Abstract
In this study, NiFe2O4/hydroxyapatite/graphene quantum dots (NiFe2O4/HAP/GQDs) as nano-adsorbent to remove cadmium (Cd2+) from aqueous solution were synthesized. This nano-adsorbent was depicted by some techniques such as X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM) and Fourier transform infrared spectroscopy (FT-IR). Adsorption studies were conducted to investigate the effect of contact time, pH, adsorbent dosage and initial cadmium ion concentration on removal efficiency. To determine the most fitting kinetic model, the suitability of pseudo-first-order and pseudo-second-order models was compared. The adsorption isotherm was described well by the Langmuir isotherm and maximum equilibrium uptake capacity (qe) was 344.83 mg g−1. Studies revealed that adsorption process is not a physical process and formation of complexes (bidentate and monodentate complexes) between—COO− and—OH functional groups on the surface of NiFe2O4/HAP/GQDs and Cd2+ ions may occur. In addition, the adsorbent can simply be removed by an ordinary filtration process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Poisonous heavy metals like cadmium have been classified as the most destructive pollutants in the environment, because it is not degradable and highly harmful to health [1]. Cadmium has been shown to cause kidney failure [2]. Due to its hazardous impacts on human healthiness, removing cadmium from water and wastewater is a major environmental problem [3,4,5,6].
Different techniques have been used to remove metals from waters and wastewaters including ion exchange, reverse osmosis, chemical precipitation and electrolysis [7,8,9,10,11]. The above-mentioned methods have some disadvantages, for instance, time consuming, high cost, and secondary waste [12]. The use of nanotechnology is a major method for solving the problems of these methods [13,14,15,16,17,18]. Magnetic nanoparticles (NPs) have some unique properties such as great surface area, high adsorption capability, and rapid adsorption process. In last years, these materials have been more significant due to their particular characteristics. One of their specific characteristics is that almost all atoms are on the area of the nanoparticles. So, they can attach with more atoms that present superior chemical activity. Therefore, the nanoparticles can attract metal ions with a large adsorption rate [19]. Between different nanoparticles, hydroxyapatite (HAP) and graphene quantum dots (GQDs) are of great importance for their excellent usages. HAP (Ca10(PO4)6(OH)2) has been utilized for medical applications [20]. Considering its high specific surface area, slight solubility in water, numerous hydroxyl groups, availability, cheapness, environmental benign and easy synthesis method, it has been widely used as a sorbent for the removal of toxic elements from wastewaters [21].
GQDs, as a member of carbon-based nanomaterial, possess special properties including chemical inertness, slight toxicity, marvelous bio consistency, and excellent photo stability [22]. GQDs are graphene sheets similar to single, double and multiple layers all smaller than 10 nm deep and 100 nm in sideways size. Therefore, GQDs have ultrahigh specific surface area owing to the character of nano-sized graphene sheets; they have the ability for effective separation and removal of pollutants from environments. One can assume that by these types modified NiFe2O4 nanoparticles can improve the adsorption efficiency of cadmium.
In this project, a new magnetic nano-adsorbent, NiFe2O4/HAP/GQDs, was introduced to remove cadmium from aqueous solutions. Therefore, nano-adsorbent was synthesized (Scheme 1) and characterized by various techniques. Next, the properties of cadmium adsorption were investigated. Eventually, the efficacy of contact time, pH, NiFe2O4/HAP/GQDs dose and cadmium solution concentration on adsorption process was tested.
Materials and methods
Chemicals and reagents
Calcium nitrate tetrahydrate, sodium hydroxide, ammonium hydroxide, ferric nitrate 9-hydrate and nickel nitrate hexahydrate were obtained from Sigma-Aldrich. Acetic acid, hydrochloric acid and phosphoric acid were obtained from BDH. Paraffin oil was obtained from Iran paraffin Co. Cadmium sulfate hydrate was purchased from Merck Company of Germany. A 1000 mg L−1 stock solution of the salt was prepared in deionized water.
Instrumentation
The crystalline phase was tested by a diffractometer of the Philips Company with X’PertPro monochromatized Cu Kα radiation (λ = 1.54 Å). The microscopic structures and particle size were examined using SEM (Hitachi S-4800). Transmission electron microscopy (TEM) was carried out with a Leo 912AB (120 kV) microscope (Zeiss, Germany). FT-IR spectrum was specified on a Bruker model 470 spectrophotometer as KBr pellets. The magnetic characteristic was investigated by vibrating sample magnetometer (VSM, LDJ9600). The concentrations of cadmium ions were measured by an atomic absorption spectrophotometer (Varian AA240FS.)
Preparation of NiFe2O4/HAP/GQDs NPs
GQDs were prepared by pyrolysis of citric acid [23]. Concisely, 0.2 g of citric acid was added to a 100 ml round-bottomed flask and was heated at 200 °C for 5 min, until the white powder turned into a dark yellow viscous liquid. After the liquid was made, 20 ml of NaOH solution (0.25 M) was poured into a beaker, and while solution was stirred on the magnetic stirrer (at ambient temperature), the as-resulted liquid was added by pipette gradually into NaOH solution and allow to mix well together for 30 min. After 30 min, graphene dots were made and stored in a refrigerator (4 °C). 60 ml of egg white was poured into a beaker and mixed with 40 ml of distilled water. The mixture was stirred strongly at room temperature to completely mix each other and make a clear, uniform solution. Then 2.9081 g of Ni(NO3)2·6H2O and 8.0800 g of Fe(NO3)3·9H2O were added slowly to the previous solution and the solution was stirred strongly at laboratory temperature for 2 h until the salts were completely dissolved. In the next step, while stirring the solution, the solution was heated to 80° C for several hours until dried. The resulted powder was milled and calcined in a furnace at 700 °C for 3 h [24].
The dispersed NiFe2O4 in solution was then mixed with 10 mL Ca(NO3)2. 4H2O solution (0.835 mol L−1) and 10 mL NH4OH (28% w/v) to control pH close to 11. Next, 10 mL H3PO4 (0.5 mol L−1) was added gradually (Ca/P molar ratio was about 1.67) [25]. After incubation at 80 °C for 30 min, the mixture was mixed with GQDs (20 mL) and stirred at 80 °C for 30 min.
Then, the resulting magnetic nanoparticles were cooled to room temperature and left for 2 h without stirring. The obtained solid was separated by a magnet; several steps were washed with water to neutralize and then dried at 90 °C, and crushed. Then, the NiFe2O4/HAP/GQDs were obtained.
Cadmium adsorption by NiFe2O4/HAP/GQDs
Adsorption of Cd2+ from aqueous solution by NiFe2O4/HAP/GQDs was examined in batch experiments. The effect of several effective parameters, i.e., reaction time, pH, adsorbent dosage and starting concentration was studied. In these experiments, all vessels were shaken at 200 rpm and at lab temperature (25 ± 1 °C). The pHs of solutions were regulated with proper concentrations of HCl and NaOH by a pH meter. Kinetic experiments were conducted with constant NiFe2O4/HAP/GQDs dose (0.1 g) at certain time intervals (1.0, 3.0, 5.0, 7.0, 10, 15, 20, 30, 40 and 60 min) at pH = 6. The uptake isotherm experiments were done by mixing 0.03 g of NiFe2O4/HAP/GQDs in a jar containing 100 mL of Cd2+ solutions with varying concentrations (5, 10, 20, 40, 50 and 100 mg L−1) at pH = 6 for 10 min to reach equilibrium. Kinetic experiments demonstrated that contact time of 10 min was enough to attain equilibrium. So, this period of time was chosen for all isotherm experiments. After that, the nano-adsorbent was separated magnetically from solutions and then remaining cadmium concentration was measured by atomic absorption spectrophotometer. Then, amount of adsorbed per unit mass of adsorbent, qe (mg g−1), was calculated using Eq. (1) [26]:
where C0, Ce, V and M are initial Cd concentration (mg L−1), equilibrium concentration of Cd (mg L−1), volume of solution (L) and adsorbent dosage (g), respectively.
Results and discussion
Characterization of NiFe2O4/HAP/GQDs
The particle size of the NiFe2O4/HAP/GQDs was examined by the SEM and TEM. The SEM image of the NiFe2O4/HAP/GQDs in Fig. 1 reveals that the average size of adsorbent is about 25 nm. The TEM image of the NiFe2O4/HAP/GQDs in Fig. 2 shows that monotonous and compatible spherical shapes were achieved with an average diameter of about less than 25 nm. They were aggregated with several nanoparticles, and create a rough surface. Also, to analyze the relative amount of the principal elements of the NiFe2O4/HAP/GQDs nanocomposite containing Ni, Fe, O, Ca, P and C, EDX spectrum was handled to verify their elemental compositions (Fig. 1). According to EDX determinations, Fe (33.36%), Ni (2.64%), O (51.19%), Ca (2.37%), P (1.44%) and C (6.49%) were present in NiFe2O4/HAP/GQDs. The Ca:P ratio calculated from the EDX was 1.65 which is near to the stoichiometric ratio (1.67) that occurs in the natural hydroxyapatite [27, 28]. This showed that the hydroxyapatite was formed with the suggested process.
The FT-IR spectra of NiFe2O4, NiFe2O4/HAP, and NiFe2O4/HAP/GQDs are illustrated in Fig. 3. In FT-IR spectra, there are two typical peaks at 555 and 1440 cm−1 are correlated with Fe–O vibrations [29]. The bands approximately 971 and 1116 cm−1 are due to stretching mode of phosphate (P–O, \({\text{PO}}_{4}^{3 - }\)) [30]. They are the main attributes of HAP bands. The absorption band appearing around 3300 cm−1 represents O–H vibrations. Moreover, the peak located at 3100 cm−1 in spectrum indicates the asymmetric stretching and symmetric vibrations of C–H. The occurrence of the C=C bending vibrations peak at 1574 cm−1 and C=O stretching vibration band at 1668 cm−1 gives a strong proof of GQDs and synthesis of NiFe2O4/HAP/GQDs [31].
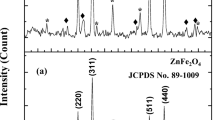
To verify the formation of NiFe2O4 in the prepared nanocomposite, the XRD patterns of the samples were investigated (Fig. 4). The XRD patterns (Fig. 4a) confirm that these nanoparticles are spinel shaped, and all of the main peaks corresponding the standard pattern of bulk NiFe2O4 (JCPDS 08-0234). Additionally, the existence of hydroxyapatite is proved in Fig. 4b. As can be shown in Fig. 4c, no signal about GQDs can be detected, which is described by the small amounts, high dispersion and low crystallinity of GQDs in NiFe2O4/HAP/GQDs nanocomposite.
The magnetic characteristics of the NiFe2O4/HAP/GQDs nanocomposite were identified using vibrating sample magnetometer (VSM). As can be seen in Fig. 5, M(H) hysteresis loop was completely reversible for the sample, indicating that the NiFe2O4/HAP/GQDs nanocomposite shows superparamagnetic characteristics. The hysteresis loops of them reached saturation up to the ultimate applied magnetic field. At room temperature, the magnetic saturation value of the NiFe2O4/HAP/GQDs is 24.88 emu g−1. The high permeability of particles in the magnetization showed that they could be separated by a typical magnetometer.
Effect of contact time
The impact of contact time on the Cd2+ adsorption value by NiFe2O4/HAP/GQDs was studied at 10 mg L−1, to optimize the adsorption time and to investigate the kinetics of the uptake process. As contact time enhances, the concentration of Cd2+ in the solution reduced quickly at first and later decelerates until it stayed constant at around 10 min, which was taken as the equilibrium time (Fig. 6). The adsorption specification suggested a rapid uptake of the cadmium. The fast level of Cd2+ adsorption at the initial minutes of adsorption process may be owing to the great number of empty sites accessible at the early period of the adsorption [32].
Effect of pH
The acidic property of a solution is one of the determining factors in the uptake process, specifically adsorption capacity [29]. To assess the impact of pH on the removal efficiency, the tests were accomplished in the pH range of 3–7. The results are shown in Fig. 7.
In lower pHs, high concentrations of hydrogen ions exist and, based on the theory of surface complex formation, hydrogen ions are absorbed instead of the metal ions.
So, as shown in Fig. 6 the removal of Cd2+ in low pHs is slighter than that of in high-level pHs. The amount of Cd2+ adsorbed increased with increasing the pH value of solution from 3 to 6.0 (approximately); this aspect can be interpreted by precipitation of Cd2+ as insoluble Cd(OH)2 precipitate. In other words, in these pH values (˃ 6), the Cd(II) ions begin to hydrolyze and then form a small quantity of cadmium hydroxyl species [33]. In other words, at lower pH (less than 3), the low metal adsorption can be assigned to the protonated surfaces of NiFe2O4/HAP/GQDs, electron repulsion, and competition between Cd2+ and H + ions. For the pH ranges from 3 to 6, the equilibrium absorption of Cd (II) ions increased dramatically as the pH increased.
This treatment can be attributed to a reduction in the amount of protonation by increasing the pH, which minimizes the competition between H+ and Cd2+ions on active sites and increase the Cd(II) ions. Additional increment in solution pH, beyond 7, brings about precipitation of cadmium as cadmium hydroxide. In comparison with Cd(II) ions, these species are undesirable for adsorption, which is a reason for a slight reduction in removal efficiency percent and adsorption capacity. Thus, the maximum absorption was around pH 6.0, and therefore selected for all absorption experiments in this study.
Effect of adsorbent dosage
The uptake productivity rises with enhancing adsorbent dosage on the adsorption processes. It is of vital importance to achieve the best removal efficiency with low adsorbent dose as possible. Therefore, the most efficient adsorbent dose measurement for adsorption processes is necessary. The effect of the adsorbent dosage was investigated by repeating experiments with different adsorbent dosage (from 0.01 to 0.2 g/100 mL). Accordingly, the values of other parameters were applied as contact time 10 min, the initial pH value of solution 6, initial Cd(II) ions concentrations 10 mg L−1, and temperature 25 °C. The outcomes are illustrated in Fig. 8. According to the results obtained from this study, the adsorption efficacy increased significantly with rising adsorbent dosage. As adsorbent amount in solution is increased, the removal efficiency also rapidly rises. The greatest removal efficiency was found to be 97.6% at the adsorbent mass of 0.03 g/100 mL. The increase in cadmium removal with increasing adsorbent dosage can be ascribed to enhanced surface area and the adsorption sites. This situation may be explained by the fact that the higher the concentrations of adsorbent, the more sorbent surface and pore volume will be accessible for the adsorption (Fig. 8).
Effect of initial Cd(II) ions concentration
Batch adsorption tests were carried out at various initial Cd2+ concentrations (5, 10, 20, 40, 50, 100, 200 and 300 mg L−1), at pH 6. 0.03 g/100 mL of NiFe2O4/HAP/GQDs was used for each adsorption test, with a contact time of 10 min. Figure 9 illustrated that with a rise in the initial cadmium concentration from 5.0 to 300 mg L−1, the amount of cadmium adsorption decreased. Removal percentage was greater for low initial Cd2+ concentration owing to accessibility of vacant binding sites on the adsorbent. Since, at high Cd2+ concentrations the binding sites were approximately entirely covered, removal efficiency decreased with increasing metal concentration.
Effect of initial Cd(II) ions concentration on % Cd(II) ions removal efficiency (contact time = 10 min., initial pH = 6, adsorbent dose = 0.03 g/100 mL and t Fig. 11 The Langmuir (a), Freundlich (b) and Dubinin − Radushkevich (c) isotherms for the Cd2+ on the NiFe2O4/HAP/GQDs surface
Effect of ionic strength
To investigate the effect of ionic strength on the efficiency of removal of Cd(II) by NiFe2O4/HAP/GQDs, tests were conducted under optimum conditions, and five different times (5, 10, 20, 30 and 60 min) with three different ionic strengths (0.1, 0.3 and 3 mol L−1). The ionic strength in each experiment was set using sodium sulfate. The results of each experiment are calculated and shown in Fig. 10. The ionic strength of the solution in the adsorption process of cadmium ions is very important. Because solutions with high ionic strength are similar and comparable to wastewaters, such solutions result in more absorption through electromagnetic interactions between cadmium ions and adsorbent surface. In accord with the surface chemistry theory, when NiFe2O4/HAP/GQDs is in contact with Cd(II) ions in solution, they are encircled by an electrical diffused double layer, the thickness of which is remarkably extended in the presence of electrolyte [34]. In this study, to evaluate the effect of ionic strength on the adsorption process by NiFe2O4/HAP/GQDs, the ability of three different ionic strengths (0.1, 0.3 and 3 mol L−1) were chosen. As Fig. 10 displays, with rising of ionic strength from 0.1 to 3 mol L−1, Cd(II) ions removal efficiency reduces. This phenomenon occurs because increasing the concentration of sodium ions in the solution increases the ionic strength of the solution too. The sodium ions are located near the surface of NiFe2O4/HAP/GQDs. The aggregation of these positive ions throughout the adsorbent leads to a reduction in the exposure between Cd(II) ions and NiFe2O4/HAP/GQDs and finally, Cd(II) ions adsorption capability by the adsorbent reduces. On the other hand, the high concentration of sodium ions competes with Cd2+ ions and place on the active sites present on the surface of NiFe2O4/HAP/GQDs. Therefore, the active sites on the surface of the NiFe2O4/HAP/GQDs fill completely and cause a reduction in Cd(II) ions removal efficiency.
Adsorption isotherms
An adsorption isotherm model shows the equilibrium correlation between the absorption of matter per unit mass of adsorbent at constant temperature and its concentration in solution [35, 36]. The experimental outcomes of this study were investigated by Langmuir, Freundlich and Dubinin–Radushkevich (D–R) models, as indicated in Table 1 and Fig. 11.
The Langmuir isotherm model, which proposes that adsorption takes place at homogeneous sites by monolayer uptake with no considerable interaction between adsorbed ions, is given as follows:
where \(q_{\text{e}}\) is the value of Cd2+ adsorbed per unit mass at equilibrium (mg g−1), \(q_{\text{m}}\) denotes the maximum value of adsorbent that can be adsorbed per unit mass adsorbent (mg g−1), \(C_{\text{e}}\) is the concentration of adsorbent in the solution at equilibrium (mg L−1) and KL (L mg−1) represents the Langmuir isotherm constant. A linear plot of Ce/qe against Ce, yields a straight line with a slope of 1/qm and an intercept of 1/KLqm [37].
The main features of the Langmuir isotherm can be stated in terms of a dimensionless constant separation factor RL which is defined as [38]:
where C0 the initial concentration of Cd2+ ions (mg L−1), and KL (L mg−1) is the Langmuir constant. The amount of RL indicates the shape of the isotherm to be either unfavorable (RL > 1), linear (RL = 1), favorable (0 < RL < 1), or irreversible (RL < 0). In this study, the value of RL is 0.042 and shows the favorable adsorption between NiFe2O4/HAP/GQDs and Cd2+.
The Freundlich isotherm is used for heterogeneous adsorption with various surface energy sites and considers the alteration of adsorption with exponential distribution of adsorption sites and energies. The Freundlich model is represented as follows [39]:
where qe is the amount of adsorbent adsorbed per unit mass of adsorbent (mg g−1), Ce denotes the equilibrium concentration of adsorbent in the solution (mg L−1), KF (mg1–(1/n) L1/n g−1) and n depict Freundlich constants which indicate the uptake capacity for the adsorbent and uptake intensity, respectively. Freundlich parameters KF and n can be calculated from the intercept and slope of a linear plot with logqe versus logCe.
If the value of 1/n < 1, then the adsorption intensity and the type of isotherm to be desirable and the adsorption capacity rise with the appearance of new adsorption sites. But if 1/n > 1, the absorption bond weakens and its absorption is very difficult, which reduces the absorption capacity. In this study, the value of 1/n (0.415) displays the favorable adsorption of Cd2+on NiFe2O4/HAP/GQDs.
Dubinin–Radushkevich (D–R) equation was used to determine whether chemical or physical absorption was observed. This equation defined as:
where qm is the maximum adsorption capacity of metal ions (mg g−1), β is a constant related to the mean energy of adsorption (mol2 kJ−2), ε is the Polanyi potential given as follows:
where T is the temperature (K) and R is the gas constant (8.314 J K−1 mol−1). A linear plot of
ln qe against \(\varepsilon^{2}\), gives a straight line with a slope of and an intercept of ln qm. With the value of β, the mean energy E, which is the free energy transfer of one mole of solute from infinity to absorbing surface, can be evaluated by Eq. (7):
If E < 8 kJ mol−1, then the adsorption process might be performed physically, but if E > 8 kJ mol−1 chemical adsorption may occur [40].
Table 1 shows the values of Langmuir, Freundlich and (D–R) constants and the correlation coefficients obtained from the linear regression. Based on the results, it can be derived from R2 that both Langmuir and Freundlich models hold high linearity as they are very close to each other. Thus, two criterions (MSE and χ2) were used to check the accuracy of the Langmuir and Freundlich models as calculated by following equations [41]:
where qexp and qcal represent the experimental and calculated uptake values, respectively (mg g−1).
Table 2 shows that the Langmuir model has low values of MSE and χ2, while the Freundlich model has the highest error rate. These results supported the validity of the Langmuir model for the absorption process. Although both models were almost identical to R2, there was a significant difference in MSE and χ2 values between the two models, which indicates that R2 can be used only for the use of the trend, and the two equal values of R2 inevitably mean errors are not the same.
From the Table 1, it is denoted that the predicted qm value from D–R isotherm is not conforming with the formerly determined Langmuir isotherm qm value. The correlation coefficient for the D–R isotherm is lower than the values of the two isotherm models (Table 1), which indicates that the Cd(II) ions uptake on NiFe2O4/HAP/GQDs is not a physical process [42].
Adsorption kinetics
Different kinetic models are proposed for precise design of the absorption process [32, 43]. In this study, the equations of Lagergren’s pseudo-first-order kinetic model and Ho’s pseudo-second-order kinetic model were used. The pseudo-first order model is given as [44]:
where qe and qt (mg g−1) are the adsorption capacities at equilibrium and at time t (min), respectively. k1 (min−1) is the rate constant for the pseudo-first-order model.
The pseudo-second-order equation is defined as [45]:
where k2 (g mg−1 min−1) is the rate constant for the pseudo-second-order rate equation, and other symbols have their usual meanings. The amounts of qe and k2 can be derived from the slope and intercept of the plot of t/qt versus t. The kinetics parameters of the pseudo-first- and pseudo-second-order models and their corresponding coefficients of correlation, R2, are indicated in Table 3 and Fig. 12.
The results of Table 3 showed that the correlation coefficient was very high (R2 = 0.999). Additionally, the calculated equilibrium absorption capacity was consistent with experimental results. This result showed that kinetic data were better arranged with a pseudo-second kinetic model. Based on the pseudo-second order model, the chemical absorption is a limiting step, so reliable results suggest that the absorption behavior may be in the sharing of electrons or exchange between the Cd2+ ions and NiFe2O4/HAP/GQDs. Similar findings have been reported for the absorption of Cd2+ ions [46, 47]. Therefore, the results from the interpretation of isotherms are consistent with the results of kinetics studies.
Considering the above results, the Cd2+ adsorption by NiFe2O4/HAP/GQDs probably can occur in the pathway [48,49,50], which was demonstrated in Scheme 2. The formation of complexes (bidentate and monodentate complexes) between –COO— and –OH functional groups on the surface of NiFe2O4/HAP/GQDs and Cd2+ ions has been occurred. Generally, the results showed that in this study the main route in the adsorption process between NiFe2O4/HAP/GQDs and Cd2+ may be chemical absorption.
Absorption capacity is an important parameter that determines the behavior of an adsorbent. Table 4 compares the maximum absorption capacity of NiFe2O4/HAP/GQDs for Cd(II) ions and other adsorbents in previous publications.
As shown in Table 4, the experimental data of the present study are comparable with the reported values in some cases. The absorption capacity varies and depends on the individual absorbent properties, the extent of surface/surface modification and the initial concentration of the adsorbate. However, current experiments are conducted to find a low-cost absorbent for Cd(II) treatment.
Conclusion
The results of present study show that the NiFe2O4/HAP/GQDs nano-adsorbent could be successfully used to remove cadmium ions from aqueous solutions. The results indicate that HAP/GQDs have been supported well on the NiFe2O4. The derived outcomes revealed that the equilibrium time for the adsorption of the cadmium ions onto adsorbent was 10 min and the most efficient condition for removal of cadmium at pH = 6.0 was obtained with a maximum absorption capacity of 344.83 mg g−1 at 25 °C. Increasing the concentration of cadmium reduced the absorption of the nano-adsorbent. The Langmuir isotherm model was more suitable than Freundlich isotherm for determining the equilibrium.
The kinetic studies show that the absorption process is well arranged by a pseudo-second-order kinetic model. Accordingly, NiFe2O4/HAP/GQDs nano-adsorbent is an effective absorbent adsorbent and can be used as a useful and valuable agent for removing cadmium ions from aqueous solutions.
References
Zeng, G., Pang, Y., Zeng, Z., Tang, L., Zhang, Y., Liu, Y., Zhang, J., Lei, X., Li, Z., Xiong, Y., Xie, G.: Removal and recovery of Zn2+ and Pb2+ by imine-functionalized magnetic nanoparticles with tunable selectivity. Langmuir 28, 468–473 (2011)
Jean, J., Sirot, V., Hulin, M., Le Calvez, E., Zinck, J., Noël, L., Vasseur, P., Nesslany, F., Gorecki, S., Guérin, T., Rivière, G.: Dietary exposure to cadmium and health risk assessment in children–results of the French infant total diet study. Food Chem. Toxicol. 115, 358–364 (2018)
Govender, S., Przybylowicz, W., Swart, P.: Removal of heavy metals from solution using biocompatible polymers. Desalin. Water Treat. 9, 272–278 (2009)
Shamohamadi, S., Bustanian, M., Tavakol, H.: Removing Cd2+ from water and wastewater by blowy sand; the effects of total hardness and pH. Desalin. Water Treat. 51, 3463–3471 (2013)
Mhamdi, M., Elaloui, E., Trabelsi-Ayadi, M.: Kinetics of cadmium adsorption by smectite of Oued Tfal (Gafsa Basin). Desalin. Water Treat. 52, 4245–4256 (2014)
Yang, G., Tang, L., Lei, X., Zeng, G., Cai, Y., Wei, X., Zhou, Y., Li, S., Fang, Y., Zhang, Y.: Cd(II) removal from aqueous solution by adsorption on α-ketoglutaric acid-modified magnetic chitosan. Appl. Surf. Sci. 292, 710–716 (2014)
Lee, S.M., Laldawngliana, C., Tiwari, D.: Iron oxide nano-particles-immobilized-sand material in the treatment of Cu(II), Cd(II) and Pb(II) contaminated waste waters. Chem. Eng. J. 195, 103–111 (2012)
Gong, R., Cai, W., Li, N., Chen, J., Liang, J., Cao, J.: Preparation and application of thiol wheat straw as sorbent for removing mercury ion from aqueous solution. Desalin. Water Treat. 21, 274–279 (2010)
Yogesh Kumar, K., Muralidhara, H.P., Arthoba Nayaka, Y., Balasubramanyam, J.: Low-cost synthesis of mesoporous Zn (II) − Sn (II) mixed oxide nanoparticles for the adsorption of dye and heavy metal ion from aqueous solution. Desalin. Water Treat. 52, 4568–4582 (2014)
Tang, L., Yang, G., Zeng, G.M., Cai, Y., Li, S.S., Zhou, Y., Pang, Y., Liu, Y.Y., Zhang, Y., Luna, B.: Synergistic effect of iron doped ordered mesoporous carbon on adsorption-coupled reduction of hexavalent chromium and the relative mechanism study. Chem. Eng. J. 239, 114–122 (2014)
Li, J., Wang, X., Zhao, G., Chen, C., Chai, Z., Alsaedi, A., Hayat, T., Wang, X.: Metal–organic framework-based materials: superior adsorbents for the capture of toxic and radioactive metal ions. Chem. Soc. Rev. 47, 2322–2356 (2018)
Acharya, J., Kumar, U., Rafi, P.M.: Removal of Heavy metal ions from wastewater by chemically modified agricultural waste material as potential adsorbent-a review. Int. J. Curr. Trends Eng. Technol. 8, 526–530 (2018)
Zhao, G., Li, J., Ren, X., Chen, C., Wang, X.: Few-layered graphene oxide nanosheets as superior sorbents for heavy metal ion pollution management. Environ. Sci. Technol. 45, 10454–10462 (2011)
Gu, P., Zhang, S., Li, X., Wang, X., Wen, T., Jehan, R., Alsaedi, A., Hayat, T., Wang, X.: Recent advances in layered double hydroxide-based nanomaterials for the removal of radionuclides from aqueous solution. Environ. Pollut. 240, 493–505 (2018)
Zhao, G., Huang, X., Tang, Z., Huang, O., Niu, F., Wang, X.: Polymer-based nanocomposites for heavy metal ions removal from aqueous solution: a review. Polym. Chem. 9, 3562–3582 (2018)
Siddiqui, S.I., Chaudhry, S.A.: Nigella sativa plant based nanocomposite-MnFe2O4/BC: an antibacterial material for water purification. J. Clean. Prod. 200, 996–1008 (2018)
Siddiqui, S.I., Chaudhry, S.A., Islam, S.: Green adsorbents from plant sources for the removal of arsenic an emerging wastewater treatment technology. In: Islam, S.U. (ed.) Plant-based natural products derivatives and applications, pp. 193–215. Wiley, Hoboken (2017)
Siddiqui, S.I., Ravi, R., Rathi, G., Tara, N., ul-Islam, S.: Decolorization of textile wastewater using composite materials. In: Islam, S.U., Butola, B.S. (eds.) Nanomaterials in the wet processing of textiles, pp. 187–218. Wiley, Hoboken (2018)
Zhai, Y., Chang, X., Cui, Y., Lian, N., Lai, S., Zhen, H., He, Q.: Selective determination of trace mercury (II) after preconcentration with 4-(2-pyridylazo)-resorcinol-modified nanometer-sized SiO2 particles from sample solutions. Microchim. Acta 154, 253–259 (2006)
Zou, Z., Lin, K., Chen, L., Chang, J.: Ultrafast synthesis and characterization of carbonated hydroxyapatite nanopowders via sonochemistry-assisted microwave process. Ultrason. Sonochem. 19, 1174–1179 (2012)
Saber-Samandari, S., Gazi, M.: Cellulose-graft-polyacrylamide/hydroxyapatite composite hydrogel with possible application in removal of Cu (II) ions. React. Funct. Polym. 73, 1523–1530 (2013)
Hallaj, T., Amjadi, M., Manzoori, J.L., Shokri, R.: Chemiluminescence reaction of glucose-derived graphene quantum dots with hypochlorite, and its application to the determination of free chlorine. Microchim. Acta 182, 789–796 (2015)
Benítez-Martínez, S., Valcárcel, M.: Fluorescent determination of graphene quantum dots in water samples. Anal. Chim. Acta 896, 78–84 (2015)
Maensiri, S., Masingboon, C., Boonchom, B., Seraphin, S.: A simple route to synthesize nickel ferrite (NiFe2O4) nanoparticles using egg white. Scripta Mater. 56, 797–800 (2007)
Brundavanam, R.K., Jiang, Z.T., Chapman, P., Le, X.T., Mondinos, N., Fawcett, D., Poinern, G.E.J.: Effect of dilute gelatine on the ultrasonic thermally assisted synthesis of nano hydroxyapatite. Ultrason. Sonochem. 18, 697–703 (2011)
Awual, M.R., Khraisheh, M., Alharthi, N.H., Luqman, M., Islam, A., Karim, M.R., Rahman, M.M., Khaleque, M.A.: Efficient detection and adsorption of cadmium (II) ions using innovative nano-composite materials. Chem. Eng. J. 343, 118–127 (2018)
Dil, E.A., Ghaedi, M., Asfaram, A.: The performance of nanorods material as adsorbent for removal of azo dyes and heavy metal ions: application of ultrasound wave, optimization and modeling. Ultrason. Sonochem. 34, 792–802 (2017)
Sricharoen, P., Limchoowong, N., Techawongstien, S., Chanthai, S.: A novel extraction method for-carotene and other carotenoids in fruit juices using air-assisted, low-density solvent-based liquidliquid microextraction and solidified floating organic droplets. Food Chem. 15, 386–393 (2015)
Pol, V.G., Thiyagarajan, P., Calderon Moreno, J.M., Popa, M.: Solvent-free fabrication of ferromagnetic Fe3O4 octahedra. Ind. Eng. Chem. Res. 49, 920–924 (2009)
Moeinpour, F., Khojastehnezhad, A.: Cesium carbonate supported on hydroxyapatite coated Ni0.5Zn0.5Fe2O4 magnetic nanoparticles as an efficient and green catalyst for the synthesis of pyrano [2, 3-c] pyrazoles. Chin. Chem. Lett. 26, 575–579 (2015)
Alvand, M., Shemirani, F.: A Fe3O4@ SiO2@ graphene quantum dot core-shell structured nanomaterial as a fluorescent probe and for magnetic removal of mercury (II) ion. Microchim. Acta 184, 1621–1629 (2017)
Yousef, R.I., El-Eswed, B., Ala’a, H.: Adsorption characteristics of natural zeolites as solid adsorbents for phenol removal from aqueous solutions kinetics, mechanism, and thermodynamics studies. Chem. Eng. J. 171, 1143–1149 (2011)
Omidvar-Hosseini, F., Moeinpour, F.: Removal of Pb(II) from aqueous solutions using Acacia Nilotica seed shell ash supported Ni0.5Zn0.5Fe2O4 magnetic nanoparticles. J. Water Reuse Desal. 6, 562–573 (2016)
Osipow, L.I.: Surface chemistry: theory and industrial applications. Reinhold Pub, New York (1962)
Siddiqui, S.I., Rathi, G., Chaudhry, S.A.: Acid washed black cumin seed powder preparation for adsorption of methylene blue dye from aqueous solution: thermodynamic, kinetic and isotherm studies. J. Mol. Liq. 264, 275–284 (2018)
Khan, T.A., Chaudhry, S.A., Ali, I.: Equilibrium uptake, isotherm and kinetic studies of Cd(II) adsorption onto iron oxide activated red mud from aqueous solution. J. Mol. Liq. 202, 165–175 (2015)
Ardejani, F.D., Badii, K.H., Yousefi Limaee, N., Shafaei, S.Z., Mirhabibi, A.R.: Adsorption of direct red 80 dye from aqueous solution onto almond shells: effect of pH, initial concentration and shell type. J. Hazard. Mater. 151, 730–737 (2008)
Ay, Ç., Özcan, A.S., Erdoğan, Y., Özcan, A.: Characterization and lead (II) ions removal of modified Punica granatum L. peels. Int. J. Phytoremediation 19, 327–339 (2017)
Kerkez, Ö., Bayazit, Ş.S.: Magnetite decorated multi-walled carbon nanotubes for removal of toxic dyes from aqueous solutions. J. Nanoparticle Res. 16, 2431 (2014)
Tan, Y., Chen, M., Hao, Y.: High efficient removal of Pb(II) by amino-functionalized Fe3O4 magnetic nano-particles. Chem. Eng. J. 191, 104–111 (2012)
Beyki, M.H., Fazli, Y.: Polyhydroxyquinoline-carbon nanotube chelating resin for selective adsorption of lead ions: multivariate optimization, isothermic, and thermodynamic study. Res. Chem. Intermed. 43, 737–754 (2017)
Boparai, H.K., Joseph, M., O’Carroll, D.M.: Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles. J. Hazard. Mater. 186, 458–465 (2011)
Siddiqui, S.I., Chaudhry, S.A.: A review on graphene oxide and its composites preparation and their use for the removal of As3+ and As5+ from water under the effect of various parameters: application of isotherm, kinetic and thermodynamics. Process Saf. Environ. J. 119, 138–163 (2018)
Mostafa, M., Chen, Y.H., Jean, J.S., Liu, C.C., Lee, Y.C.: Kinetics and mechanism of arsenate removal by nanosized iron oxide-coated perlite. J. Hazard. Mater. 187, 89–95 (2011)
Khorzughy, S.H., Eslamkish, T., Doulati Ardejani, F., Heydartaemeh, M.R.: Cadmium removal from aqueous solutions by pumice and nano-pumice. Korean J. Chem. Eng. 32, 88–96 (2015)
Shan, R.R., Yan, L.G., Yang, K., Hao, Y.F., Du, B.: Adsorption of Cd(II) by Mg− Al− CO3 − and magnetic Fe3O4/Mg− Al− CO3 − layered double hydroxides: kinetic, isothermal, thermodynamic and mechanistic studies. J. Hazard. Mater. 299, 42–49 (2015)
Mallakpour, S., Behranvand, V.: Water sanitization by the elimination of Cd2+ using recycled PET/MWNT/LDH composite: morphology, thermal, kinetic, and isotherm studies. ACS Sustain Chem. Eng. 5, 5746–5757 (2017)
Sun, W., Jiang, B., Wang, F., Xu, N.: Effect of carbon nanotubeson Cd(II) adsorption by sediments. Chem. Eng. J. 264, 645–653 (2015)
Tiwari, A., Sharma, N.: Kinetic and thermodynamic studies of adsorption of Cd2+ by superparamagnetic nano iron oxide-loaded poly(acrylonitrile-Co-acrylic acid) hydrogel. Res. Chem. Intermed. 41, 2043–2062 (2015)
Tran, H.N., You, S.-J., Chao, H.-P.: Thermodynamic parameters of cadmium adsorption onto orange peel calculated from various methods: a comparison study. J. Environ. Chem. Eng. 4, 2671–2682 (2016)
Kataria, N., Garg, V.: Green synthesis of Fe3O4 nanoparticles loaded sawdust carbon for cadmium (II) removal from water: regeneration and mechanism. Chemosphere 208, 818–828 (2018)
Chansuvarn, W., Pandee, Y., Saechim, A., Habumee, K.: Adsorption of cadmium (II) ion from aqueous solution onto a raw material of bamboo powder and its surface modification. Appl. Mech. Mater. 879, 131–136 (2018)
Borah, R., Gogoi, A., Biswas, S., Goswami, R., Shim, J., Ara Begum, N., Kumar, M.: Efficacy and field applicability of Burmese grape leaf extract (BGLE) for cadmium removal: an implication of metal removal from natural water. Ecotox. Environ. Saf. 147, 585–593 (2018)
Xiong, T., Yuan, X., Chen, X., Wu, Z., Wang, H., Leng, L., Wang, H., Jiang, L., Zeng, G.: Insight into highly efficient removal of cadmium and methylene blue by eco-friendly magnesium silicate-hydrothermal carbon composite. Appl. Surf. Sci. 427, 1107–1117 (2018)
Li, R., Liang, W., Huang, H., Jiang, S., Guo, D., Li, M., Zhang, Z., Ali, A., Wang, J.J.: Removal of cadmium (II) cations from an aqueous solution with aminothiourea chitosan strengthened magnetic biochar. J. Appl. Polym. Sci. 135, 46239–46246 (2018)
Qu, J., Meng, X., Jiang, X., You, H., Wang, P., Ye, X.: Enhanced removal of Cd (II) from water using sulfur-functionalized rice husk: characterization, adsorptive performance and mechanism exploration emperature = 25°C. J. Clean. Prod. 183, 880–886 (2018)
Acknowledgements
The authors appreciate the Islamic Azad University-Bandar Abbas Branch for financial support of this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kahrizi, P., Mohseni-Shahri, F.S. & Moeinpour, F. Adsorptive removal of cadmium from aqueous solutions using NiFe2O4/hydroxyapatite/graphene quantum dots as a novel nano-adsorbent. J Nanostruct Chem 8, 441–452 (2018). https://doi.org/10.1007/s40097-018-0284-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40097-018-0284-3