronary plaque assessment. Similarly, advanced computational modelling and machine learning approaches or hybrid imaging approaches could offer promise in improving the identification of high-risk plaque upon imaging. For example, a CCTA study using a machine learning-based profile of perivascular adipose tissue remodelling and adverse fibrosis was better able to predict CV events in comparison to existing processes [96]. Molecular imaging probes, including 18F-NaF, are improving, and several trials are ongoing that might be useful in identifying novel high-risk plaque features. These include the PREFFIR study, which aims to perform CCTA and PET with 18F-NaF on 700 patients with ACS. As these imaging strategies improve, the potential for coronary plaque imaging to be used as a screening tool also increases, for both patients with established coronary disease and in a population setting. Concurrently, treatments aimed at reducing CV events have also seen substantial developments, and greater understanding of high-risk plaque features that could be targeted by novel pharmacological therapies will encourage ongoing research and drug development in this area. Similarly, the PROSPECT 2 trial demonstrated promising results for prophylactic stenting for high plaque burden lesions, and the results of the PREVENT trial are eagerly awaited.
Conclusions
Advances in coronary plaque imaging over the last two decades have led to an increased interest in the identification of novel high-risk plaque features. Multiple studies have demonstrated that there are an array of plaque features that are associated with ACS risk in addition to patient factors and haemodynamic factors that also mediate risk. Advancements in imaging identification of high-risk plaque and treatment options for such features may lead to a new paradigm in CV care.
References
Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;141(9):e139–596.
Tomaniak M, Katagiri Y, Modolo R, et al. Vulnerable plaques and patients: state-of-the-art. Eur Heart J. 2020;41(31):2997–3004.
Arbab-Zadeh A, Fuster V. From detecting the vulnerable plaque to managing the vulnerable patient: JACC state-of-the-art review. J Am Coll Cardiol. 2019;74(12):1582–93.
Li J, Montarello NJ, Hoogendoorn A, et al. Multimodality intravascular imaging of high-risk coronary plaque. JACC Cardiovasc Imaging. 2022;15(1):145–59.
Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47(8 Suppl):C13–8.
Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20(5):1262–75.
Narula J, Nakano M, Virmani R, et al. Histopathologic characteristics of atherosclerotic coronary disease and implications of the findings for the invasive and noninvasive detection of vulnerable plaques. J Am Coll Cardiol. 2013;61(10):1041–51.
Jang IK, Tearney GJ, MacNeill B, et al. In vivo characterization of coronary atherosclerotic plaque by use of optical coherence tomography. Circulation. 2005;111(12):1551–5.
Yonetsu T, Kakuta T, Lee T, et al. In vivo critical fibrous cap thickness for rupture-prone coronary plaques assessed by optical coherence tomography. Eur Heart J. 2011;32(10):1251–9.
Räber L, Koskinas KC, Yamaji K, et al. Changes in coronary plaque composition in patients with acute myocardial infarction treated with high-intensity statin therapy (IBIS-4): a serial optical coherence tomography study. JACC Cardiovasc Imaging. 2019;12(8 Pt 1):1518–28.
Kubo T, Maehara A, Mintz GS, et al. The dynamic nature of coronary artery lesion morphology assessed by serial virtual histology intravascular ultrasound tissue characterization. J Am Coll Cardiol. 2010;55(15):1590–7.
Motreff P, Rioufol G, Finet G. Seventy-four-month follow-up of coronary vulnerable plaques by serial gray-scale intravascular ultrasound. Circulation. 2012;126(24):2878–9.
Uemura S, Ishigami K, Soeda T, et al. Thin-cap fibroatheroma and microchannel findings in optical coherence tomography correlate with subsequent progression of coronary atheromatous plaques. Eur Heart J. 2012;33(1):78–85.
Dawson LP, Lum M, Nerleker N, Nicholls SJ, Layland J. Coronary atherosclerotic plaque regression: JACC state-of-the-art review. J Am Coll Cardiol. 2022;79(1):66–82.
Stary HC, Chandler AB, Dinsmore RE, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1995;92(5):1355–74.
Badimon L, Vilahur G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J Intern Med. 2014;276(6):618–32.
Camaré C, Pucelle M, Nègre-Salvayre A, Salvayre R. Angiogenesis in the atherosclerotic plaque. Redox Biol. 2017;12:18–34.
Sandfort V, Lima JA, Bluemke DA. Noninvasive imaging of atherosclerotic plaque progression: status of coronary computed tomography angiography. Circ Cardiovasc Imaging. 2015;8(7):e003316.
Kiran Munnur R, Andrews J, Kataoka Y, et al. Serial coronary plaque assessment using computed tomography coronary angiography. Circ Cardiovasc Imaging. 2019;12(3):e008404.
Shaw LJ, Blankstein R, Bax JJ, et al. Society of Cardiovascular Computed Tomography/North American Society of Cardiovascular Imaging—expert consensus document on coronary CT imaging of atherosclerotic plaque. J Cardiovasc Comput Tomogr. 2021;15(2):93–109.
Goeller M, Achenbach S, Duncker H, Dey D, Marwan M. Imaging of the pericoronary adipose tissue (PCAT) using cardiac computed tomography: modern clinical implications. J Thorac Imaging. 2021;36(3):149–61.
Evans NR, Tarkin JM, Chowdhury MM, Warburton EA, Rudd JH. PET imaging of atherosclerotic disease: advancing plaque assessment from anatomy to pathophysiology. Curr Atheroscler Rep. 2016;18(6):30.
Robson PM, Dweck MR, Trivieri MG, et al. Coronary artery PET/MR imaging: feasibility, limitations, and solutions. JACC Cardiovasc Imaging. 2017;10(10 Pt A):1103–12.
Moss AJ, Doris MK, Andrews JPM, et al. Molecular coronary plaque imaging using (18)F-fluoride. Circ Cardiovasc Imaging. 2019;12(8):e008574.
Adamson PD, Newby DE. Non-invasive imaging of the coronary arteries. Eur Heart J. 2019;40(29):2444–54.
Reimann C, Brangsch J, Kaufmann JO, et al. Dual-probe molecular MRI for the in vivo characterization of atherosclerosis in a mouse model: simultaneous assessment of plaque inflammation and extracellular-matrix remodeling. Sci Rep. 2019;9(1):13827.
Garcia-Garcia HM, Costa MA, Serruys PW. Imaging of coronary atherosclerosis: intravascular ultrasound. Eur Heart J. 2010;31(20):2456–69.
Honda S, Kataoka Y, Kanaya T, Noguchi T, Ogawa H, Yasuda S. Characterization of coronary atherosclerosis by intravascular imaging modalities. Cardiovasc Diagn Ther. 2016;6(4):368–81.
Prati F, Regar E, Mintz GS, et al. Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Eur Heart J. 2010;31(4):401–15.
Suter MJ, Nadkarni SK, Weisz G, et al. Intravascular optical imaging technology for investigating the coronary artery. JACC Cardiovasc Imaging. 2011;4(9):1022–39.
Yang X, Luo W, Han S, et al. Prevalence of high-risk coronary plaques in patients with and without metabolic syndrome and the relationship with prognosis. BMC Cardiovasc Disord. 2020;20(1):73.
Yamamoto H, Kitagawa T, Ohashi N, Utsunomiya H, Kunita E, Oka T, Urabe Y, Tsushima H, Awai K, Kihara Y. Noncalcified atherosclerotic lesions with vulnerable characteristics detected by coronary CT angiography and future coronary events. J Cardiovasc Computed Tomogr. 2013;7:192–9.
Feuchtner G, Kerber J, Burghard P, Dichtl W, Friedrich G, Bonaros N, Plank F. The high-risk criteria low-attenuation plaque <60 HU and the napkin-ring sign are the most powerful predictors of MACE: a long-term follow-up study. Eur Heart J Cardiovasc Imaging. 2017;18:772–9.
Nakanishi K, Fukuda S, Tanaka A, Otsuka K, Jissho S, Taguchi H, Yoshikawa J, Shimada K. Persistent epicardial adipose tissue accumulation is associated with coronary plaque vulnerability and future acute coronary syndrome in nono-bese subjects with coronary artery disease. Atherosclerosis. 2014;237:353–60.
Otsuka K, Fukuda S, Tanaka A, Nakanishi K, Taguchi H, Yoshiyama M, Shimada K, Yoshikawa J. Prognosis of vulnerable plaque on computed tomographic coronary angiography with normal myocardial perfusion image. Eur Heart J Cardiovasc Imaging. 2014;15:332–40.
Conte E, Annoni A, Pontone G, Mushtaq S, Guglielmo M, Baggiano A, Volpato V, Agalbato C, Bonomi A, Veglia F, Formenti A, Fiorentini C, Bartorelli AL, Pepi M, Andreini D. Evaluation of coronary plaque characteristics with coronary computed tomography angiography in patients with non-obstructive coronary artery disease: a long-term follow-up study. Eur Heart J Cardiovasc Imaging. 2017;18:1170–8.
Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014;114(12):1852–66.
Bittencourt MS, Hulten E, Ghoshhajra B, et al. Prognostic value of nonobstructive and obstructive coronary artery disease detected by coronary computed tomography angiography to identify cardiovascular events. Circ Cardiovasc Imaging. 2014;7(2):282–91.
Chang HJ, Lin FY, Lee SE, et al. Coronary atherosclerotic precursors of acute coronary syndromes. J Am Coll Cardiol. 2018;71(22):2511–22.
Lee SE, Sung JM, Rizvi A, et al. Quantification of coronary atherosclerosis in the assessment of coronary artery disease. Circ Cardiovasc Imaging. 2018;11(7):e007562.
Ahmadi A, Argulian E, Leipsic J, Newby DE, Narula J. From subclinical atherosclerosis to plaque progression and acute coronary events: JACC state-of-the-art review. J Am Coll Cardiol. 2019;74(12):1608–17.
Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364(3):226–35.
Erlinge D, Maehara A, Ben-Yehuda O, et al. Identification of vulnerable plaques and patients by intracoronary near-infrared spectroscopy and ultrasound (PROSPECT II): a prospective natural history study. Lancet. 2021;397(10278):985–95.
Halon DA, Lavi I, Barnett-Griness O, et al. Plaque morphology as predictor of late plaque events in patients with asymptomatic type 2 diabetes: a long-term observational study. JACC Cardiovasc Imaging. 2019;12(7 Pt 2):1353–63.
Prati F, Romagnoli E, Gatto L, et al. Relationship between coronary plaque morphology of the left anterior descending artery and 12 months clinical outcome: the CLIMA study. Eur Heart J. 2020;41(3):383–91.
Shah PK. Mechanisms of plaque vulnerability and rupture. J Am Coll Cardiol. 2003;41(4 Suppl S):15s–22s.
Leber AW, Knez A, Becker A, et al. Accuracy of multidetector spiral computed tomography in identifying and differentiating the composition of coronary atherosclerotic plaques: a comparative study with intracoronary ultrasound. J Am Coll Cardiol. 2004;43(7):1241–7.
Nerlekar N, Ha FJ, Cheshire C, et al. Computed tomographic coronary angiography-derived plaque characteristics predict major adverse cardiovascular events: a systematic review and meta-analysis. Circ Cardiovasc Imaging. 2018;11(1): e006973.
Puchner SB, Liu T, Mayrhofer T, et al. High-risk plaque detected on coronary CT angiography predicts acute coronary syndromes independent of significant stenosis in acute chest pain: results from the ROMICAT-II trial. J Am Coll Cardiol. 2014;64(7):684–92.
Motoyama S, Sarai M, Harigaya H, et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol. 2009;54(1):49–57.
Oemrawsingh RM, Cheng JM, García-García HM, et al. Near-infrared spectroscopy predicts cardiovascular outcome in patients with coronary artery disease. J Am Coll Cardiol. 2014;64(23):2510–8.
Madder RD, Husaini M, Davis AT, et al. Large lipid-rich coronary plaques detected by near-infrared spectroscopy at non-stented sites in the target artery identify patients likely to experience future major adverse cardiovascular events. Eur Heart J Cardiovasc Imaging. 2016;17(4):393–9.
Schuurman AS, Vroegindewey M, Kardys I, et al. Near-infrared spectroscopy-derived lipid core burden index predicts adverse cardiovascular outcome in patients with coronary artery disease during long-term follow-up. Eur Heart J. 2017;39(4):295–302.
Madder RD, Goldstein JA, Madden SP, et al. Detection by near-infrared spectroscopy of large lipid core plaques at culprit sites in patients with acute ST-segment elevation myocardial infarction. JACC Cardiovasc Interv. 2013;6(8):838–46.
Waksman R, Di Mario C, Torguson R, et al. Identification of patients and plaques vulnerable to future coronary events with near-infrared spectroscopy intravascular ultrasound imaging: a prospective, cohort study. Lancet. 2019;394(10209):1629–37.
Pu J, Mintz GS, Biro S, et al. Insights into echo-attenuated plaques, echolucent plaques, and plaques with spotty calcification: novel findings from comparisons among intravascular ultrasound, near-infrared spectroscopy, and pathological histology in 2,294 human coronary artery segments. J Am Coll Cardiol. 2014;63(21):2220–33.
Mortensen MB, Gaur S, Frimmer A, et al. Association of age with the diagnostic value of coronary artery calcium score for ruling out coronary stenosis in symptomatic patients. JAMA Cardiol. 2022;7(1):36–44.
Ehara S, Kobayashi Y, Yoshiyama M, et al. Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction: an intravascular ultrasound study. Circulation. 2004;110(22):3424–9.
Nicholls SJ, Puri R, Anderson T, et al. Effect of evolocumab on coronary plaque composition. J Am Coll Cardiol. 2018;72(17):2012–21.
Puri R, Nicholls SJ, Shao M, et al. Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol. 2015;65(13):1273–82.
Jin HY, Weir-McCall JR, Leipsic JA, et al. The relationship between coronary calcification and the natural history of coronary artery disease. JACC Cardiovasc Imaging. 2021;14(1):233–42.
Kedhi E, Berta B, Roleder T, et al. Thin-cap fibroatheroma predicts clinical events in diabetic patients with normal fractional flow reserve: the COMBINE OCT-FFR trial. Eur Heart J. 2021;42(45):4671–9.
Otsuka K, Fukuda S, Tanaka A, et al. Napkin-ring sign on coronary CT angiography for the prediction of acute coronary syndrome. JACC Cardiovasc Imaging. 2013;6(4):448–57.
Kashiwagi M, Tanaka A, Kitabata H, et al. Feasibility of noninvasive assessment of thin-cap fibroatheroma by multidetector computed tomography. JACC Cardiovasc Imaging. 2009;2(12):1412–9.
Maurovich-Horvat P, Hoffmann U, Vorpahl M, Nakano M, Virmani R, Alkadhi H. The napkin-ring sign: CT signature of high-risk coronary plaques? JACC Cardiovasc Imaging. 2010;3(4):440–4.
Joshi NV, Vesey AT, Williams MC, et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet. 2014;383(9918):705–13.
Andreou I, Antoniadis AP, Shishido K, et al. How do we prevent the vulnerable atherosclerotic plaque from rupturing? Insights from in vivo assessments of plaque, vascular remodeling, and local endothelial shear stress. J Cardiovasc Pharmacol Ther. 2015;20(3):261–75.
Bajraktari A, Bytyçi I, Henein MY. High coronary wall shear stress worsens plaque vulnerability: a systematic review and meta-analysis. Angiology. 2021;72(8):706–14.
Stone PH, Saito S, Takahashi S, et al. Prediction of progression of coronary artery disease and clinical outcomes using vascular profiling of endothelial shear stress and arterial plaque characteristics: the PREDICTION Study. Circulation. 2012;126(2):172–81.
Stone PH, Maehara A, Coskun AU, et al. Role of low endothelial shear stress and plaque characteristics in the prediction of nonculprit major adverse cardiac events: the PROSPECT study. JACC Cardiovasc Imaging. 2017;11(3):462–71.
Dai J, Xing L, Jia H, et al. In vivo predictors of plaque erosion in patients with ST-segment elevation myocardial infarction: a clinical, angiographical, and intravascular optical coherence tomography study. Eur Heart J. 2018;39(22):2077–85.
Gijsen FJ, Wentzel JJ, Thury A, et al. A new imaging technique to study 3-D plaque and shear stress distribution in human coronary artery bifurcations in vivo. J Biomech. 2007;40(11):2349–57.
Franck G, Mawson T, Sausen G, et al. Flow perturbation mediates neutrophil recruitment and potentiates endothelial injury via TLR2 in mice: implications for superficial erosion. Circ Res. 2017;121(1):31–42.
Libby P, Loscalzo J, Ridker PM, et al. Inflammation, immunity, and infection in atherothrombosis: JACC review topic of the week. J Am Coll Cardiol. 2018;72(17):2071–81.
Nowak WN, Deng J, Ruan XZ, Xu Q. Reactive oxygen species generation and atherosclerosis. Arterioscler Thromb Vasc Biol. 2017;37(5):e41–52.
Kong P, Cui ZY, Huang XF, Zhang DD, Guo RJ, Han M. Inflammation and atherosclerosis: signaling pathways and therapeutic intervention. Signal Transduct Target Ther. 2022;7(1):131.
Antoniades C, Antonopoulos AS, Deanfield J. Imaging residual inflammatory cardiovascular risk. Eur Heart J. 2020;41(6):748–58.
Meier CR, Jick SS, Derby LE, Vasilakis C, Jick H. Acute respiratory-tract infections and risk of first-time acute myocardial infarction. Lancet. 1998;351(9114):1467–71.
Nagarajan V, Fonarow GC, Ju C, et al. Seasonal and circadian variations of acute myocardial infarction: findings from the Get With The Guidelines-Coronary Artery Disease (GWTG-CAD) program. Am Heart J. 2017;189:85–93.
Oikonomou EK, Marwan M, Desai MY, et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): a post-hoc analysis of prospective outcome data. Lancet. 2018;392(10151):929–39.
Nerlekar N, Brown AJ, Muthalaly RG, et al. Association of epicardial adipose tissue and high-risk plaque characteristics: a systematic review and meta-analysis. J Am Heart Assoc. 2017;6(8):e006379. https://doi.org/10.1161/JAHA.117.006379.
Cheng VY, Dey D, Tamarappoo B, et al. Pericardial fat burden on ECG-gated noncontrast CT in asymptomatic patients who subsequently experience adverse cardiovascular events. JACC Cardiovasc Imaging. 2010;3(4):352–60.
Sugiyama T, Kanaji Y, Hoshino M, et al. Determinants of pericoronary adipose tissue attenuation on computed tomography angiography in coronary artery disease. J Am Heart Assoc. 2020;9(15): e016202.
Yuvaraj J, Lin A, Nerlekar N, et al. Pericoronary adipose tissue attenuation is associated with high-risk plaque and subsequent acute coronary syndrome in patients with stable coronary artery disease. Cells. 2021;10(5):1143.
Goeller M, Tamarappoo BK, Kwan AC, et al. Relationship between changes in pericoronary adipose tissue attenuation and coronary plaque burden quantified from coronary computed tomography angiography. Eur Heart J Cardiovasc Imaging. 2019;20(6):636–43.
Ward NC, Watts GF, Eckel RH. Statin toxicity. Circ Res. 2019;124(2):328–50.
Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097–107.
Nicholls SJ, Kataoka Y, Nissen SE, et al. Effect of evolocumab on coronary plaque phenotype and burden in statin-treated patients following myocardial infarction. JACC Cardiovasc Imaging. 2022. https://doi.org/10.1016/j.jcmg.2022.03.002.
Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387–97.
Bowman L, Mafham M, Wallendszus K, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379(16):1529–39.
Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–31.
Tong DC, Bloom JE, Quinn S, et al. Colchicine in patients with acute coronary syndrome: two-year follow-up of the Australian COPS randomized clinical trial. Circulation. 2021;144(19):1584–6.
Tardif JC, Kouz S, Waters DD, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. 2019;381(26):2497–505.
Hung OY, Molony D, Corban MT, et al. Comprehensive assessment of coronary plaque progression with advanced intravascular imaging, physiological measures, and wall shear stress: a pilot double-blinded randomized controlled clinical trial of nebivolol versus atenolol in nonobstructive coronary artery disease. J Am Heart Assoc. 2016;5(1):e002764. https://doi.org/10.1161/JAHA.115.002764.
Stone GW, Maehara A, Ali ZA, et al. Percutaneous coronary intervention for vulnerable coronary atherosclerotic plaque. J Am Coll Cardiol. 2020;76(20):2289–301.
Oikonomou EK, Williams MC, Kotanidis CP, et al. A novel machine learning-derived radiotranscriptomic signature of perivascular fat improves cardiac risk prediction using coronary CT angiography. Eur Heart J. 2019;40(43):3529–43.
Acknowledgements
Funding
No funding was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author contributions
All authors contributed to the study conception and design, material preparation, data collection and analysis. All authors read and approved the final manuscript.
Disclosures
Luke Dawson and Jamie Layland both have nothing to disclose.
Compliance with ethics guidelines
The article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Dawson, L.P., Layland, J. High-Risk Coronary Plaque Features: A Narrative Review. Cardiol Ther 11, 319–335 (2022). https://doi.org/10.1007/s40119-022-00271-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40119-022-00271-9