Abstract
A novel, efficient and magnetically recoverable nanomaterial consisting of heteropoly acid supported on ionic liquid-modified copper ferrite nanoparticle was prepared and performed as a heterogeneous catalyst in the fast and convenient synthesis of 1H-pyrazolo[1,2-b]phthalazine-5,10-dione derivatives under mild and solvent-free conditions. The synthesized nanomaterial was characterized with FT-IR, XRD, FESEM, TEM, ICP and VSM. Furthermore, the obtained nanomaterial displayed striking reusability in the titled catalytic reaction. Compared with the various previously reported catalysts, the newly synthesized nanocatalyst is found to be most efficient with regard to operation simplicity, reaction time, yield and ease of catalyst separation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, considerable attention has been devoted to multi-component reactions (MCRs) due to their widespread applications in organic, medicinal and green chemistry. MCRs reduce the number of steps, reaction time, use of solvents and by-products [1]. Furthermore, their simplicity, efficiency and cost-effectivity make them more attractive and useful in drug design [2]. MCRs have now been well used as an excellent synthetic method for the preparation of heterocyclic compounds. Nitrogen-containing heterocyclic compound, especially heterocycles containing bridgehead hydrazine, has attracted (immense or considerable) attention because of their pharmaceutical and biological properties. For example, 1H-pyrazolo[1,2-b]phthalazine-5,10-diones were reported as the anti-inflammatory, analgesic, anti-hypoxic, anti-pyretic agents [3].
There are several methods and different catalysts reported in the literature for the synthesis of 1H-pyrazolo[1,2-b]phthalazine-5,10-diones such as use of ultrasonic irradiation [4], supported caesium carbonate [5], CuI nanoparticles [6], Al-KIT-6 [7], NiCl2.6H2O [8], InCl3 [9]. SBA-supported sulfonic acid [10], p-toluenesulfonic acid [11] and basic ionic liquid [12]. Most of the aforementioned techniques suffer from drawbacks such as prolonged reaction time, poor yields, pollution caused by toxic catalysts and solvents, numerous tedious steps and use of expensive catalysts or reagents which lead to restricted use of these derivatives in practical applications. Therefore, research on 1H-pyrazolo[1,2-b]phthalazine-5,10-diones and finding a novel catalyst would be highly desirable.
On the other hand, room temperature ionic liquids (RTIls) are suitable reaction media for synthesis [12], catalysis [13], separation and extraction [14] which have attracted much interest among chemists. However, cost and viscosity are two major obstacles for their widespread industrial applications. Therefore, immobilization of ionic liquids on the surface of solid supports could be a good methodology to overcome these problems.
The choice of an efficient support is one of the most crucial steps in catalyst systems because an efficient support could significantly improve the activity, selectivity and recycling of catalyst. Recent interest in some type of metal oxide nanoparticles as a catalyst support stems from their magnetic property. Among the various magnetic nanoparticles, magnetite (Fe3O4) is the most widely used catalysts support. However, the acidic environment reduces the magnetization of Fe3O4 because its Fe2+ content is readily oxidized. So, Fe3O4 nanoparticles must be synthesized under N2 atmosphere to prevent the air oxidation of Fe2+. Therefore, copper ferrite (CuFe2O4) as a typical ferromagnetic oxide with spinel structure has high thermal stability, moderate magnetization and considerable chemical stability and could be excellent support for homogeneous catalysts. So, by immobilization of ionic liquids on the surface of copper ferrite nanoparticles, the advantage such as high surface area and reusability could be achieved.
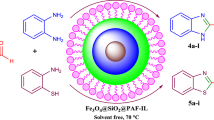
To the best of our knowledge, there is no report of magnetic ionic liquid-supported H5BW12O40 as a heterogeneous catalyst. In this paper, we present the preparation and characterization of magnetically recoverable 1-(copperferritesiloxypropyl)-3-methylimidazolium heteropolytungstate ionic liquid nanoparticles and evaluate its catalytic activity in the synthesis of 1H-pyrazolo[1,2-b]phthalazine-5,10-diones under solvent-free conditions (Scheme 1).
Experimental
Material and instrumentation
All reagents and starting materials were purchased from Merck and Sigma-Aldrich chemical companies and were used as received. Fourier transform infrared spectra were recorded on 8700 Shimadzu Fourier transform spectrophotometer using KBr pellets in the region of 400-4000 cm−1. The powder X-ray diffraction (XRD) pattern was recorded with Philips PW1730 X-ray diffractometer. Field emission scanning electron microscopy (FESEM) photographs were obtained using Hitachi S-4160 microscope. Magnetic susceptibility measurements of nanoparticles were taken using a vibrating sample magnetometer (VSM) (BHV-55, Riken, Japan) at room temperature. Elemental analysis was performed by inductivity coupled plasma on Varian, Australia, ICP-OES, model Vista Pro spectrometry. Melting points were measured using the capillary tube method with a Barnstead electrothermal type 9200 melting points apparatus. The progress of the catalytic reaction was monitored by thin layer chromatography.
Catalyst preparation
Synthesis of H5BW12O40 heteropoly acid
First, the K5[BW12O40] salt was synthesized according to reported method by Rocchiccioli-deltcheff et al. [15]. Then, the prepared heteropoly anion, K5[BW12O40], was converted into the corresponding acid by passing it through a column of Dowex-50W-X8 ion exchange resin. A sample of K5[BW12O40] (3 g) was dissolved in 60 ml water; then, the solution was passed through the resin column in the H+ form. This process was repeated for two times. Slow evaporation of the final solution gives the pure H5BW12O40 heteropoly acid.
Synthesis of copper ferrite nanoparticles (CuFe2O4)
CuFe2O4 (magnetic) nanoparticles were prepared by thermal decomposition of Cu(NO3)2·3H2O and Fe(NO3)3·9H2O with molar ratio of 1:2 in basic solution, as reported by Dandia et al. [16].
Preparation of ionic liquid-modified magnetic nanoparticles (ILMNPs)
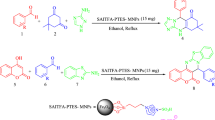
First of all, CuFe2O4 (1 g) was dispersed in 100 ml of toluene and ultrasonicated for 10 min, and then, chloropropyl trimethoxysilane (2 ml) was added dropwise to the mixture and refluxed under dry nitrogen atmosphere for 24 h. The resulted chloropropyl-modified magnetic nanoparticles (ClpMNPs) were magnetically separated and washed with ethanol and dried at 70 °C. Secondly, the obtained ClpMNPs (2 g) was suspended in 100 ml of acetonitrile, and then, 1-methylimidazole (4 mmol) and triethylamine (0.5 ml) were added to the mixture and refluxed for 24 h. The obtained ionic liquid-modified magnetic nanoparticles (ILMNPs) were collected by magnetic field and washed abundantly with ethanol and dried at 70 °C .
Finally, ILMNPs (1 g) were suspended in 70 ml deionized water. Then, a solution of H5BW12O40 (2 mmol) in 30 ml deionized water was added to the above mixture and stirred for 12 h. The resulted solid was collected by magnetic field and washed with ethanol and dried at 60 °C.
Catalytic reaction
General procedure for the preparation of 1H-pyrazolo[1,2-b]phthalazine-5,10-diones
0.05 g of BW12-ILMNPs was added to a mixture of aromatic aldehyde (1.0 mmol), malononitrile (1.0 mmol) and phthalhydrazide (1.0 mmol). Then, the mixture was stirred at 80 °C in an oil bath under solvent-free condition for the appropriate time as indicated in Table 4. After the end of the reaction, which was monitored by TLC, the mass was cooled to 25 °C and then was dissolved in boiling ethanol, and catalyst was separated by an external magnet. Consequently, the liquor was poured into ice. After the crude product was separated by simple filtration, the pure 1H-pyrazolo[1,2-b]phthalazine-5,10-dione derivative was obtained through recrystallization from ethanol. The product purity was determined by comparison with their physical data (melting point and FT-IR spectrum) with known compounds in articles.
Result and discussion
Catalyst preparation and characterization
Scheme 2 illustrates the procedure used for the preparation of magnetic nanocatalyst. In the first step, CuFe2O4 nanoparticles have been prepared by the thermal decomposition of Fe3+ and Cu2+ in basic solution [16]. Then, treatment of CuFe2O4 with (3-chloropropyl)trimethoxysilane affords ClpMNPs and substitution of chloro groups from ClpMNPS with 1-methyl imidazole yielded ILMNPs. At the last step, addition of H5BW12O40 heteropoly acid to ILMNPs affords BW12-ClpMNPs. Imidazolium cations bounds electrostatically to the produced heteropoly anion. It has been shown that heteropoly anion salt of organic cations are generally insoluble in water [17]. Therefore, the excess of HPA can be simply removed by washing the resulting material with water.
FT-IR spectroscopy
FT-IR spectra of the prepared CuFe2O4 (a), ClpMNPs (b), ILMNPs (c) and BW12-ILMNPs (d) are shown in Fig. 1. The appeared peaks around 450 and 580 cm−1 which were attributed to the Fe2O3 and CuO vibrational modes demonstrate the formation of CuFe2O4 magnetic core in the prepared nanomaterials [18]. The broad peak at about 1080 cm−1 assigns to Si–O–Si stretching vibrations which indicates survival of SiO2 layer around the CuFe2O4 nanoparticles [19]. The peak at 3400 cm−1 is attributed to the O–H stretching. The stretching vibrations at about 2935 cm−1 in the spectrum of ClpMNPs confirm the anchored propyl groups. In the FT-IR spectrum of ILMNPs, two new peaks at 1656 and 1533 cm−1 are belonging to stretching vibration of aromatic ring. Keggin-type HPAs have four characteristic peaks in wavenumber region 750–1100 cm−1, which in the BW12-ILMNPS spectra (Fig. 1d) become manifest at 900 and 792 cm−1 (corner and edge sharing W–O–W vibrations) [20]. The attributed peak to the W=O stretching has interference from Si–O–Si stretching absorbance. The increase in the W–O–W stretching vibrations frequencies of prepared nanomaterial in comparison with neat HPAs is due to strong interaction between heteropoly anion and organic cation [21]. Accordingly, the FT-IR spectroscopy confirms the surface modification of magnetic nanoparticles and preparation of BW12-ILMNPs.
Chemical analysis
Table 1 shows the chemical analysis of the prepared nanomaterial. It demonstrates the loading of heteropoly acid, where its loading amount is equal to the W content of the prepared nanomaterial. Accordingly, the loading amount of heteropoly acid is 0.145 mmol/g. On the other hand, the loading amount of nitrogen in BW12-ILMNPs is 1.41 mmol/g. Therefore, the amount of supported 1-methylimidazole is 0.705 mmol/g and the ratio of imidazolium group to heteropoly acid is approximately 1:5. Furthermore, acid–base titration was performed using a Metrohm 809 Titrando for evaluating the acidity of catalyst. The titration of acidic groups was accomplished using the catalyst slurry containing 50 mg of catalyst in deionized water, and a solution containing 0.01 M NaOH was used as titrant. The amount of titrant consumed to reach pH 7.5 revealed that the amount of acidic groups is about 0.697 mmol/g.
X-ray diffraction (XRD) study
Figure 2 depicts the X-ray diffraction of BW12-ILMNPs. The marked diffraction peaks can be assigned to the planes of cubic spinel structured CuFe2O4 (JCPDS No. 250283). The broad peak appeared at 2 \(\theta\) of ca. 23° indicates that the silica layer around the CuFe2O4 nanoparticles is in amorphous form. The prepared BW12-ILMNPs displays common diffraction peaks of CuFe2O4 nanoparticles and silica layer around them. Generally, it indicates that heteropoly anion species are well dispersed on the surface of ILMNPs.
FESEM study
The surface morphology and particle size of BW12-ILMNPs were studied by FESEM as shown in Fig. 3a. According to the FESEM images, it was confirmed that prepared nanoparticles are approximately spherical. Also, the aggregation gives rise to the increasing size of observed nanoparticles. The presented FESEM images of BW12-ILMNPs display that the average size of these nanoparticles is in the range of 70–80 nm. Furthermore, Fig. 3b shows the size distribution of nanoparticles which is determined by measuring diameters of one hundred nanoparticles randomly selected on the FESEM images. As can be seen, the distribution is symmetric around 70–80 nm and the most of the particles have the size between 60 and 90 nm.
The chemical identity of ILMNPs and BW12-ILMNPs was confirmed by EDX analysis (Fig. 4). The EDX analysis of ILMNPs (Fig. 4a) showed the presence of Fe, Cu, O, Si and Cl, whereas the EDX analysis of BW12-ILMNPs (Fig. 4b) indicated the coexistence of Fe, Cu, O, Si, B and W in the nanoparticles and proved that the heteropoly acid replaced with Cl and BW12-ILMNPs has formed.
The study of magnetic property
Magnetic measurement of CuFe2O4 and BW12-ILMNPs is shown in Fig. 5. No remanence effect was observed in the magnetization diagram of nanomaterials, which indicating the superparamagnetic property. Furthermore, magnetization saturation values for CuFe2O4 and BW12-ILMNPs are 48 and 25 emu/g, respectively. The mass saturation magnetization reduction would be attributed to the presence of the silica shell and functionalized groups around the CuFe2O4 core. The importance of superparamagnetic property of resulted BW12-ILMNPs is due to its applications, which prevents aggregation and enables it to redisperse swiftly after removal of magnetic field.
The study of thermal reliability
Figure 6 shows TGA curves of the ILMNPs and BW12-ILMNPs heated under N2 atmosphere at 10 °C/min. As can be seen, the ILMNPs (Fig. 6a) degrade in two steps, whereas the BW12-ILMNPs (Fig. 6b) degrade in three steps. It demonstrates that the third step in Fig. 6b is due to decomposition of heteropoly acid. The ILMNPs and BW12-ILMNPs start to lose weight at approximately 50 °C which could be due to loss of water. The observed slope around 200–400 °C leads to loss of weight that could be assigned to decomposition of anchored propyl and imidazolium group. Above 400 °C, the supported heteropoly acid starts to decompose and the mass loss of it is about 18%.
Studying catalytic activity of BW12-ILMNPs in 1H-pyrazolo[1,2-b]phthalazine-5,10-diones synthesis
The best experimental route was procured via optimization of the catalysts amount (Table 2), reaction temperature (Table 3), reaction time (Table 4) and various catalysts (Table 5).
In order to peruse the appropriate amount of catalyst, 0–0.1 g of catalyst was added to a mixture of benzaldehyde (1 mmol), malononitrile (1 mmol) and phthalhydrazide (1 mmol) at 80 °C under solvent-free conditions as the model reaction. Then, the mixture was stirred for appropriate time in an oil bath and the reaction was monitored by TLC.
After the completion of reaction, the crude product was purified as explained in “Experimental” section. The reaction did not occur in the absence of catalyst (Table 2 entry 1) which indicates that catalyst plays an important role in the reaction progress.
As is foretaste, yield % was increasing with raising catalyst amount; therefore, 0.05 g of catalyst was chosen as the optimal quantity.
Then the effect of temperature was scrutinized under solvent-free conditions for 10 min in the presence of 0.05 g of nanocatalyst, and 80 °C was considered as optimal temperature (Table 3).
Eventually the effect of time was studied and revealed that the excellent condition was solvent-free at 80 °C in the presence of 0.05 g of catalyst for 10 min (Table 4).
The catalytic performance of prepared nanomaterial was compared with previously reported methods (Table 5). The superiority of the present catalyst in terms of reaction time and yield % is quite obvious. Furthermore, ease of catalyst separation, reaction conditions and economical aspects are other remarkable advantages of present methodology.
As given in Table 5 (entry 13), no reaction was done when ILMNPs was used as a catalyst. It demonstrates supported heteropoly acid is the active site for catalytic activity.
The obtained optimal conditions were exerted to a series of aromatic aldehydes containing either electron-donating or electron-withdrawing functional group in the ortho-, meta- and para-positions. The results are given in Table 6. It appears that electron-withdrawing groups give rise to supreme yield of the products. Moreover, the applicability of reaction with aliphatic aldehydes under the optimized conditions was checked and observed that this reaction could not be done when the aliphatic aldehyde was used as a starting material.
Finally, the reusability of nanocatalyst was studied in model reaction under optimized conditions. After the separation of nanocatalyst by an external magnetic field at the end of the reaction, the catalyst was washed with ethanol and dried at 70 °C under vacuum for 2 h. The recycled nanocatalyst was reused in another condensation reaction. Findings revealed that the nanocatalyst could be reused for at least five runs without significant loss of its catalytic activity as shown in Fig. 7.
Conclusion
In this study, a novel and efficient nanocatalyst was synthesized by the reaction of ionic liquid-modified magnetic nanoparticles with heteropoly anion. The obtained nanocatalyst displayed supreme catalytic efficiency in one-pot three component condensation for the preparation of 1H-pyrazolo[1,2-b]phthalazine-5,10-diones. In comparison with previously reported methods, the present nanocatalyst provides an easy and convenient methodology for the preparation of 1H-pyrazolo[1,2-b]phthalazine-5,10-diones. Some advantages of this procedure are operation simplicity, high yields, low reaction time, simple work-up, reusability and recyclability of magnetic nanocatalyst, avoidance of organic toxic solvents.
References
Zhu, J., Bienaymé, H.: Multicomponent reactions. Wiley, Hoboken (2006)
Nefzi, A., Ostresh, J.M., Houghten, R.A.: The current status of heterocyclic combinatorial libraries. Chem. Rev. 97, 449–472 (1997)
Al’-Assar, F., Zelenin, K., Lesiovskaya, E., Bezhan, I., Chakchir, B.: Synthesis and pharmacological activity of 1-hydroxy-, 1-amino-, and 1-hydrazino-substituted 2, 3-dihydro-1H-pyrazolo [1, 2-a] pyridazine-5, 8-diones and 2, 3-dihydro-1H-pyrazolo [1, 2-b] phthalazine-5, 10-diones. Pharm. Chem. J. 36, 598–603 (2002)
Nabid, M.R., Rezaei, S.J.T., Ghahremanzadeh, R., Bazgir, A.: Ultrasound-assisted one-pot, three-component synthesis of 1H-pyrazolo [1, 2-b] phthalazine-5, 10-diones. Ultrason. Sonochem. 17, 159–161 (2010)
Maleki, B., Chalaki, S.B.N., Ashrafi, S.S., Seresht, E.R., Moeinpour, F., Khojastehnezhad, A., Tayebee, R.: Caesium carbonate supported on hydroxyapatite-encapsulated Ni0∙5Zn0∙5Fe2O4 nanocrystallites as a novel magnetically basic catalyst for the one-pot synthesis of pyrazolo [1, 2-b] phthalazine-5, 10-diones. Appl. Organomet. Chem. 5, 290–295 (2015)
Safaei-Ghomi, J., Shahbazi-Alavi, H., Ziarati, A., Teymuri, R., Saberi, M.R.: A highly flexible green synthesis of 1H-pyrazolo [1, 2-b] phthalazine-5, 10-dione derivatives with CuI nanoparticles as catalyst under solvent-free conditions. Chin. Chem. Lett. 25, 401–405 (2014)
Karthikeyan, G., Pandurangan, A.: Post synthesis alumination of KIT-6 materials with Ia3d symmetry and their catalytic efficiency towards multicomponent synthesis of 1H-pyrazolo [1, 2-] phthalazine-5, 10-dione carbonitriles and carboxylates. J. Mol. Catal. A Chem. 361, 58–67 (2012)
Song, S.-H., Zhong, J., He, Y.-H., Guan, Z.: One-pot four-component synthesis of 1H-pyrazolo [1, 2-b] phthalazine-5, 10-dione derivatives. Tetrahedron Lett. 53, 7075–7077 (2012)
Reddy, M.V., Jeong, Y.T.: InCl 3-catalyzed green synthesis of 1H-pyrazolo [1, 2-b] phthalazine-5, 10-diones under solvent-free conditions. Tetrahedron Lett. 54, 3546–3549 (2013)
Ziarani, G.M., Mohtasham, N.H., Badiei, A., Lashgari, N.: Efficient one-pot solvent-free synthesis of 1H-pyrazolo [1, 2-b] phthalazine-5, 10-diones catalyzed by sulfonic acid functionalized nanoporous silica (SBA-Pr-SO3H). J. Chin. Chem. Soc. 61, 990–994 (2014)
Ghahremanzadeh, R., Shakibaei, G.I., Bazgir, A.: An efficient one-pot synthesis of 1H-pyrazolo [1, 2-b] phthalazine-5, 10-dione derivatives. Synlett 2008, 1129–1132 (2008)
Shaterian, H.R., Mohammadnia, M.: Mild basic ionic liquids catalyzed new four-component synthesis of 1H-pyrazolo [1, 2-b] phthalazine-5, 10-diones. J. Mol. Liq. 173, 55–61 (2012)
Zhao, D., Wu, M., Kou, Y., Min, E.: Ionic liquids: applications in catalysis. Catal. Today 74, 157–189 (2002)
Li, M., Pham, P.J., Pittman, C.U., Li, T.: SBA-15-supported ionic liquid compounds containing silver salts: novel mesoporous π-complexing sorbents for separating polyunsaturated fatty acid methyl esters. Microporous Mesoporous Mater. 117, 436–443 (2009)
Rocchiccioli-Deltcheff, C., Fournier, M., Franck, R., Thouvenot, R.: Vibrational investigations of polyoxometalates. 2. Evidence for anion-anion interactions in molybdenum (VI) and tungsten (VI) compounds related to the Keggin structure. Inorg. Chem. 22, 207–216 (1983)
Dandia, A., Jain, A.K., Sharma, S.: CuFe2O 4 nanoparticles as a highly efficient and magnetically recoverable catalyst for the synthesis of medicinally privileged spiropyrimidine scaffolds. RSC Adv. 3, 2924–2934 (2013)
Kozhevnikov, I.: Catalysts for fine chemical synthesis, catalysis by polyoxometalates, vol. 2. Wiley, Hoboken (2002)
Sezgin, N., Sahin, M., Yalcin, A., Koseoglu, Y.: Synthesis, characterization and the heavy metal removal efficiency of MFe2O4 (M=Ni, Cu) Nanoparticles. Ekoloji 89, 89–96 (2013)
Alizadeh, A., Khodaei, M.M., Beygzadeh, M., Kordestani, D., Feyzi, M.: Biguanide-functionalized Fe3O4/SiO2 magnetic nanoparticles: an efficient heterogeneous organosuperbase catalyst for various organic transformations in aqueous media. Bull. Korean Chem. Soc. 33, 2546–2552 (2012)
Juan, J.C., Zhang, J., Yarmo, M.A.: 12-Tungstophosphoric acid supported on MCM-41 for esterification of fatty acid under solvent-free condition. J. Mol. Catal. A Chem. 267, 265–271 (2007)
Mosa, J., Larramona, G., Durán, A., Aparicio, M.: Synthesis and characterization of P2O5–ZrO2–SiO2 membranes doped with tungstophosphoric acid (PWA) for applications in PEMFC. J. Membr. Sci. 307, 21–27 (2008)
Acknowledgements
We are grateful to the University of Hakim Sabzevari Research council for the partial support of this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Saadati-Moshtaghin, H.R., Zonoz, F.M. Synthesis and characterization of magnetically recoverable 1-(copperferritesiloxypropyl)-3-methylimidazolium heteropolytungstate ionic liquid as a new nanocatalyst for the preparation of 1H-pyrazolo[1,2-b]phthalazine-5,10-diones. J Nanostruct Chem 7, 317–325 (2017). https://doi.org/10.1007/s40097-017-0241-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40097-017-0241-6