Abstract
Introduction
Frailty is associated with changes in inflammation, coagulation, and possibly platelet function. Aspirin is still prescribed for stroke prevention in older patients with atrial fibrillation, although not recommended by current guidelines. In frail older people, it is unclear whether platelet aggregability and response to aspirin are altered. This study aims to investigate the effects of frailty and chronological age on platelet aggregability and on responses to aspirin in older patients with atrial fibrillation.
Methods
Inpatients with atrial fibrillation aged ≥65 years were recruited from a tertiary referral hospital in Sydney, Australia. Frailty was determined using the Reported Edmonton Frail Scale. Platelet aggregation studies were performed using whole blood impedance aggregometry.
Results
Data from 115 participants were analyzed (mean age 85 ± 6 years, 41% female, 52% frail). Spearman correlation coefficients found no significant associations of platelet aggregation with chronological age or with frailty score. Comparison between frail and non-frail groups showed that there was no impact of frailty status on aggregation assays amongst participants who were not taking any antiplatelet drugs. Amongst participants taking aspirin, the frail had higher adjusted arachidonic acid agonist (ASPI) test measures (AU per platelet) than the non-frail (0.11 ± 0.11 vs. 0.05 ± 0.04; p = 0.04), suggesting that in frail participants, platelet aggregation is less responsive to aspirin than in non-frail.
Conclusions
We found no effect of chronological age or frailty status on platelet aggregation amongst older patients with atrial fibrillation in this pilot study. However, frailty could be associated with reduced aspirin responsiveness among older patients with atrial fibrillation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is marked heterogeneity amongst people aged over 65 years. Some of this may be captured by increasing chronological age. However, much of this variability is thought to be due to biological age or frailty [1]. Frailty is a state of vulnerability that carries an increased risk of poor outcomes in older adults [1]. The prevalence and clinical importance of frailty are increasing with ageing of the population [1, 2]. Frailty is associated with changes in inflammation, coagulation, and possibly platelet function [3, 4].
Atrial fibrillation (AF) is a common cardiac arrhythmia in older adults. The prevalence of AF in published studies in Western countries ranges from 0.5% to 3% in the general population, 5–6% in people older than 65 years, and up to 5–15% among those aged 80 years or older [5–7]. Treatment of AF aims at stroke prevention with antithrombotic therapy, reducing symptoms with rate-control or rhythm-control strategies, and management of associated medical conditions [8]. According to the current guidelines, aspirin is not recommended for stroke prevention in AF unless patients refuse the use of any oral anticoagulant [9, 10]. International drug utilization studies show that, in practice, 17–45% of older adults use aspirin for stroke prevention in AF [11–14]. The evidence for stroke prevention in AF with aspirin is weak and the risk of major bleeding with aspirin is not significantly different to that of oral anticoagulants, especially in older people [9, 15, 16].
The efficacy of antiplatelet drugs has not been rigorously tested in older people and older people are generally more vulnerable to adverse drug effects due to changes in pharmacokinetics and pharmacodynamics associated with aging and an increased risk of drug–drug and drug–disease interactions in the presence of polypharmacy and multimorbidity [17]. In frail older people, it is unclear whether response to antiplatelet therapies is altered. Some studies have suggested that platelet aggregability may increase in old age [4, 18, 19] and plasma aspirin esterase activity is reduced in frail people [20–22]. However, there has been no study exploring the association between frailty and platelet aggregation. Therefore, the aims of this study were to investigate the effects of frailty and chronological age on platelet aggregability and on platelet responses to aspirin in older patients with AF.
Methods
A total of 302 inpatients aged ≥65 years with AF at Royal North Shore Hospital, a tertiary referral teaching hospital in Sydney, Australia, were recruited for a study of anticoagulant utilization and outcomes in frail and non-frail older inpatients with AF. Of these patients, 134 participated in this sub-study on platelet aggregation. Among these patients, 82 who were not taking any antiplatelet drugs for at least a week before blood samples were taken for testing and 33 patients who were taking regular aspirin (100 mg daily) and no other antiplatelet agents were eligible for this analysis. Informed consent was obtained from all participants or their caregivers. The study was approved by The Northern Sydney Local Health District Human Research Ethics Committee and The University of Sydney Human Research Ethics Committee. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013.
Frailty was determined using the Reported Edmonton Frail Scale [23]. This scale, which was adapted from the Edmonton Frail Scale for use in Australian acute inpatients, assesses nine frailty domains: cognition, general health status, functional independence, social support, medication use, nutrition, mood, continence, and reported functional performance. With a maximum score of 18, a score of 0–5 indicates robust, 6–7 indicates apparently vulnerable status, 8–9 mild frailty, 10–11 moderate frailty, and 12 or higher indicates severe frailty. The cut-off point to identify frailty is 8 [23].
Blood was collected from the participants in the morning from the antecubital vein into tubes containing hirudin. Platelet aggregation studies were performed between 30 min and 2 h after blood was taken, using whole blood impedance aggregometry (WBIA, Multiplate Analyser, Roche Diagnostics). The Multiplate Analyser measures aggregation in whole blood samples through changes in electrical impedance between two electrodes and has been applied to detect platelet inhibition by aspirin in many studies [24–29]. More details about the test have been described elsewhere [30, 31]. Platelet agonists used in this assay were arachidonic acid (ASPItest) to trigger arachidonic acid-induced platelet aggregation, which is affected by aspirin; adenosine diphosphate (ADPtest) to trigger ADP-induced platelet aggregation, which is affected by thienopyridines (e.g., clopidogrel, prasugrel, ticlopidine); and Thrombin Receptor Activating Peptide 6 (TRAPtest) to trigger TRAP-6 induced platelet aggregation, which is only affected by glycoprotein IIb/IIIa receptor antagonists (e.g., tirofiban, abciximab, eptifibatide). ADPtest and TRAPtest were used as positive controls for platelet reactivity. Platelet aggregation is defined by the area under the aggregation-time curve, which represents the aggregation over 6 min, and values are reported in arbitrary aggregation units (AU). Suggested normal ranges in healthy blood donors as provided by the manufacturer are 71–115 AU for the ASPItest, 57–113 AU for the ADPtest, and 84–128 AU for the TRAPtest [32].
Analysis of the data was performed using SPSS for Windows 20.0 (IBM Corp., Armonk, NY, USA). Continuous variables are presented as mean ± standard deviation, and categorical variables as frequency and percentage. Clinical characteristics and laboratory parameters were compared between frailty and treatment groups using the Mann–Whitney U test for continuous variables, and Chi-square or Fisher’s exact test for binary variables. Correlation of platelet aggregation with age, frailty score, and other variables that have previously been shown to have an impact on platelet aggregation [33] was assessed with Spearman correlation. Two-sided p values <0.05 were considered significant. Platelet function was considered separately for each treatment regimen and by frailty status. The platelet counts in this study showed a marked degree of variation (mean 220 ± 95 × 109/l, median 202 × 109/l, range 30–502 × 109/l). Twenty-five patients had platelet counts below the normal range, and, as expected, these patients had significantly lower AU values than patients with platelet counts in the normal range (p < 0.001). Spearman correlation also showed that platelet count had a very strong association with platelet aggregation (r = 0.59, p < 0.001 for ASPI test; r = 0.63, p < 0.001 for ADP test; r = 0.69, p < 0.001 for TRAP test). Therefore, we adjusted the test results to control for the effect of the platelet counts and provide a purer representation of platelet aggregability by dividing the AU value by the platelet count, giving a value of AU per platelet. We compared aggregability between frail and non-frail participants with and without aspirin treatment based on these adjusted values. Sensitivity analyses were also performed to assess the robustness of the finding after excluding those participants with platelet counts <100 × 109/l or >400 × 109/l.
Results
A total of 115 participants were included in the study (mean age 85 ± 6 years, age range 71–97 years, 41% female, 52% frail). Among the 82 participants who did not take any antiplatelet therapy in the week prior to sampling (Table 1), mean age was 84 ± 6 years and 49% of the participants were frail. Compared to the non-frail, frail participants had a significantly higher score on the Charlson Comorbidity Index, with a higher prevalence of heart failure and renal impairment. There was no quantitative difference in any of the platelet-aggregation assays between frail and non-frail participants. Spearman correlation coefficients were performed for each test of platelet aggregation with age, frailty score, and other variables that may impact platelet aggregation (Table 2). There were no significant correlations between platelet aggregation and any of these variables.
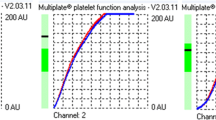
Among the 33 participants who were taking aspirin, the frail (n = 20) had higher ASPI test results than the non-frail (0.11 ± 0.11 AU per platelet in the frail versus 0.05 ± 0.04 AU per platelet in the non-frail; p = 0.04), suggesting that platelets in the frail are less responsive to aspirin (Table 3). Representative curves from the ASPI tests of a frail and non-frail participant are shown in Fig. 1. Spearman correlation coefficients of the ASPI test results with age, frailty score and other variables found that the only significant correlation was of the presence of a diagnosis of heart failure with increased AU (correlation coefficient 0.40, p = 0.02) (Table 4). Sensitivity analyses showed that the difference between the frail and the non-frail remained significant amongst the participants with platelet counts from 100–400 × 109/l (n = 26), consistent with the analyses amongst those with platelet counts from 30–502 × 109/l (n = 33) (Table 5).
Arachidonic acid-induced platelet aggregation (ASPItest) in participants taking aspirin. a From a representative frail participant. b From a representative non-frail participant. (One Multiplate test cell includes two independent sensor units. The increase of impedance due to the attachment of platelets to the electrodes is detected for each sensor unit separately and transformed to arbitrary aggregation units that are plotted against time. The duplicate sensors work as an internal control) [24]
Discussion
In this pilot study of older inpatients with AF, there was no significant relationship between platelet aggregation and chronological age. This result is different to many previous studies in which there was a trend towards increased platelet aggregation with age [4, 18, 19, 33]. However, all of these studies were designed to compare platelet aggregation between younger groups and older groups (participant age ranged from around 20 to 80 years old, with the cut-off point to determine older groups usually around 60 years old). In contrast, in our study the mean age of participants was around 84–86 years, with an age range from 71 to 97 years. Furthermore, unlike our study of acutely unwell older inpatients, previous studies demonstrating increased platelet aggregation with age were in healthy volunteers from the community without a history of cardiovascular disease. Additionally, in this study we used the Multiplate assay—a new method to evaluate platelet aggregation, which is different from light transmission aggregometry that was used in previous studies [4, 18, 19, 33].
Amongst participants not taking antiplatelet drugs, there was no association between frailty status, a marker of biological age, and platelet aggregation. Amongst those taking aspirin, there was a significant difference in platelet aggregation to arachidonic acid (ASPI test): the frail exhibited a degree of aspirin resistance compared to the non-frail. The reduced responsiveness to aspirin observed in the frail may be partly attributed to the higher prevalence of heart failure in the frail participants. In participants taking aspirin, we found a moderate positive correlation between heart failure and arachidonic acid-induced platelet aggregation, which means that compared to participants without a history of heart failure, those with heart failure tend to have a higher on-treatment platelet aggregation. The relationship between heart failure and decreased aspirin effectiveness has been reported in several studies [34, 35]. Although not comprehensively understood, this could be explained by several mechanisms such as increased levels of circulating catecholamines, angiotensin II and b-thromboglobulin, platelet factor 4, P-selectin, and platelet-endothelial cell adhesion molecules in patients with heart failure [36]. The observed reduced platelet responsiveness to aspirin in the frail supports the current guidelines that do not recommend aspirin for stroke prevention in AF, and raises a question about the risk-to-benefit ratio of aspirin prescription in older patients with AF, which ironically is more common in the frail [37], in whom prescribers may be more concerned about using anticoagulants.
The study comprised a sample of very old and frail people, who are often excluded from studies [38]. Recently, objective measures of frailty, including the Reported Edmonton Frail Scale used in our study [23], have facilitated study of the physiology and management of frailty [1]. The physiological etiology of frailty is still not comprehensively understood. Multiple physiological factors are thought to be involved in the development of frailty, including activation of inflammation, coagulation systems, and changes in pharmacokinetics and pharmacodynamics [1, 3, 21, 39]. Studies measuring individual factors in the coagulation system suggest that frailty is associated with pro-coagulant changes such as increased plasma fibrinogen, factor VIII, C-reactive protein, D-dimer, and tissue plasminogen activator (t-PA) plasma levels [40–43]. To our knowledge, there has been no previous study focusing on the impact of frailty on platelet aggregation and platelet response to antiplatelet drugs. There have only been several studies reporting the association between frailty and reduced activity of plasma aspirin esterase, a hydrolysis enzyme that helps the conversion of aspirin (acetylsalicylic acid) to salicylic and acetic acid [20, 21].
In this study, we used the Multiplate method to study platelet aggregation. Since the introduction of the bleeding time test, different methodologies have been developed to obtain the optimal platelet function test and to assess platelet reactivity in response to antiplatelet drugs [44–47]. The Multiplate is a new method for evaluating platelet aggregation and is one of the point-of-care assays for monitoring antiplatelet therapy [30]. It can be performed in whole blood, does not require specifically trained laboratory personnel, and is simple to interpret [45]. This method has been widely used in clinical trials and is also implemented in daily practice in catheterization laboratories, predominantly in Europe [44]. However, it should be noted that the correlation of this test with other tests of platelet aggregation and with clinical outcomes is not perfect [29, 48] and that this test has not been validated in very old or frail participants. The Multiplate assay provides a reproducible measure of reduced platelet aggregation in response to defined agonists. However, unlike assays measuring platelet response to very low doses of agonists, which were used in previous studies of platelet function in ageing [4, 18, 19, 33], the Multiplate assay is not designed to detect platelet hyperaggregability.
A major limitation of this study is that it was done in the acute care setting, in which platelet aggregation may be influenced by acute inflammation [49]. This is a pilot study testing the hypothesis of altered platelet aggregation with frailty that relies on a convenience sample. Small sample size may have limited the power of this study to observe small changes with age and frailty. This study sample is based on volunteers from inpatients recruited for a study on anticoagulant utilization. Approximately half of the participants in that study agreed to a blood test, so the sample may be not representative of older inpatients with AF. Furthermore, all of the participants in this study had AF, which may be procoagulant [50]. Therefore, results should be cautiously interpreted and generalized to older inpatients without AF who may be prescribed aspirin for other indications.
Conclusions
We found no effect of chronological age or frailty status on platelet aggregation amongst hospitalized older patients with AF in this pilot study. Response to aspirin is reduced in the frail and in those with heart failure. This may have implications for efficacy of aspirin in this population.
References
Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–62.
Raphael D, Cava M, Brown I, Renwick R, Heathcote K, Weir N, et al. Frailty: a public health perspective. Can J Public Health. 1995;86(4):224–7.
Kanapuru B, Ershler WB. Inflammation, coagulation, and the pathway to frailty. Am J Med. 2009;122(7):605–13.
Gleerup G, Winther K. The effect of ageing on platelet function and fibrinolytic activity. Angiology. 1995;46(8):715–8.
Chugh SS, Roth GA, Gillum RF, Mensah GA. Global burden of atrial fibrillation in developed and developing nations. Glob Heart. 2014;9(1):113–9.
Ball J, Carrington MJ, McMurray JJV, Stewart S. Atrial fibrillation: profile and burden of an evolving epidemic in the 21st century. Int J Cardiol. 2013;167(5):1807–24.
Camm AJ, Kirchhof P, Lip GYH, Schotten U, Savelieva I, Ernst S, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology. Eur Heart J. 2010;31(19):2369–429.
Camm AJ, Lip GY, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation—developed with the special contribution of the European Heart Rhythm Association. Europace. 2012;14(10):1385–413.
Hanon O, Assayag P, Belmin J, Collet JP, Emeriau JP, Fauchier L, et al. Expert consensus of the French Society of Geriatrics and Gerontology and the French Society of Cardiology on the management of atrial fibrillation in elderly people. Arch Cardiovasc Dis. 2013;106(5):303–23.
January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):2246–80.
Corvol A, Gulsvik AK, Kuper IMJA, Phylaktou P, Myrstad M, Somme D, et al. Use of anticoagulants for atrial fibrillation in older subjects across different countries: Cyprus, France, Netherlands, Norway. Eur Geriatr Med. 2014;5(1):60–5.
Ferguson C, Inglis SC, Newton PJ, Middleton S, Macdonald PS, Davidson PM. The atrial fibrillation and stroke thromboprophylaxis in heart failure (AFASTER) cohort study: 90 day outcomes. Eur J Heart Fail. 2014;16(Suppl2):282.
Gage BF, Boechler M, Doggette AL, Fortune G, Flaker GC, Rich MW, et al. Adverse outcomes and predictors of underuse of antithrombotic therapy in Medicare beneficiaries with chronic atrial fibrillation. Stroke. 2000;31(4):822–7 Epub 2001/02/07.
Lleva P, Aronow WS, Gutwein AH. Prevalence of inappropriate use of digoxin in 136 patients on digoxin and prevalence of use of warfarin or aspirin in 89 patients with persistent or paroxysmal atrial fibrillation. Am J Ther. 2009;16(6):e41–3.
Go AS, Hylek EM, Phillips KA, Chang YC, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. J Am Med Assoc. 2001;285(18):2370–5.
Mant J, Hobbs FD, Fletcher K, Roalfe A, Fitzmaurice D, Lip GY, et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet. 2007;370(9586):493–503 (Epub 2007/08/19).
Capodanno D, Angiolillo DJ. Antithrombotic therapy in the elderly. J Am Coll Cardiol. 2010;56(21):1683–92.
Kasjanovova D, Balaz V. Age-related changes in human platelet function in vitro. Mech Ageing Dev. 1986;37(2):175–82.
Terres W, Weber K, Kupper W, Bleifeld W. Age, cardiovascular risk factors and coronary heart disease as determinants of platelet function in men. A multivariate approach. Thromb Res. 1991;62(6):649–61.
Williams FM, Wynne H, Woodhouse KW, Rawlins MD. Plasma aspirin esterase: the influence of old age and frailty. Age Ageing. 1989;18(1):39–42.
Hubbard RE, O’Mahony MS, Calver BL, Woodhouse KW. Plasma esterases and inflammation in ageing and frailty. Eur J Clin Pharmacol. 2008;64(9):895–900.
Summerbell J, Yelland C, Woodhouse K. The kinetics of plasma aspirin esterase in relation to old age and frailty. Age Ageing. 1990;19(2):128–30.
Hilmer SN, Perera V, Mitchell S, Murnion BP, Dent J, Bajorek B, et al. The assessment of frailty in older people in acute care. Aust J Ageing. 2009;28(4):182–8.
Sibbing D, Schulz S, Braun S, Morath T, Stegherr J, Mehilli J, et al. Antiplatelet effects of clopidogrel and bleeding in patients undergoing coronary stent placement. J Thromb Haemost. 2010;8(2):250–6.
Von Pape KW, Dzijan-Horn M, Bohner J, Spannagl M, Weisser H, Calatzis A. Control of aspirin effect in chronic cardiovascular patients using two whole blood platelet function assays: PFA-100® and Multiplate®. Hamostaseologie. 2007;27(3):155–60.
Wurtz M, Hvas AM, Christensen KH, Rubak P, Kristensen SD, Grove EL. Rapid evaluation of platelet function using the Multiplate(R) Analyzer. Platelets. 2014;25(8):628–33 Epub 2013/11/20.
Calderaro D, Pastana AF, Flores da Rocha TR, Yu PC, Gualandro DM, DeLuccia N, et al. Aspirin responsiveness safely lowers perioperative cardiovascular risk. J Vasc Surg. 2013;58(6):1593–9 Epub 2013/11/28.
Rahe-Meyer N, Winterhalter M, Hartmann J, Pattison A, Hecker H, Calatzis A, et al. An evaluation of cyclooxygenase-1 inhibition before coronary artery surgery: aggregometry versus patient self-reporting. Anesth Analg. 2008;107(6):1791–7.
Pedersen SB, Grove EL, Nielsen HL, Mortensen J, Kristensen SD, Hvas AM. Evaluation of aspirin response by Multiplate® whole blood aggregometry and light transmission aggregometry. Platelets. 2009;20(6):415–20.
Tóth O, Calatzis A, Penz S, Losonczy H, Siess W. Multiple electrode aggregometry: a new device to measure platelet aggregation in whole blood. Thromb Haemost. 2006;96(12):781–8.
Sibbing D, Braun S, Jawansky S, Vogt W, Mehilli J, Schomig A, et al. Assessment of ADP-induced platelet aggregation with light transmission aggregometry and multiple electrode platelet aggregometry before and after clopidogrel treatment. Thromb Haemost. 2008;99(1):121–6 Epub 2008/01/25.
Reference ranges for Multiplate analysis using double wall hirudin tubes, version 1.0 (2013). Roche Diagnostics GmbH. http://www.roche.es/content/dam/roche_spain/es_ES/documents/Multiplate_Reference_ranges.pdf. Accessed 15 Jan 2016.
O’Donnell CJ, Larson MG, Feng D, Sutherland PA, Lindpaintner K, Myers RH, et al. Genetic and environmental contributions to platelet aggregation: the Framingham Heart Study. Circulation. 2001;103(25):3051–6.
Sane DC, McKee SA, Malinin AI, Serebruany VL. Frequency of Aspirin resistance in patients with congestive heart failure treated with antecedent Aspirin. Am J Cardiol. 2002;90(8):893–5.
Kaplon-Cieslicka A, Rosiak M, Postula M, Serafin A, Kondracka A, Opolski G, et al. Predictors of high platelet reactivity during aspirin treatment in patients with type 2 diabetes. Kardiol Polska. 2013;71(9):893–902.
Airee A, Draper HM, Finks SW. Aspirin resistance: disparities and clinical implications. Pharmacotherapy. 2008;28(8):999–1018.
Perera V, Bajorek BV, Matthews S, Hilmer SN. The impact of frailty on the utilisation of antithrombotic therapy in older patients with atrial fibrillation. Age Ageing. 2009;38(2):156–62.
Ridda I, MacIntyre CR, Lindley RI, Tan TC. Difficulties in recruiting older people in clinical trials: an examination of barriers and solutions. Vaccine. 2010;28(4):901–6.
Chaves PHM, Semba RD, Leng SX, Woodman RC, Ferrucci L, Guralnik JM, et al. Impact of anemia and cardiovascular disease on frailty status of community-dwelling older women: the women’s health and aging studies I and II. J Gerontol Ser A Biol Sci Med Sci. 2005;60(6):729–35.
Walston J, McBurnie M, Newman A, et al. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the cardiovascular health study. Arch Intern Med. 2002;162(20):2333–41.
Reiner AP, Aragaki AK, Gray SL, Wactawski-Wende J, Cauley JA, Cochrane BB, et al. Inflammation and thrombosis biomarkers and incident frailty in postmenopausal women. Am J Med. 2009;122(10):947–54.
Cohen HJ, Harris T, Pieper CF. Coagulation and activation of inflammatory pathways in the development of functional decline and mortality in the elderly. Am J Med. 2003;114(3):180–7.
Folsom AR, Boland LL, Cushman M, Heckbert SR, Rosamond WD, Walston JD. Frailty and risk of venous thromboembolism in older adults. J Gerontol Ser A Biol Sci Med Sci. 2007;62(1):79–82.
Karathanos A, Geisler T. Monitoring aspirin and clopidogrel response: testing controversies and recommendations. Mol Diagn Ther. 2013;17(3):123–37.
Steiner S, Moertl D. Platelet reactivity tests for assessing antiplatelet drug response: what the clinician needs to know. Expert Rev Cardiovasc Ther. 2013;11(8):975–84.
Duke WW. The relation of blood platelets to hemorrhagic disease. Description of a method for determining the bleeding time and coagulation time and report of three cases of hemorrhagic disease relieved by transfusion. J Am Med Assoc. 1983;250(9):1201–9.
Paniccia R, Priora R, Liotta AA, Abbate R. Platelet function tests: a comparative review. Vasc Health Risk Manag. 2015;11:133–48.
Grove EL, Hvas AM, Johnsen HL, Hedegaard SS, Pedersen SB, Mortensen J, et al. A comparison of platelet function tests and thromboxane metabolites to evaluate aspirin response in healthy individuals and patients with coronary artery disease. Thromb Haemost. 2010;103(6):1245–53.
Stokes KY, Granger DN. Platelets: a critical link between inflammation and microvascular dysfunction. J Physiol. 2012;590(5):1023–34.
Kamath S, Blann AD, Chin BSP, Lanza F, Aleil B, Cazenave JP, et al. A study of platelet activation in atrial fibrillation and the effects of antithrombotic therapy. Eur Heart J. 2002;23(22):1788–95.
Acknowledgments
We thank all the kind participants and their caregivers who made this study possible. We also thank the Geoff and Elaine Penney Ageing Research Unit, Royal North Shore Hospital, Australia, for their support with resources for the study. We thank Miss Natalia Tan (Research Assistant, Northern Blood Research Centre, Kolling Institute of Medical Research, The University of Sydney, Australia) for her kind help during the study. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
Tu N. Nguyen, Dominic Pepperell, Marie-Christine Morel-Kopp, Robert G Cumming, Christopher Ward, and Sarah N. Hilmer declare that they have no conflicts of interest.
Compliance with ethics guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Nguyen, T.N., Pepperell, D., Morel-Kopp, MC. et al. Effect of Frailty and Age on Platelet Aggregation and Response to Aspirin in Older Patients with Atrial Fibrillation: A Pilot Study. Cardiol Ther 5, 51–62 (2016). https://doi.org/10.1007/s40119-016-0056-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40119-016-0056-4