Abstract
In this study, two functionals (B3LYP and ωB97XD) were used for density functional theory (DFT) calculation of two major boron compounds (BH3 and BF3) adsorption on fullerene-like Al12N12 nanocluster. High values of adsorption energy, −268.6 (−244.7) for BF3 and −224.5 (−196.4) kJ/mol for BH3 were found using ωB97XD (B3LYP) functional, indicating strong chemisorption which is the result of Lewis acid–base interaction of adsorbent and adsorbates. The high negative values of ΔG (Gibbs free energy) and ΔH (enthalpy) confirm spontaneous exothermic adsorption process. Further studies were done by taking into account the charge analysis, FMO (frontier molecular orbitals), MEP (molecular electrostatic potential), density of states (DOS), and reactivity of resulted systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Borane (trihydridoboron) and boron trifluoride are two inorganic compounds with chemical formulas of BH3 and BF3, respectively. They are drab gases that are famous as substantial reagents in reaction pathway [1, 2]. In addition, they are known as significant Lewis acids (because of the electron deficiency of boron atom in their compounds) and general construction blocks for further boron complexes. Studying the interface conception of different B–N bonds is vital for energy storage viewpoint. Group III-nitrogen compounds are well-recognized as storage energy owing to their lightweight and inherent high releasing energy [3].
Although BH3 itself has not normally used as a reactant; however, it is a potential intermediate in adsorption of di-borane, B2H6, that is broadly used as a source of boron [4]. Similarly, boron halides, including BF3 and BCl3, have been used as substitute probe molecules owing to the 2p1 electronic structure of boron that provides a powerful Lewis acid when shared with a halogen atom [5, 6]. The boron atom of BF3 has an unfilled pz-like orbital in perpendicular to the molecular plane and has a propensity to receive electron pairs, and this property causes adsorption of boron compounds. For example in our recent study [7], we investigated the adsorption of some boron compounds on the surface of nitrogen-doped graphene. We found formation of new bond between nucleophilic atom (N) and electrophilic atom (B), whereas there was weak physisorption in the case of pristine graphene. There are various investigations on BF3 and BH3 in literature in this regard. For example, Xu et al. [8] used DFT to study the hydroboration of the Ge(1 0 0)−2 × 1 surface with BH3. Based on their result, the Ge(1 0 0) surface displays rather different surface reactivity to dissociative adsorption of BH3 in comparison with the C(1 0 0) and Si(1 0 0) surfaces. In another work, dissociative adsorption of BH3 on the Si(100) surface has been searched with nonlocal DFT by Konecny and Doren [4]. They revealed that a Si–B bond is constructed through a nucleophilic attack on boron, leaving BH2 and H fragments bonded to the surface.
Abee and Cox [5] searched on BF3 as a probe molecule to interrogate the basicity of Cr2O3 (101̄2) surfaces.
After a successful development of CNT and fullerene C60, various spherical fullerene-like configurations composed of inorganic non-carbon materials have been stimulated a great deal of interest [9,10,11,12,13,14,15,16,17,18,19,20]. Strout et al. revealed that the fullerene-like nano-cages X12Y12 are the most stable structures among all (XY)n (X = B, Al, Ga,… and Y = N, P, As, …) semiconductors [9]. Al12N12, Al12P12, B12N12, and B12P12 are of great significance because of their high steadiness, large energy band gap, and outstanding chemical and physical properties. Among them, B12N12 nanoclusters were synthesized by Oku et al. in 2004 [10]. Different applications of nanostructure materials have been studied in recent literature [11,12,13,14,15,16,17,18,19,20,21,22,23,24]. Because of important roles of boron compounds in different areas of study, recently we rummaged on the chemisorption property of boron trichloride (BCl3) on the surface of Al12N12 nanocluster [6]. Following successful potential of mentioned nanocluster as an ideal adsorbent, we persuaded to search on the adsorption of other boron compounds on this kind of nanocluster. As we found, there is no investigation in literature on the BH3 and BF3 adsorption on the Al12N12 nanocluster. In this paper, I have employed DFT method to investigate the adsorption of above-mentioned molecules on the surface of Al12N12 nanocluster. The attained results were analyzed by considering adsorption energies, the natural bond orbital (NBO) charge transfer, frontier molecular orbitals, density of states, and global indices of activities.
Computational details
The geometries of Al12N12 nanocluster and corresponding boron complexes were fully relaxed at the B3LYP (and ωB97XD)/6−31G** level of theory as implemented in Gaussian 09 suite of program [25]. The suitability of B3LYP functional for different nanostructures has been proved [26,27,28,29]. To consider the effect of dispersion on adsorption energies, all relaxed structures were subjected to new optimization using meta-hybrid functional (wB97XD) [30]. For each calculation, the global charge of system was neutral. Frequency calculations have been performed to ensure the stability of the interaction. Adsorption energy (Ead) of BX3 (X = F, H) on Al12N12 nanocluster is defined as
where EAl12N12-BX3 is the total energy of the adsorbed BX3 molecule on the surface of nanocluster (Al12N12−), and EAl12N12 and EBX3 are the total energies of the free Al12N12 and a free BX3 molecule, respectively.
Natural bond orbital (NBO) analysis [31] is used to follow charge distribution and charge transfer between BX3 and Al12N12. Frontier molecular description has been studied to follow the variation in the structure of nanocluster when BX3 is adsorbed. Parr et al. [32] stated the electrophilicity concept. Chemical potential (µ) is defined based on the following eq. [33]:
where EHOMO is the energy of HOMO and ELUMO is the energy of LUMO. In addition, hardness (ŋ) can be calculated using the Koopmans’ theorem [33] as:
Softness (S) [33] and electrophilicity (ω) [33] are defined as the following eqs. correspondingly.
Results and discussion
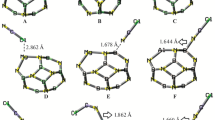
The electronic structure of Al12N12 nanocluster is pretty discussed in different investigations [11,12,13,14], so, a comprehensive explanation is not provided here. As shown in Fig. 1, this kind of nanocluster is performed of some tetragon (four membered) and hexagon (six membered) rings. Actually, there are two kinds of Al…N bond through the cluster, one is shared between two hexagon rings (b@66) and another is shared between a hexagon ring and a tetragon ring (b@64). The bond distances are calculated to be 1.79 and 1.86 Å for b@66 and b@64, respectively. The NBO charge allocation in Al12N12 nanocluster is also depicted in Fig. 1 (right). It is obvious from Fig. 1 that the charge is uniformly distributed through the nanocluster. Each Al atom has a positive charge of +1.84 e, whereas each N atom has a negative charge of −1.84 e through the structure. As a result, positive and negative sites of cluster correspond to nucleophilic and electrophilic attacks of adsorbates, respectively.
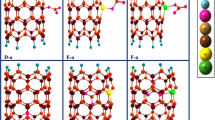
To study the interaction of BF3 and BH3 onto Al12N12 nanocluster, firstly I placed the BX3 (X = F, H) molecule at different orientations above the cluster, 1-the B atom of BX3 above the N of cluster, 2-the X atom of BX3 above the N of cluster so that X–B bond has perpendicular orientation than the surface. All these initial configurations were used to fully optimization using two before-mentioned functionals. Despite there are different initial configurations, I found that there is only one relaxed structure (output) for BF3 as well as BH3 upon optimization. Figure 2 depicts side and tope views of adsorbed BF3 and BH3 onto nanocluster. As shown in Fig. 2, adsorption of BF3 and BH3 correspond to the formation of new bond between N of cluster and B of adsorbate. The B…N bond length is 1.45 and 1.59 Å for Al12N12–BF3 and Al12N12–BH3 complexes, respectively. The lower distance in the earlier complex more likely relates to the more positively charged boron in BF3 owing to strong electron affinity of F atoms. As a result, more positively charged boron atom in BF3 corresponds to stronger Lewis acid–base interaction. This bond formation results the transferring in hybridizing of B atom from sp2 to sp3. Moreover, we can see another bond formation between the F atom of BF3 and also the H atom of BH3 with the Al atom of nanocluster (both F and H are negatively charged in their related complex). These new bonds formation is because of transferring the density of electron from F and H atoms (which are rich in electron) to unoccupied orbital of Al atom of nanocluster. It is obvious that the bond length of Al…F and Al…H are calculated to be 1.69 and 1.71 Å, respectively, which are comparable with B…N bond in their complexes.
The experimental bond length of B–H and B–F in free BH3 and BF3 molecules is reported 1.19 and 1.31 Å, respectively [5, 34], which are very close to the values of 1.194 and 1.317 Å calculated by this study. It is shown in Fig. 2 that after adsorption of BF3 molecule, fully dissociation in one of the B-F bonds is happened, whereas partial dissociation in one of the B–H bonds is resulted. In the case of BF3, one B…F bond is elongated to 2.42 Å (83.3% increase) and two other bonds are partially extended to 1.33 Å. So, because of the large change in one of the bonds in the molecule, we can conclude fully dissociation of BF3 upon adsorption on Al12N12 nanocluster. On the other hand, for BH3, the elongation is less pronounced. It is shown in Fig. 2 that one of the bond lengths of B…H reaches to 1.36 Å (13.3% increase) and two other reach to 1.20 Å upon adsorption which attribute to the partial dissociation of molecule.
The values of adsorption energy are listed in Table 1. We can see that the adsorption of BF3 and BH3 on the surface of Al12N12 nanocluster corresponds to releasing energy of −244.7 and −196.4 kJ/mol (based on B3LYP functional), and −268.6 and −224.5 kJ/mol (based on ωB97XD functional), respectively. The difference between the values of adsorption energy achieved by two functionals reveals that the dispersion parameter plays an important role in interaction. All of these values of adsorption are categorized in chemisorption region. In addition, considerable difference between the results of two functionals suggests that dispersion has important role in adsorption process of this study. The results of adsorption energies are totally in agreement with the results of bond distances, the higher adsorption energy corresponds to the lower bond distance. Moreover, calculated value of adsorption energy are greatly higher than what reported for BH3 adsorption on Si(100) surface (≃180 kJ/mole, [4]), and BF3 adsorption on α-Cr2O3 \((10\overline{{12}} )\) (≃133 kJ/mole, [5]).
Despite the high changes in the electronic structure of adsorbates, the changes in the electronic structure of nanocluster are also important. It is shown in Table 1 that the Al…N bonds in b@66 (b@64) are changed from 1.79 (1.86) Å for pristine nanocluster to 2.03 (1.92) for BF3 and 1.92 (1.87) for BH3 adsorbed systems. It is obvious that the effect of BF3 adsorption on the electronic structure of nanocluster is more pronounced compared to BH3 which is attributed to the stronger interaction in the earlier complex. By adsorption of BF3 and BH3, not only the bond lengths through the nanocluster are changed, but also the charge allocation on each atom significantly is effected (see Fig. 3). By comparing the NBO charge distribution of pristine nanocluster (Fig. 1, right) with that of complexes (Fig. 3), one can conclude significant change especially in the area of interaction, which is considerable in the case of BF3 compared to BH3 adsorption. Along with the stronger interaction of N (of nanocluster) with boron atom of BF3 rather than BH3, the higher change in the electronic structure of nanocluster upon adsorption of BF3 is also effected by the stronger Lewis acid–base interaction between F atom (of BF3) and Al atom (of nanocluster) compared to that interaction between the same Al atom and H atom (of BH3). Therefore, the difference in the NBO charge distribution of two complexes is a result of two different kinds of interaction between corresponding atoms. The values of net charge transfer are also listed in Table 1. As expected, direction of charge transfer is from nanocluster to adsorbates, confirming transfer of charge from nucleophilic atom (N) to electrophilic atom (B). On the other hand, more charge transfer for BF3 adsorption is in accordance to its higher interaction. However, direction of net charge transfer are the resultant of two revers charge transfer; one from N (of cluster) to B, and another from F (H) of adsorbate to Al (of cluster), but the value of the earlier is much higher as expected, resulting overall direction of charge transfer from Al12N12 nanocluster to adsorbates.
In order to examine the thermodynamic feasibility of BF3 and BH3 adsorption on the Al12N12 at ambient temperature and pressure (T = 298.14 K, and P = 1 atm), I have calculated free energies (ΔG) and enthalpy changes (ΔH) of each system using the results of vibrational frequency calculations,. The computed values of ΔH are −240.3 and −187.3 kJ/mol, and those of ΔG are −195.1 and −140.6 kJ/mol, respectively. However, the lower value of ΔG compared to that of ΔH is because of the entropic influence. The negative value of Gibbs free energy as well as enthalpy of each system confirms that adsorption of above-mentioned adsorbates is a spontaneous exothermic process.
To better understand the binding sites, I have calculated the molecular electrostatic potentials (MEP) of each system. For this determination, MEP of Al12N12 nanocluster and its complexes (Al12N12–BF3 and Al12N12–BH3) were compared and are given in Fig. 4. For pristine Al12N12 nanocluster, Aluminum has positive charge (blue color) while the nitrogen has negative charge. The negative charge on N in the nanocluster is not much pronounced (yellow color). After adsorption of BF3 and BH3 molecules, we can see important changes in the blueish and yellowish part of cluster, especially at the area of interaction. The red part of Al12N12–BF3 complex attributes to the electronegative F atom of BF3 while no red part can be seen in Al12N12–BH3 complex. This is another proving for happening two strong Lewis acid–base interactions (N…B and Al… F) during the earlier complex formation.
Frontier molecular orbitals are also studied to search the effect of BF3 and BH3 adsorption on the electronic properties of Al12N12 nanocluster. The outlooks of HOMOs and LUMOs are shown in Fig. 5 and their values are given in Table 2. The spreading of densities also delivers important information concerning the adsorbent capability of Al12N12 nanocluster for BF3 and BH3 molecules. As shown in Fig. 5, the HOMO in Al12N12 nanocluster is confined on nitrogen atoms, while the LUMO has density homogeneously circulated on the whole nanocluster. Upon BF3 and BH3 adsorption, the densities are redistributed confirming important alterations in the electronic structure of Al12N12 nanocluster.
The HOMO–LUMO energies of Al12N12 nanocluster are −6.472 and −2.541 eV, correspondingly. As shown in Fig. 5 and the data of Table 2, the adsorption of BF3 and BH3 causes some changes in the electronic structure of the Al12N12 nanocluster. While the energy gap of pristine Al12N12 nanocluster is 3.931 eV, upon BF3 and BH3 adsorption, the energy gaps are reduced to 3.618 and 3.869 eV, respectively.
For BF3 adsorption, the HOMO of resulted system increases while the LUMO decreases; however, the change in the LUMO is more pronounced than HOMO, resulting in a decrease in the energy gap of system. On the other hand, upon adsorption of BH3, the energy of HOMO approximately remains unchanged while the LUMO decreases, resulting in a little decrease in the energy gap of system.
Density of states (DOS) of pristine Al12N12 and Al12N12–BF3 and Al12N12–BH3 complexes is studied in order to obtain more understanding the effect of adsorption (See Fig. 6). Some new energy states appear upon adsorption of both BF3 and BH3 molecules. These new states appear near the frontier molecular orbitals, which confirm case of strong hybridizing. The alteration in electronic properties is very essential for the development of sensors. Alteration in HOMO–LUMO causes change in electrical conductivity, which is the prime factor in the design of electrochemical sensors. Association between conductivity and Eg can was determined as Eq. (6) [35].
where it could be understood that a small lessening in Eg leads to meaningfully greater electrical conductivities.
The global indices of reactivity for pristine Al12N12 and its complexes are given in Table 2. They are pretty vital parameters owing to they exemplify the reactivity and steadiness of a system. Entertainingly, the hardness of nanocluster (1.965 eV) decreases on complexation with BF3 (1.809 eV) and BH3 (1.934 eV). One can see that the change in hardness is comparatively small for BH3 adsorption (Δŋ = −0.031 eV) whereas meaningfully large alteration is observed for BF3 adsorption (Δŋ = −0.156). Meanwhile, hardness attributes to the steadiness of a complex toward distortion in the attendance of electrical field, based on the result, the Al12N12 complexes are more susceptible to deformation under electrical field than pristine nanocluster. Softness inversely changes compared to hardness, hence, it is predictable that softness for Al12N12−BF3 and Al12N12−BH3 will increase, resulting increase in the reactivity of complexes.
Conclusion
BF3 and BH3 with a low-lying empty orbital are strong Lewis acids, so their capability to accept electrons along with their planar geometries suggests that nucleophilic attack by a nucleophilic atom is performed. The goal of this research is investigation on the adsorption properties of BF3 and BH3 molecules on Al12N12 nanocluster considering DFT method. I found fully dissociative adsorption for BF3 and partial dissociative adsorption for BH3 molecules. The computed values of ΔH are −240.3 and −187.3 kJ/mol, and those of ΔG are −195.1 and −140.6 kJ/mol, for BF3 and BH3 adsorption, respectively. The negative value of Gibbs free energy approves that adsorption of above-mentioned adsorbates is a spontaneous process. Results of frontier molecular orbitals confirm some important changes in the electronic structure of the nanocluster upon adsorption.
References
Rasul, G., Prakash, S.G.K., Olah, G.A.: Complexes of CO2, COS, and CS2 with the super Lewis Acid BH4+ contrasted with extremely weak complexations with BH3: theoretical calculations and experimental relevance. J. Am. Chem. Soc. 121, 7401–7404 (1999)
Brinck, T., Murray, J.S., Politzer, P.: A Computational analysis of the bonding in boron trifluoride and boron trichloride and their complexes with ammonia”. Inorg. Chem. 32, 2622–2625 (1993)
Leskiw, B.D., Castleman Jr., A.W., Ashman, C., Khanna, S.N.: Reactivity and electronic structure of aluminum clusters: the aluminum–nitrogen system. J. Chem. Phys. 114, 1165–1169 (2001)
Konecny, R., Doren, D.J.: Adsorption of BH3 on Si (100)−(2×1). J. Phys. Chem. B 101, 10983–10985 (1997)
Abee, M.W., Cox, D.F.: BF3 Adsorption on α-Cr2O3 \((10\overline{{12}} )\): Probing the Lewis basicity of surface oxygen anions. J. Phys. Chem. B 105, 8375–8380 (2001)
Rad, A.S., Ayub, K.: DFT study of boron trichloride adsorption on the surface of Al12N12 nanocluster. Mol. Phys. 115, 879–884 (2017)
Rad, A.S., Shadravan, A., Soleymani, A.A., Motaghedi, N.: Lewis acid-base surface interaction of some boron compounds with N-doped graphene, first principles study. Curr. Appl. Phys. 15, 1271–1277 (2015)
Xu, Y.J., Li, J.Q.: The dissociative adsorption of borane on the Ge (100)–2×1 surface: a density functional theory study. Appl. Surf. Sci. 252, 5855–5860 (2006)
Strout, D.L.: Structure and stability of boron nitrides: isomers of B12N12. J. Phys. Chem. A 104, 3364–3366 (2000)
Oku, T., Nishiwaki, A., Narita, I.: Formation and atomic structure of B12N12 nano-cage clusters studied by mass spectrometry and cluster calculation. Sci. Tech. Adv. Mater. 5, 635–638 (2004)
Rad, A.S., Ayub, K.: A comparative density functional theory study of guanine chemisorption on Al12N12, Al12P12, B12N12, and B12P12 nano-cages. J. Alloys Compd. 672, 161–169 (2016)
Rad, A.S., Ayub, K.: Adsorption of pyrrole on Al12N12, Al12P12, B12N12, and B12P12 fullerene-like nano-cages, a first principles study. Vacuum 131, 135–141 (2016)
Rad, A.S.: Study on the surface interaction of Furan with X12Y12 (X = B, Al, and Y = N, P) semiconductors: DFT calculations. Heteroat. Chem. 27, 316–322 (2016)
Ayub, K.: Are phosphide nano-cages better than nitride nano-cages? A kinetic, thermodynamic and non-linear optical properties study of alkali metal encapsulated X12Y12 nano-cages. J. Mater. Chem. C 4, 10919–10934 (2016)
Wang, Q., Sun, Q., Jena, P., Kawazoe, Y.: Potential of AlN nanostructures as hydrogen storage materials. ACS Nano 3, 621–626 (2009)
Rad, A.S., Ayub, K.: Ni adsorption on Al12P12 nano-cage: a DFT study. J. Alloys Compd. 678, 317–324 (2016)
Rad, A.S., Ayub, K.: Detailed surface study of adsorbed nickel on Al12N12 nano-cage. Thin Solid Films 612, 179–185 (2016)
Rad, A.S., Ayub, K.: Enhancement in hydrogen molecule adsorption on B12N12 nano-cluster by decoration of nickel. Int. J. Hydrogen Energy 41, 22182–22191 (2016)
Rad, A.S., Ayub, K.: Coordination of nickel atoms with Al12X12 (X = N, P) nano-cages enhances H2 adsorption: a surface study by DFT. Vacuum 133, 70–80 (2016)
Iqbal, J., Ayub, K.: Theoretical study of the non linear optical properties of alkali metal (Li, Na, K) doped aluminum nitride nanocages. RSC Advances 687, 94228–94235 (2016)
Rad, A.S., Ayub, K.: Adsorption properties of acetylene and ethylene molecules onto pristine and Nickel-decorated Al12N12 nanoclusters. Mat. Chem. Phys 194, 337–344 (2017)
Rad, A.S., Ayub, K.: Adsorption of thiophene on the surfaces of X12Y12 (X = Al, B, and Y = N, P) nanoclusters; A DFT study. J. Mol. Liq. 238, 303–309 (2017)
Rad, A.S., Ayub, K.: O3 and SO2 sensing concept on extended surface of B12N12 nanocages modified by Nickel decoration: a comprehensive DFT study. Solid State Sci. 69, 22–30 (2017)
Rad, A.S., Zareyee, D.: Adsorption properties of SO2 and O3 molecules on Pt-decorated graphene: a theoretical study. Vacuum 130, 113–118 (2016)
Frisch, M. J.: Gaussian 09, Revision E.01, Wallingford CT (2009)
Chen, L., Xu, C., Zhang, X.-F., Zhou, T.: Raman and infrared-active modes in MgO nanotubes. Physica E 41, 852–855 (2009)
Rad, A.S.: Study of dimethyl ester interaction on the surface of Ga-doped graphene: Application of density functional theory. J. Mol. Liq. 229, 1–5 (2017)
Rad, A.S., Sani, E., Binaeian, E., Peyravi, M., Jahanshahi, M.: DFT study on the adsorption of diethyl, ethyl methyl, and dimethyl ethers on the surface of gallium doped graphene. Appl. Surf. Sci. 401, 156–161 (2017)
Rad, A.S., Jouibary, Y.M., Foukolaei, V.P., Binaeian, E.: Study on the structure and electronic property of adsorbed guanine on aluminum doped graphene: first principles calculations. Curr. Appl. Phys. 16, 527–533 (2016)
Chai, J.D., Head-Gordon, M.: Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 10, 6615–6620 (2008)
Reed, A.E., Weinstock, R.B., Weinhold, F.: Natural population analysis. J. Chem. Phys. 83, 735–746 (1985)
Parr, R.G., Szentpaly, L., Liu, S.: Electrophilicity Index. J. Am. Chem. Soc. 121, 1922–1924 (1999)
Koopmans, T.: Ordering of wave functions and eigenenergies to the individual electrons of an Atom. Physica 1, 104–113 (1934)
Kawaguchi, K.: Fourier transform infrared spectroscopy of the BH3 ν3 band”. The J. Chem. Phys. 96, 3411 (1992)
Li, S.: Semiconductor Physical Electronics, 2nd edn. Springer, Berlin (2006)
Acknowledgements
I highly acknowledge the financial support received from the Iran Nanotechnology Initiative Council, Iran.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Rad, A.S. Chemisorption of BH3 and BF3 on aluminum nitride nanocluster: quantum-chemical investigations. J Nanostruct Chem 7, 207–215 (2017). https://doi.org/10.1007/s40097-017-0231-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40097-017-0231-8