Abstract
Recently, small (<2 nm) and monodispersed Pt clusters has gained much attention due to their high catalytic activity in the aerobic oxidations. However, the chemical synthesis of small Pt clusters is not trivial; high temperature is often required to completely reduce the Pt4+/2+ ions to Pt0, which accelerates the growth of the Pt clusters. Here, we discussed a very simple microfluidic reduction of Pt4+ to Pt0 by NaBH4 in the presence of PVP that produces <2 nm Pt clusters in any variable reduction conditions. The microfluidic reduction conditions were optimized for the synthesis of possible smallest Pt clusters in terms of five reaction parameters: (1) temperature, (2) concentration of H2PtCl6, (3) molar ratio of NaBH4 to Pt4+ ions, (4) molar ratio of PVP-monomer to Pt4+ ions, and (5) molecular weight/chain length of PVP. We found that possible smallest particles with average diameter 1.3 ± 0.3 nm were produced when aqueous solutions of H2PtCl6 (4 mM) and NaBH4 (40 mM) containing PVP (160 mM) were injected into the micromixer placed in an icebath at a flow rate of 200 mL/h. The produced particles were characterized by UV–visible absorption spectrophotometry, powder X-ray diffractometry and transmission electron microscopy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Colloidal clusters of noble metal in the 1–10 nm size regime have recently attracted considerable attention in many areas of research due to their nobility in physical and chemical properties [1, 2]. Those noble properties are of fundamental interest to both homogeneous and heterogeneous catalysis, which are significantly different from their bulk counterparts [3, 4]. Both catalytic activity [5] and selectivity [6] are known to be influenced by the size and as well as the shape of the clusters [7–11], and therefore the synthesis of well-controlled sizes and shapes of particles could be critical for this purpose.
Many procedures have been reported for the synthesis of colloidal Pt clusters. Commonly used procedures include Pt4+ or Pt2+ salts reduction by borohydride [12–16], hydrogen [17, 18], alcohol [19–24], glycol [25, 26], or ethylene glycol [27, 28] in the presence of a stabilizer or on a solid support. Despite a large choice of synthetic protocols, an accurate control of the particle size, simple and reproducible synthetic strategies are important to investigate their physical and chemical properties.
Typically, metal clusters provide highly active centers, and due to their higher surface energies and larger surfaces, they are not in a thermodynamically stable state [29, 30]. To produce homogeneous and stable metal particles usually soluble polymers are widely employed as supports because of their availability, enhanced stabilization properties and resistance to particle sintering or agglomeration [31]. Poly(vinylpyrrolidone) (PVP) is the most widely used capping agent to control not only the size but also the shape of the metal clusters where PVP molecules interact strongly through their carbonyl group by oxygen atom with the metal surface for their enhanced stabilization [32, 33]. Chemical syntheses offer a versatile route by assembling atoms and particles from the atomic or molecular state to the macroscopic scale [34]. The characteristics of the crystals can be controlled by the thermodynamics and kinetics of the synthesis [35]. Even though great progress has been made, there is still a necessity to develop chemical synthetic methods that can tailor the morphology of Pt crystals at different scales.
In this article, a simple microfluidic reduction of Pt4+ to Pt0 by NaBH4 in the presence of PVP were discussed that can provide a large-scale synthesis of Pt clusters hydrosol. The sizes of the produced clusters were <2 nm in any variable condition of the reaction parameters. The reduction conditions were optimized for the synthesis of possible smallest Pt clusters. The as produced particles were characterized by UV–visible absorption spectrophotometry, powder X-ray diffractometry (XRD) and transmission electron microscopy (TEM).
Experimental
Chemicals and solvents
Hexachloroplatinicacid hexahydrate (H2PtCl6·6H2O, Sigma-Aldrich), PVP (average molecular weight 3500–3,60,000, E-Merck) and sodium borohydride (NaBH4, Wako Pure Chemical Industries Ltd.) of an analytical grade were purchased and used as received. Deionized water was used to prepare aqueous solutions and final washings of glassware’s.
Preparation of Pt:PVP clusters
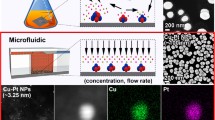
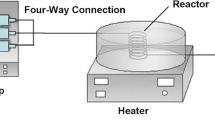
The microfluidic preparation of Pt clusters was described in detail previously [22]. In brief, freshly prepared ice-chilled aqueous solution of H2PtCl6 (4 mM, 35 mL) containing PVP (80 mM with respect to monomer) and ice-chilled aqueous solution of reducing agent NaBH4 (20 mM) also containing PVP (80 mM with respect to monomer) were injected into the micromixer with the help of automatically actuated two syringe pumps with a constant flow rate of 200 mL/h by placing the micromixer in an ice bath. The Pt4+ ion to PVP-monomer final molar ratio was 1:40. The eluted hydrosol of produced Pt:PVP through the outlet were collected in a conical flask equipped with a magnetic stirrer that was also placed in an ice bath. The schematic view of the microfluidic preparation of Pt:PVP clusters is shown in Fig. 1. The as produced particles were purified by the diafiltration technique where hemodialyzer was used as an ultrafiltration membrane for the removal of unwanted ions, molecules or solvents.
Characterization
Using quartz cuvette, UV–visible absorption spectra of colloidal solutions were carried out on a Shimadzu UV-1800 spectrophotometer (from 200 to 1100 nm). Transmission electron micrographs (TEM) were performed using a JEM-2100F instrument operated at an accelerating voltage of 200 kV. The samples for the TEM analysis were prepared by casting a drop of ethanolic dispersion (~0.3 mM, on Pt-atom basis) on a carbon-coated TEM grid and left to air dry. X-ray diffraction (XRD) measurements of the clusters were performed using a diffractometer (D8 ADVANCE, Bruker) with Cu Kα radiation (1.5418 Å) operated at 40 kV and 40 mA. Fine powder samples were used for these measurements. The diffraction patterns were simulated using a TOPAS-4 program.
Results and discussion
Optimization of reduction conditions
For the efficient production of small and monodisperse PVP-stabilized Pt clusters in microfluidic method, reduction conditions were optimized for five reaction parameters, namely (1) temperature, (2) concentration of precursor metal ions, H2PtCl6, (3) molar ratio of NaBH4 to Pt4+ ions, (4) molar ratio of PVP-monomer to Pt4+ ions, and (5) molecular weight of PVP.
A temperature effect on microfluidic synthesis was observed at 0, 20, 40, 60 and 80 °C where both of the solutions were met together at this temperature inside micromixer. It was found that the average diameters of the metal clusters were increased gradually with increasing the synthetic temperature from 0 to 80 °C as shown in Fig. 2. With increasing temperate, nucleation rate usually increases and results in increased number of cluster seeds and also increases the normal degradation rate of borohydride. At elevated temperature, formation of larger clusters indicated that higher temperature enhanced the relative population of stable particles by degradation of other less-stable particles and reunited them via thermal degradation as shown in Scheme 1 [37]. This can be explained with Ostwald ripening process, which is a thermodynamically driven spontaneous process because larger particles are more energetically stable than the smaller one [38, 39]. As the system tries to lower its overall energy, molecules on the surface of small (energetically unfavorable) particles will tend to detach and diffuse through the solution and then attach to the surface of larger particles. Therefore, the number of smaller particles continues to shrink, while larger particles continue to grow. On the other hand at higher temperature, the rate of degradation of borohydride in aqueous solution was increased which resulted incomplete reduction of Pt4+ ions where particles growth step was prolonged by slowed down the reduction step.
An effect of H2PtCl6 concentration on the size of final Pt clusters was studied ranging from 1.0 to 10.0 mM. It is relevant to know an optimum concentration, which will provide high and quality yield with the view of an efficient reduction and large amount preparation. No significant difference was observed in Pt clusters size up to 4.0 mM H2PtCl6 solution concentration as shown in Fig. 3. Similar size (1.3 ± 0.3 nm) and monodispersed clusters were produced. However, as the concentration was increased (10 mM), comparatively larger Pt clusters (1.6 ± 0.5 nm) were produced. In addition, unreacted Pt2+ ions were also detected in the ultrafiltrate of cluster hydrosol. The higher concentration of H2PtCl6 may not provide homogeneous solutions, which led into an incomplete reduction. We determined the amount of unreduced Pt ions in the ultrafiltrate by ICP measurement. We observed that nearly 90 % of precursor Pt4+ ions were reduced to Pt0 whereas about 10 % was unreduced which remained in the ultrafiltrate as Pt2+ ions. At much higher concentration of Pt4+, bulk Pt was formed along with unreduced Pt2+ ions. That was probably due to the higher concentration of NaBH4 that destabilized the protective nature of PVP, which resulted bulk platinum formation.
NaBH4 is a strong reducing agent that readily reduces H2PtCl6 to colloidal platinum. The proposed overall chemical reactions are:
According to the above-mentioned balance chemical equation [40, 41], for complete reduction of all Pt4+ ions present in solution to Pt0, a 1:1 molar ratio is required between borohydride and Pt4+ ions. Unreduced Pt2+ ions were detected even at 1:3 molar ratio of Pt4+ to NaBH4, although according to the balance chemical equation this ratio was much higher for the complete reduction of all Pt ions present in the solution. The formed Pt0 acts as a highly active catalysts for the hydrolysis of NaBH4 [22], so that a large excess of NaBH4 could not ensure the complete reduction of all precursor ions as shown in Fig. 4. We observed that minimum molar ratio 4 is required for complete reduction of all Pt4+ ions present in the solution by microfluidic method. This higher amount of NaBH4 is required because of the degradation during reduction although produced Pt0 were separated and fresh borohydride and Pt4+ ions were always fed. But at much higher ratio of NaBH4 >20 resulted bulk platinum formation. Probably higher ratio of NaBH4 destabilized the protective nature of PVP, which resulted bulk platinum formation.
Reduction of H2PtCl6 to Pt0 by MeOH in the presence of PVP is suggested to be proceeded in two steps where first step is the conversion of [Pt+IVCl6]2− to [Pt+IICl4]2− [42]. Although we did not studied the reaction mechanism, the presence of Pt2+ ions in the ultrafiltrate even after addition of 100 times NaBH4 compared to microfluidic system is one of the proof to follow the reaction accordance with the two-step reduction mechanism, steps (3) and (4) as was suggested by Ingelsten et al. [43]:
The molar ration of PVP-monomer to Pt4+ from 5 to 200 was studied in microfluidic reduction, which is shown in Fig. 5 where the average gram molecular weight of PVP was 40,000. It was found that average size of the Pt clusters was increased with decreasing the PVP-monomer to Pt4+ ratio from 20 to 5. The degree of monodispersity of the clusters was also decreased. It was probably, this amount of PVP was not sufficient to well stabilize the smaller Pt clusters during preparation. On the other hand, there were no significant differences between the size and size distribution of clusters were observed when the molar ratios of PVP-monomer to Pt4+ ions were tuned from 40 to 100. But at much higher ratio of PVP-monomer, larger particles were observed. It was probably because of the higher viscosity of the solution, which hindered the well mixing of both solutions. With increasing the PVP content, the probability of bare surface for accessing the substrates to the clusters surface decreases, so the minimum ratio 40 was chosen for smaller particles formation and as well as maximizing the bare surfaces of the clusters. Interestingly, it was observed that at much lower PVP-monomer to Pt4+ ratio (10–5), clusters were less stable for long time storage, usually for more than a year. After purification of the produced particles, one part was freeze dried, pulverized and stored in an airtight vial at room temperature. Another part was colloidal hydrosol, which was also stored in an airtight vial at room temperature. The stability of the clusters was tested by UV–visible absorption spectrophotometry and TEM micrographs observation. Basically, there was no difference between before and after storage.
An effect of the average gram molecular weight (MW) of the stabilizing polymer that is chain length of PVP was investigated on the size and size distribution of Pt clusters from 3500 to 360,000 for a constant PVP-monomer to Pt-atom ratio of 40, which is shown in Fig. 6. It was found that a molecular weight of 40,000 provided the smallest particles of 1.3 ± 0.3 nm. With increasing or decreasing the average gram MW of PVP, produced particles were larger than 1.3 ± 0.3 nm. It was assumed that in case of small-chained PVP, the relative number of polymer chain per seed of clusters was much higher than required to well stabilize that initially produced small but less stable particles by steric hindrance those were finally reunited to produce larger clusters. On the other hand, in case of longer chain polymer, insufficient number of polymer chain per cluster that was actually required destabilized the smaller particles formation, which is required for sufficient stabilization.
Size distributions
Figure 7 shows the representative TEM image of Pt:PVP clusters where particles were found to be comprised of spherical in shape. More than 300 clusters diameter was measured and plotted as histogram. An average diameter (d TEM) and distribution of the clusters were determined from the best fitting of the histograms using Gaussian function. To the best of our knowledge, this is the unique method for the preparation of the smallest Pt:PVP clusters in aqueous phase reduction of Pt4+/2+ ions by NaBH4 with an average diameter 1.3 ± 0.3 nm reported so far [2–8, 16–18, 22, 36].
Optical spectra for cluster formation
Figure 8 shows the normalized UV–visible absorption spectra of the Pt clusters hydrosol measured at 0.2 mM concentration of Pt0 and precursor solution spectra (Pt4+ ions) was measured at 1.0 mM concentration. Before reduction, the H2PtCl6 solution with or without PVP were pale yellow. After reduction with NaBH4, it was instantly turned into dark brown regardless of the particles size and shape. The formed Pt:PVP cluster hydrosol exhibited an exponential-like profile in the UV–visible absorption spectrum with no obvious absorption peak, which is in consistence with earlier studies, whereas the original precursor solution optical spectra were completely different. This feature is attributed for intra- and inter-band optical transitions, suggesting the formation of Pt clusters [20, 22, 29, 30]. These measurements further confirmed that the Pt4+ ions were completely reduced to Pt0 forming the nano-clusters.
Aqueous phase reduction of Pt4+/2+ ions to Pt0 by NaBH4 is the most suitable method for synthesizing small and monodisperse Pt clusters but the produced Pt0 readily reacts with borohydride (BH4 −) causing incomplete reduction of the precursor ions, which results a very low yield along with larger particles formation [19–22]. Hence, fresh BH4 − should be supplied continuously to reduce metal ions and separate the produced Pt0 from the reaction mixture. Using a fluidic system, the hydrolysis of BH4 − can be prevented by separating the produced Pt0, which will facilitate the reduction of the metal ions. In addition, microfluidic system ensures a homogeneous mixing of metal ions and BH4 −, which is expected to produce monodispersed Pt clusters.
Structural characterization
The XRD pattern of produced Pt:PVP clusters and a bulk Pt metal is shown in Fig. 9. The observed four peaks in the spectrum can be designated to (111), (200), (220) and (311) planes of platinum clusters with an fcc phase structure, which suggests the purity of the produced platinum crystals and no platinum oxide or reaction residue were detectable. The average diameters of the Pt crystallites (d XRD) were calculated using the Scherrer equation to the Pt (111) diffraction peak, which was found to be 1.2 nm. The XRD diffraction patterns were simulated using TOPAS-4 program.
where k is a dimensionless constant called shape factor (for spherical particles its value is equal to 0.89), λ is the X-ray wavelength in nanometer (nm), θ is the Bragg diffraction angle of the Pt (111) plane, and β is the full width at half-maximum (FWHM) of the Pt (111) diffraction peak. The value of the crystallite size determined from XRD was consistent with the value determined from TEM analysis. To the best of our knowledge, this is the smallest Pt:PVP crystallite synthesized in aqueous phase reduction of Pt ions reported so far [19–21].
The broadened and low diffraction peak at Pt (111) as well as little shift to the smaller angle (from 40°) suggests the formation of a large number of vacancies associated with higher population of low-coordination sites in smaller Pt clusters [32]. The shifting of diffraction peak to the smaller angle also suggests the expansion of the lattice parameter (interatomic distances) from the perfect single crystal lattice (for bulk Pt a 0 = 0.39231 nm) and also increase in the unit cell volume [44]. Lattice expansion with crystal size reduction has also been observed for Pd nanoparticles [45] and Ag nanoparticles [46]. A probable explanation for this effect might be the use of PVP as stabilizer, which has the ability to donate electron to the vacant d-orbital of metal atoms, hence leading to Pt–Pt bond expansion of the surface-near atoms by increasing electron density [2, 22, 47].
A change of interatomic distances and unit cell parameters considerably influences the catalytic activity of Pt-based nanoparticles. An expansion of the Pt–Pt interatomic distances demonstrated an enhanced catalytic activity on oxide reduction, as well as a significantly improved resistance to CO poisoning [48]. Similar beneficial effects were also reported for Pd particles [49]. We also expect the similar enhanced catalytic activity for our microfluidic Pt:PVP particles.
Conclusion
Mixing is a crucial factor for producing small and monodispersed metal clusters. Hence, micromixer is a simple and powerful tool that helps to mix solutions homogeneously nearly at the molecular level. It also prevents the degradation of NaBH4 by preventing the mixing of produced clusters from reactants by separating them through fluidic system that enables to produce small and monodispersed Pt clusters. From the reduction behavior and nucleation/growth mechanism based on an empirical model, the microfluidic-borohydride reduction for Pt4+ ions occurs in two steps. In the first step, Pt4+ converts to Pt2+, which is subsequently reduced to Pt0 by the produced H2 gas in the second step. Optimizing the reduction conditions, Pt nano-clusters of possible smallest diameter was synthesized and that was 1.3 ± 0.3 nm of TEM diameter. The crystal size determined by XRD was consistent with TEM diameter and that was 1.2 nm having fcc crystal geometry same as bulk platinum. Utilizing this technique, other nano-clusters of transition metals could be synthesized.
References
Hirai, H., Toshima, N.: In: Tailored Metal Catalysts. Iwasawa Y., (Ed) Reidel Publishing, Dordrecht (1986)
Tsunoyama, H., Ichikuni, N., Sakurai, H., Tsukuda, T.: Effect of electronic structures of Au clusters stabilized by poly (N-vinyl-2-pyrrolidone) on aerobic oxidation catalysis. J. Am. Chem. Soc. 131, 7086–7093 (2009)
Xiao, C.X., Cai, Z.P., Wang, T., Kou, Y., Yan, N.: Aqueous-phase Fischer–Tropsch synthesis with a ruthenium nanocluster catalyst. Angew. Chem. 120, 758–761 (2008)
Xiao, C.X., Wang, H.Z., Mu, X.D., Kou, Y.: Ionic-liquid-like copolymer stabilized nanocatalysts in ionic liquids I. Platinum catalyzed selective hydrogenation of o-chloronitrobenzene. J. Catal. 250, 25–32 (2007)
Song, S., Liu, R., Zhang, Y., Feng, J., Liu, D., Xing, Y., Zhao, F., Zhang, H.: Colloidal noble-metal and bimetallic alloy nanocrystals: a general synthetic method and their catalytic hydrogenation properties. Chem. A Eur. J. 16, 6251–6256 (2010)
Yan, N., Zhao, C., Luo, C., Dyson, P.J., Liu, H., Kou, Y.: One-step conversion of cellobiose to C6-alcohols using a ruthenium nanocluster catalyst. J. Am. Chem. Soc. 128, 8714–8715 (2006)
Mu, X.D., Meng, J.Q., Li, Z.C., Kou, Y.: Rhodium nanoparticles stabilized by ionic copolymers in ionic liquids: long lifetime nanocluster catalysts for benzene hydrogenation. J. Am. Chem. Soc. 127, 9694–9695 (2005)
Zhao, C., Wang, H.Z., Yan, N., Xiao, C.X., Mu, X.D., Dyson, P.J., Kou, Y.: Ionic-liquid-like copolymer stabilized nanocatalysts in ionic liquids: II. Rhodium Catal. Hydrog. Arenes J. Catal. 250, 33–40 (2007)
Zhao, C., Gan, W., Fan, X., Cai, Z., Dyson, P.J., Kou, Y.: Aqueous-phase biphasic dehydroaromatization of bio-derived limonene into p-cymene by soluble Pd nanocluster catalysts. J. Catal. 254, 244–250 (2008)
He, L., Liu, H., Xiao, C.X., Kou, Y.: Liquid-phase synthesis of methyl formate via heterogeneous carbonylation of methanol over a soluble copper nanocluster catalyst. Green Chem. 10, 619–622 (2008)
Somorjai, G.A.: Introduction to Surface Chemistry and Catalysis. Wiley, New York (1994)
White, R.J., Luque, R., Budarin, V.L., Clark, J.H., Macquarrie, D.J.: Supported metal nanoparticles on porous materials. Methods and applications. Chem. Soc. Rev. 38, 481–494 (2009)
Astruc, D., Lu, F., Aranzaes, J.R.: Nanoparticles as recyclable catalysts: the frontier between homogeneous and heterogeneous catalysis. Angew. Chem. Int. Ed. 44, 7852–7872 (2005)
Rioux, R.M., Song, H., Grass, M., Habas, S., Niesz, K., Hoefelmeyer, J.D., Yang, P., Somorjai, G.A.: Monodisperse platinum nanoparticles of well-defined shape: synthesis, characterization, catalytic properties and future prospects. Top. Catal. 39, 167–174 (2006)
Chen, A., Holt-Hindle, P.: Platinum-based nanostructured materials: synthesis, properties, and applications. Chem. Rev. 110, 3767–3804 (2010)
Xu, S., Yang, Q.: Well-dispersed water-soluble Pd nanocrystals: Facile reducing synthesis and application in catalyzing organic reactions in aqueous media. J. Phys. Chem. C 112, 13419–13425 (2008)
Zhang, Q., Xie, J., Yang, J., Lee, J.Y.: Monodisperse icosahedral Ag, Au, and Pd nanoparticles: size control strategy and superlattice formation. ACS Nano 3, 139–148 (2008)
Wiley, B., Sun, Y., Xia, Y.: Synthesis of silver nanostructures with controlled shapes and properties. Acc. Chem. Res. 40, 1067–1076 (2007)
Van Rheenen, P.R., McKelvy, M.J., Glaunsinger, W.S.: Synthesis and characterization of small platinum particles formed by the chemical reduction of chloroplatinic acid. J. Solid State Chem. 67, 151–169 (1987)
Knecht, M.R., Weir, M.G., Myers, V.S., Pyrz, W.D., Ye, H., Petkov, V., Buttrey, D.J., Frenkel, A.I., Crooks, R.M.: Synthesis and characterization of Pt dendrimer-encapsulated nanoparticles: effect of the template on nanoparticle formation. Chem. Mater. 20, 5218–5228 (2008)
Borodko, Y., Thompson, C.M., Huang, W., Yildiz, H.B., Frei, H., Somorjai, G.A.: Spectroscopic study of platinum and rhodium dendrimer (PAMAM G4OH) compounds: structure and stability. J. Phys. Chem. C 115, 4757–4767 (2011)
Hossain, M.J., Tsunoyama, H., Yamauchi, M., Ichikuni, N., Tsukuda, T.: High-yield synthesis of PVP-stabilized small Pt clusters by microfluidic method. Catal. Today 183, 101–107 (2012)
Gobby, D., Angeli, P., Gavriilidis, A.: Mixing characteristics of T-type microfluidic mixers. J. Micromech. Microeng. 11, 126 (2001)
Soleymani, A., Kolehmainen, E., Turunen, I.: Numerical and experimental investigations of liquid mixing in T-type micromixers. Chem. Eng. J. 135, S219–S228 (2008)
Schwesinger, N., Frank, T., Wurmus, H.: A modular microfluid system with an integrated micromixer. J. Micromech. Microeng. 6, 99 (1996)
Munson, M.S., Yager, P.: Simple quantitative optical method for monitoring the extent of mixing applied to a novel microfluidic mixer. Anal. Chim. Acta 507, 63–71 (2004)
Hessel, V., Hardt, S., Löwe, H., Schönfeld, F.: Laminar mixing in different interdigital micromixers: I. Experimental characterization. AIChE J. 49, 566–577 (2003)
Hardt, S., Schönfeld, F.: Laminar mixing in different interdigital micromixers: II. Numerical simulations. AIChE J. 49, 578–584 (2003)
Rioux, R.M., Song, H., Hoefelmeyer, J.D., Yang, P., Somorjai, G.A.: High-surface-area catalyst design: synthesis, characterization, and reaction studies of platinum nanoparticles in mesoporous SBA-15 silica. J. Phys. Chem. B 109, 2192–2202 (2005)
Teranishi, T., Hosoe, M., Tanaka, T., Miyake, M.: Size control of monodispersed Pt nanoparticles and their 2D organization by electrophoretic deposition. J. Phys. Chem. B 103, 3818–3827 (1999)
Campelo, J.M., Luna, D., Luque, R., Marinas, J.M., Romero, A.A.: Sustainable preparation of supported metal nanoparticles and their applications in catalysis. Chem. Sus. Chem. 2, 18–45 (2009)
Toshima, N., Harada, M., Yonezawa, T., Kushihashi, K., Asakura, K.: Structural analysis of polymer-protected palladium/platinum bimetallic clusters as dispersed catalysts by using extended X-ray absorption fine structure spectroscopy. J. Phys. Chem. 95, 7448–7453 (1991)
Borodko, Y., Humphrey, S.M., Tilley, T.D., Frei, H., Somorjai, G.A.: Charge-transfer interaction of poly(vinylpyrrolidone) with platinum and rhodium nanoparticles. J. Phys. Chem. C 111, 6288–6295 (2007)
Elechiguerra, J.L., Reyes-Gasga, J., Yacaman, M.J.: The role of twinning in shape evolution of anisotropic noble metal nanostructures. J. Mater. Chem. 16, 3906–3919 (2006)
Teranishi, T., Miyake, M.: Size control of palladium nanoparticles and their crystal structures. Chem. Mater. 10, 594–600 (1998)
Lim, B., Jiang, M., Tao, J., Camargo, P.H., Zhu, Y., Xia, Y.: Shape-controlled synthesis of Pd nanocrystals in aqueous solutions. Adv. Funct. Mater. 19, 189–200 (2009)
Leszczyńska, A., Njuguna, J., Pielichowski, K., Banerjee, J.R.: Polymer/montmorillonitenanocomposites with improved thermal properties: part I. Factors influencing thermal stability and mechanisms of thermal stability improvement. Thermochim. Acta 453, 75–96 (2007)
Shao-Horn, Y., Sheng, W.C., Chen, S., Ferreira, P.J., Holby, E.F., Morgan, D.: Instability of supported platinum nanoparticles in low-temperature fuel cells. Top. Catal. 46, 285–305 (2007)
Chen, Z., Waje, M., Li, W., Yan, Y.: Supportless Pt and PtPd nanotubes as electrocatalysts for oxygen-reduction reactions. Angew. Chem. Int. Ed. 46, 4060–4063 (2007)
Garron, A., Świerczyński, D., Bennici, S., Auroux, A.: New insights into the mechanism of H2 generation through NaBH4 hydrolysis on Co-based nanocatalysts studied by differential reaction calorimetry. Int. J. Hydrog. Energy 34, 1185–1199 (2009)
Gonçalves, A., Castro, P., Novais, A., Fernandes, V.R., Rangel, C.M., Matos, H.: Dynamic modeling of hydrogen generation via hydrolysis of sodium borohydride. Reactions 1, 2 (2007)
Duff, D.G., Edwards, P.P., Johnson, B.F.: Formation of a polymer-protected platinum sol: a new understanding of the parameters controlling morphology. J. Phys. Chem. 99, 15934–15944 (1995)
Ingelsten, H.H., Bagwe, R., Palmqvist, A., Skoglundh, M., Svanberg, C., Holmberg, K., Shah, D.O.: Kinetics of the formation of nano-sized platinum particles in water-in-oil microemulsions. J. Colloid Interface Sci. 241, 104–111 (2001)
Leontyev, I.N., Kuriganova, A.B., Leontyev, N.G., Hennet, L., Rakhmatullin, A., Smirnova, N.V., Dmitriev, V.: Size dependence of the lattice parameters of carbon supported platinum nanoparticles: X-ray diffraction analysis and theoretical considerations. RSC Adv. 4, 35959–35965 (2014)
Kuhrt, C., Anton, R.: On the origin of a lattice expansion in palladium and Pd@Au vapour deposits on various substrates. Thin Solid Films 198, 301–315 (1991)
Onodera, S.: Lattice parameters of fine copper and silver particles. J. Phys. Soc. Jpn. 61, 2190–2193 (1992)
Qiu, L., Liu, F., Zhao, L., Yang, W., Yao, J.: Evidence of a unique electron donor-acceptor property for platinum nanoparticles as studied by XPS. Langmuir 22, 4480–4482 (2006)
Alayoglu, S., Nilekar, A.U., Mavrikakis, M., Eichhorn, B.: Ru–Pt core–shell nanoparticles for preferential oxidation of carbon monoxide in hydrogen. Nat. Mater. 7, 333–338 (2008)
Suo, Y., Zhuang, L., Lu, J.: First-principles considerations in the design of Pd-alloy catalysts for oxygen reduction. Angew. Chem. Int. Ed. 46, 2862–2864 (2007)
Acknowledgements
This research work was done under the Postdoctoral Fellowship Programme of Bangladesh Council of Science and Industrial Research (BCSIR), Bangladesh and financial support thereby. Thanks to BFRI authority for their kind cooperation. Finally, special thanks to Sophisticated Analytical Instrument Facility (SAIF), Shillong, India for the TEM micrographs.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hossain, M.J., Rahman, M.S., Rahman, M.S. et al. Optimized reduction conditions for the microfluidic synthesis of 1.3 ± 0.3 nm Pt clusters. J Nanostruct Chem 6, 49–56 (2016). https://doi.org/10.1007/s40097-015-0179-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40097-015-0179-5