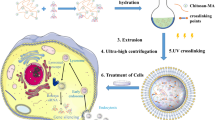
Abstract
The preparation of safe and efficient siRNA carriers remains a challenge that has limited the therapeutic applications of siRNA. In this study, the design of a new small interfering RNA (siRNA) carrier based on diisopropylaminoethyl-chitosan was devised for application in non-viral gene therapy. Polycations having varied proportions (11–32%) of diisopropylethylamine groups (DIPEA) and grafted with polyethylene glycol (1–3%) were synthesized and characterized. The physicochemical and biological properties of the polymers and their nanoparticles were evaluated at pH 6.3 and pH 7.4. The degrees of ionization at pH 7.4 were precisely controlled by the composition and increased from 13% for chitosan to 47% for the more substituted derivative. Nanoparticles with very low toxicities and sizes in the range of 100–200 nm, remained stable up to 24 h after their preparation in both the evaluated pHs under plasma osmolality. As probed by scanning electron and confocal microscopies, an efficient cell uptake of spherical nanoparticles mediated a TNFα knockdown of almost 60% in RAW 264.7 macrophages, and mRNA silence levels higher than the Lipofectamine (up to 90%) in HeLa cells. Overall, the results showed that these derivatives are promising vectors for in vivo studies under physiological conditions.
Graphical abstract









Similar content being viewed by others
References
Mirzaei, S., Mahabady, M.K., Zabolian, A., et al.: Small interfering RNA (siRNA) to target genes and molecular pathways in glioblastoma therapy: current status with an emphasis on delivery systems. Life Sci. 275, 119368 (2021)
Mirzaei, S., Gholami, M.H., Hashemi, F., et al.: Employing siRNA tool and its delivery platforms in suppressing cisplatin resistance: approaching to a new era of cancer chemotherapy. Life Sci. 277, 119430 (2021)
Rossi, J.J., Rossi, D.J.: siRNA drugs: here to stay. Mol. Ther. 29, 431 (2021)
Tavakoli, N., Divsalar, A., Haertlé, T., et al.: Milk protein-based nanodelivery systems for the cancer treatment. J. Nanostruct. Chem. 11, 483–500 (2021)
Ashrafizadeh, M., Delfi, M., Hashemi, F., et al.: Biomedical application of chitosan-based nanoscale delivery systems: potential usefulness in siRNA delivery for cancer therapy. Carbohydr. Polym. 260, 117809 (2021)
Lavertu, M., Méthot, S., Tran-Khanh, N., Buschmann, M.D.: High efficiency gene transfer using chitosan/DNA nanoparticles with specific combinations of molecular weight and degree of deacetylation. Biomaterials 27, 4815 (2006)
Jennings, J.A., Bumgarden, J.D.: Chitosan Based Biomaterials Volume 2: Tissue Engineering and Therapeutics. Woodhead Publishing, Amsterdam (2017)
Alameh, M., Lavertu, M., Tran-Khanh, N.: siRNA delivery with chitosan: influence of chitosan molecular weight, degree of deacetylation, and amine to phosphate ratio on in vitro silencing efficiency, hemocompatibility, biodistribution, and in vivo efficacy. Biomacromol 19, 112–131 (2018)
Dehousse, V., Garbacki, N., Jaspart, S., et al.: Comparison of chitosan/siRNA and trimethylchitosan/siRNA complexes behaviour in vitro. Int. J. Biol. Macromol. 46, 342–349 (2010)
Soliman, O.Y., Alameh, M.G., De Cresenzo, G., et al.: Efficiency of chitosan/hyaluronan-based mRNA delivery systems in vitro: influence of composition and structure. J. Pharm. Sci. 109, 1581–1593 (2020)
Oliveira, F.D.P.P., Picola, I.P.D., Shi, Q., et al.: Synthesis and evaluation of diethylethylamine-chitosan for gene delivery: composition effects on the in vitro transfection efficiency. Nanotechnology 24, 055101 (2013)
Picola, I.P.D., Shi, Q., Fernandes, J.C., et al.: Chitosan derivatives for gene transfer: effect of phosphorylcholine and diethylaminoethyl grafts on the in vitro transfection efficiency. J. Biomater. Sci. Polym. Ed. 27, 1611–1630 (2016)
De Souza, R.H.F.V., Picola, I.P.D., Shi, Q., et al.: Diethylaminoethyl-chitosan as an efficient carrier for siRNA delivery: improving the condensation process and the nanoparticles properties. Int. J. Biol. Macromol. 119, 186–197 (2018)
Jones, C.H., Chen, C.K., Ravikrishnan, A., et al.: Overcoming nonviral gene delivery barriers: perspective and future. Mol. Pharm. 10, 4082–4098 (2013)
Giacomelli, F.C., Stepánek, P., Giacomelli, C., et al.: pH-triggered block copolymer micelles based on a pH-responsive PDPA (poly[2-(diisopropylamino)ethyl methacrylate]) inner core and a PEO (poly(ethylene oxide)) outer shell as a potential tool for the cancer therapy. Soft Matter 7, 9316–9325 (2011)
Zhou, K., Liu, H., Zhang, S., et al.: Multicolored pH-tunable and activatable fluorescence nanoplatform responsive to physiologic pH stimuli. J. Am. Chem. Soc. 134, 7803–7811 (2012)
Zhou, G., Xu, Y., Chen, M., et al.: Tumor-penetrating peptide modified and pH-sensitive polyplexes for tumor targeted siRNA delivery. Polym. Chem. 7, 3857–3863 (2016)
Cao, Y., Tan, Y.F., Wong, Y.S., et al.: Recent advances in chitosan-based carriers for gene delivery. Mar. Drugs 17, 381 (2019)
Tiera, M.J., Shi, Q., Winnik, F.M., Fernandes, J.C.: Polycation-based gene therapy: current knowledge and new perspectives. Curr. Gene Ther. 11, 288–306 (2011)
Blanco, E., Shen, H., Ferrari, M.: Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 33, 941–951 (2015)
Abbaszadeh, F., Moradi, O., Norouzi, M., Sabzevari, O.: Improvement single-wall carbon nanotubes (SWCNTs) based on functionalizing with monomers 2-hydroxyethylmethacryate (HEMA) and N-vinylpyrrolidone (NVP) for pharmaceutical applications as cancer therapy. J. Ind. Eng. Chem. 20, 2895–2900 (2014)
Partikel, K., Korte, R., Stein, N.C., et al.: Effect of nanoparticle size and PEGylation on the protein corona of PLGA nanoparticles. Eur. J. Pharm. Biopharm. 141, 70–80 (2019)
Tang, G.P., Zeng, J.M., Gao, S.J., et al.: Polyethylene glycol modified polyethylenimine for improved CNS gene transfer: effects of PEGylation extent. Biomaterials 24, 2351–2362 (2003)
Rheiner, S., Bae, Y.: Increased poly(ethylene glycol) density decreases transfection efficacy of siRNA/poly(ethylene imine) complexes. AIMS Bioeng. 3, 454–467 (2016)
Yang, C., Gao, S., Dagnæs-Hansen, F., et al.: Impact of PEG chain length on the physical properties and bioactivity of PEGylated chitosan/siRNA nanoparticles in vitro and in vivo. ACS Appl. Mater. Interfaces 9, 12203–12216 (2017)
Cabral, H., Miyata, K., Osada, K., Kataoka, K.: Block copolymer micelles in nanomedicine applications. Chem. Rev. 118, 6844–6892 (2018)
Agirre, M., Zarate, J., Ojeda, E., et al.: Low molecular weight chitosan (LMWC)-based polyplexes for pDNA delivery: from bench to bedside. Polymers 6, 1727–1755 (2014)
Du, B., Jiang, X., Huang, Y., et al.: Tailoring kidney transport of organic dyes with low-molecular-weight PEGylation. Bioconj. Chem. 31, 241–247 (2019)
Zhang, Y., Satterlee, A., Huang, L.: In vivo gene delivery by nonviral vectors: overcoming hurdles? Mol. Ther. 20, 1298–1304 (2012)
Tiera, M.J., Shi, Q., Barbosa, H.F.G., Fernandes, J.C.: Polymeric systems as nanodevices for siRNA delivery. Curr. Gene Ther. 13, 358–369 (2013)
Tamura, A., Yui, N.: Lysosomal-specific cholesterol reduction by biocleavable polyrotaxanes for ameliorating niemann-pick type C disease. Sci. Rep. 4, 1–8 (2014)
Wang, X., Cai, X., Hu, J., et al.: Glutathione-triggered “off-on” release of anticancer drugs from dendrimer-encapsulated gold nanoparticles. J. Am. Chem. Soc. 135, 9805–9810 (2013)
Adriaansen, J., Vervoordeldonk, M.J.B.M., Tak, P.P.: Gene therapy as a therapeutic approach for the treatment of rheumatoid arthritis: innovative vectors and therapeutic genes. Rheumatology 45, 656–668 (2006)
Ta, W., Chawla, A., Pollard, J.W.: Origins and hallmarks of macrophages: development, homeostasis, and disease. Nature 496, 445–455 (2013)
Pandi, P., Jain, A., Raju, S., Khan, W.: Therapeutic approaches for the delivery of TNF-α siRNA. Ther. Deliv. 8, 343–355 (2017)
Staudacher, A.H., Al-Ejeh, F., Fraser, C.K., et al.: The La antigen is over-expressed in lung cancer and is a selective dead cancer cell target for radioimmunotherapy using the La-specific antibody APOMAB®. EJNMMI Res. 4, 1–13 (2014)
Tiera, M.J., Qiu, X.P., Bechaouch, S., et al.: Synthesis and characterization of phosphorylcholine-substituted chitosans soluble in physiological pH conditions. Biomacromol 7, 3151–3156 (2006)
Martins, G.O., Petrônio, M.S., Lima, A.M.F., et al.: Amphipathic chitosans improve the physicochemical properties of siRNA-chitosan nanoparticles at physiological conditions. Carbohydr. Polym. 216, 332–342 (2019)
Gunn, J., Paranji, R.K., Zhang, M.: A simple and highly sensitive method for magnetic nanoparticle quantitation using 1H-NMR spectroscopy. Biophys. J. 97, 2640–2647 (2009)
Gabriel, J.D.S., Tiera, M.J., Tiera, V.A.D.O.: Synthesis, characterization, and antifungal activities of amphiphilic derivatives of diethylaminoethyl chitosan against Aspergillus flavus. J. Agric. Food Chem. 63, 5725–5731 (2015)
Richard, I., Thibault, M., De Crescenzo, G., et al.: Ionization behavior of chitosan and chitosan-DNA polyplexes indicate that chitosan has a similar capability to induce a proton-sponge effect as PEI. Biomacromol 14, 1732–1740 (2013)
Rai, R., Alwani, S., Badea, I.: Polymeric nanoparticles in gene therapy: new avenues of design and optimization for delivery applications. Polymers 11, 745 (2019)
Barreto, J.C.G., Tita, D.L., Orlandia, M.O.: Development of an automated method to perform a quantitative study of particle size distribution and the effect of a conductive layer in scanning electron microscopy. Quim. Nova 42, 447–452 (2019)
Fernandes, J.C., Qiu, X., Winnik, F.M., et al.: Low molecular weight chitosan conjugated with folate for siRNA delivery in vitro: optimization studies. Int. J. Nanomed. 7, 5833 (2012)
Fujita, S., Sakairi, N.: Water soluble EDTA-linked Chitosan as a zwitterionic flocculant for pH sensitive removal of Cu(II) ion. RSC Adv. 6, 10385–10392 (2016)
Fiamingo, A., Campana-Filho, S.P.: Structure, morphology and properties of genipin-crosslinked carboxymethylchitosan porous membranes. Carbohydr. Polym. 143, 155–163 (2016)
Zhang, K., Helm, J., Peschel, D.: NMR and FT Raman characterisation of regioselectively sulfated chitosan regarding the distribution of sulfate groups and the degree of substitution. Polymer 51, 4698–4705 (2010)
Zhang, J., Xia, W., Liu, P., et al.: Chitosan modification and pharmaceutical/biomedical applications. Mar. Drugs 8, 1962–1987 (2010)
Jain, A., Gulbake, A., Shilpi, S., et al.: A new horizon in modifications of chitosan: syntheses and applications. Crit. Rev. Ther. Drug Carr. Syst. 30, 91 (2013)
Thomas, D.P., William, C.E., Jennifer, L.S., Graham, T.J.: Cell Biology, 3rd edn. Elsevier, Philadelphia (2016)
Vermeulen, L.M., De Smedt, S.C., Remaut, K., Braeckmans, K.: The proton sponge hypothesis: fable or fact? Eur. J. Pharm. Biopharm. 129, 184–190 (2018)
Casey, J.R., Grinstein, S., Orlowski, J.: Sensors and regulators of intracellular pH. Nat. Rev. Mol. Cell Biol. 11, 50–61 (2010)
Doriti, A., Brosnan, S.M., Weidner, S.M., Schlaad, H.: Synthesis of polysarcosine from air and moisture stable N-phenoxycarbonyl-N-methylglycine assisted by tertiary amine base. Polym. Chem. 7, 3067–3070 (2016)
Serrano-Sevilla, I., Artiga, Á., Mitchell, S.G., et al.: Natural polysaccharides for siRNA delivery: nanocarriers based on chitosan, hyaluronic acid, and their deerivatives. Molecules 24, 2570 (2019)
Layek, B., Singh, J.: Chitosan for DNA and gene therapy. In: Jennings, J.A., Bumgarden, J.D. (eds.) Chitosan Based Biomaterials Volume 2: Tissue Engineering and Therapeutics, pp. 209–244. Woodhead Publishing, Amsterdam (2017)
Chen, H., Cui, S., Zhao, Y., et al.: Grafting chitosan with polyethylenimine in an ionic liquid for efficient gene delivery. PLoS ONE 10, e0121817 (2015)
Kean, T., Roth, S., Thanou, M.: Trimethylated chitosans as non-viral gene delivery vectors: cytotoxicity and transfection efficiency. J. Control. Release 103, 643–653 (2005)
Layek, B., Singh, J.: Caproic acid grafted chitosan cationic nanocomplexes for enhanced gene delivery: effect of degree of substitution. Int. J. Pharm. 447, 182–191 (2013)
Liu, X., Howard, K.A., Dong, M., et al.: The influence of polymeric properties on chitosan/siRNA nanoparticle formulation and gene silencing. Biomaterials 28, 1280–1288 (2007)
Yue, Z.G., Wei, W., Lv, P.P., et al.: Surface charge affects cellular uptake and intracellular trafficking of chitosan-based nanoparticles. Biomacromol 12, 2440–2446 (2011)
Makvandi, P., Chen, M., Sartorius, R., et al.: Endocytosis of abiotic nanomaterials and nanobiovectors: Inhibition of membrane trafficking. Nano Today 40, 101279 (2021)
Picola, I.P.D., Busson, K.A.N., Casé, A.H., et al.: Effect of ionic strength solution on the stability of chitosan-DNA nanoparticles. J. Exp. Nanosci. 8, 703–7016 (2013)
Behzadi, S., Serpooshan, V., Tao, W., et al.: Cellular uptake of nanoparticles: journey inside the cell. Chem. Soc. Rev. 46, 4218–4244 (2017)
Saptarshi, S.R., Duschl, A., Lopata, A.L.: Interaction of nanoparticles with proteins: relation to bio-reactivity of the nanoparticle. J. Nanobiotechnology. 11, 1–12 (2013)
Carlos, M.I.S., Zheng, K., Garrett, N., et al.: Limiting the level of tertiary amines on polyamines leads to biocompatible nucleic acid vectors. Int. J. Pharm. 526, 106–124 (2017)
Gormley, A.J., Ghandehari, H.: Evaluation of toxicity of nanostructures in biological systems. In: Sahu, S.C., Casciano, D.A. (eds.) Nanotoxicity: From In Vivo and In Vitro Models to Health Risks, pp. 115–159. Wiley, Hoboken (2009)
Kim, B., Park, J.H., Sailor, M.J.: Rekindling RNAi therapy: materials design requirements for in vivo siRNA delivery. Adv. Mater. 31, 1903637 (2019)
Hao, G., Xu, Z.P., Li, L.: Manipulating extracellular tumour pH: an effective target for cancer therapy. RSC Adv. 8, 22182–22192 (2018)
Guţoaia, A., Schuster, L., Margutti, S., et al.: Fine-tuned PEGylation of chitosan to maintain optimal siRNA-nanoplex bioactivity. Carbohydr. Polym. 143, 25–34 (2016)
Ping, Y., Liu, C., Zhang, Z., et al.: Chitosan-graft-(PEI-β-cyclodextrin) copolymers and their supramolecular PEGylation for DNA and siRNA delivery. Biomaterials 32, 8328–8341 (2011)
Germershaus, O., Mao, S., Sitterberg, J., et al.: Gene delivery using chitosan, trimethyl chitosan or polyethylenglycol-graft-trimethyl chitosan block copolymers: establishment of structure-activity relationships in vitro. J. Control. Release 125, 145–154 (2008)
Ragelle, H., Riva, R., Vandermeulen, G., et al.: Chitosan nanoparticles for siRNA delivery: optimizing formulation to increase stability and efficiency. J. Control. Release 176, 54–63 (2014)
Acknowledgements
This research was supported by a grant from the São Paulo Research Foundation, FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) grant n. 2017/10331-5. A. M. Martinez-Junior acknowledge the support of the National Council for the Improvement of Higher Education, CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) grant n. 2017/10331-5. A. M. Martinez-Junior and M. S. Petrônio acknowledge the support of FAPESP (grants 2019/27801-0 and 2015/05148-1). J. Fernandes and M. Benderour would like to thank the Ministère de l’Économie, de la Science et de l’Innovation du Québec, (PSR-SIIRI-960) and the Chaire de Recherche en Orthopédie de l’Université de Montréal à l’Hôpital du Sacré-Cœur de Montréal). The authors would also like to thank: the CMIB-UNESP (EMU-FAPESP project nº 2009/53989-4) for instrumentation access and Dr. F. R. de Moraes for his help with 13C NMR analysis; Dr. S. C. M. Agustinho and A. L. Tognon (MSc) for help with 1H NMR analysis (IQSC-USP); the LMA-IQ (UNESP) laboratory for providing SEM facilities and Dr. D. Tita for his assistance with Scanning Electron Microscopy; Dr. M. F. Lima (LQBOA Group-UNESP/IBILCE), Dr. M. P. S Cabrera (Peptides Research Group-UNESP/IBILCE) and Dr. C. R. B. Domingos (LHGDH Group-UNESP/IBILCE) for access to facilities and instrumentation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
MJT, JCF, AMMJ, RHFVS and MB have filed for a patent on the nanoparticles described in this study. The authors report no other conflicts of interest in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
40097_2022_487_MOESM1_ESM.docx
Supplementary file1 1H and 13C NMR spectra, dynamic light scattering measurements, cytotoxicity in RAW 264.7 cells, 3D confocal images, GPC chromatograms, and titrations data can be found in the supplementary information of the manuscript. (DOCX 5337 KB)
Rights and permissions
About this article
Cite this article
Martinez Junior, A.M., de Souza, R.H.F.V., Petrônio, M.S. et al. Double-grafted chitosans as siRNA nanocarriers: effects of diisopropylethylamine substitution and labile-PEG coating. J Nanostruct Chem 13, 605–624 (2023). https://doi.org/10.1007/s40097-022-00487-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40097-022-00487-0