Abstract
We present an investigation on magnetic and magnetocaloric properties of nano-sized La0.8Ca0.2Mn1−x Fe x O3 (x = 0, 0.01, 0.15, 0.2) manganites synthesized by sol–gel process. X-ray diffraction and magnetization measurements were performed to investigate both crystallographic structure and magnetocaloric properties, respectively. All samples show an orthorhombic structure with Pnma space group. Ferromagnetic–paramagnetic transition sensitive to iron doping is observed at Curie temperature (T C) ranging from 223 K (x = 0) to 70 K (x = 0.2). In addition, a large magnetocaloric effect near T C is observed. Under a magnetic field change of 5 T, a maximum of magnetic entropy \(\Delta S_{\text{M}}^{ \hbox{max} }\) reaches 4.42, 4.32, 1.6, and 0.54 J kg−1 K−1, for x = 0, x = 0.01, x = 0.15, and x = 0.2, respectively. The relative cooling power (RCP) values were computed as well. RCP values of 164 and 117 J kg−1 were found for La0.8Ca0.2MnO3 (LCM) and La0.8Ca0.2Mn.0.99Fe0.01O3 (LCMFe0.01), respectively. The large values of entropy changes and related RCP allow concluding that our material could be a highly attractive candidate for magnetic refrigeration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The modern society is increasingly relying on refrigeration technology. The vapor compression refrigerators have been mainly used for cooling applications. However, the compression and expanding processes in the refrigerators of a gas are not sufficiently efficient. On the other hand, the use of gases such as chlorofluorocarbons and hydro-chlorofluorocarbons is damaging to our living environment. For these reasons, exploring a new type refrigeration technology that is environmentally friendly and energy efficient becomes an urgent necessity. Comparing to the conventional gas compression (CGC), magnetic refrigeration (MR) based on the magnetocaloric effect (MCE) [1] exhibits several advantages [2, 3]. Indeed, the MR does not use global warming gases and therefore is an environmentally friendly cooling technology [4, 5]. So an ongoing research is necessary to find appropriate materials with a large enough magnetic entropy at moderate magnetic fields near room temperature [5, 6]. The gadolinium (Gd) rare earth metal has been considered as the most obvious material exhibiting a large MCE in room-temperature magnetic refrigerators [2, 7, 8]. However, the use of the Gd is limited due to its expensive cost price. Nevertheless, some other candidates have been found to exhibit large MCE, such as Gd5 (Si x Ge1−x )4 [9], La (Fe1−x Si x )13 [10], MnFeP1−x As x [11], and Tb1−x Gd x Al2 [12].
In the last few years, manganites with a general formula R1−x A x MnO3 (R = rare earth, A = alkali earth) have attracted more attention as alternative candidates for MR near room temperature. Compared to Gd, they show several advantages such as higher chemical stability, higher resistivity, and lower cost. Their preparation can be achieved without substantial difficulties. As other advantages, they present the possibility to tune their magnetic transition temperature by the substitution on both R-sites and Mn-sites. The MCE of La1−x A x MnO3 (A = Ca, Sr, Ba) manganites was first studied by Moreli et al. [13]. A large MCE in La1−x A x MnO3 polycrystalline samples (0.2 ≤ x ≤ 0. 33) is reported by Guo et al. [14, 15]. In fact, for ΔH = 1.5 T, the \(\left| {\Delta S_{\text{M}} } \right|\) reaches a maximum of about 5.5 J/(kg K) at 230 K, 4.7 J/(kg K) at 224 K, and 4.3 J/(kg K) at 260 K for x = 0.2, 0.25, and 0.33, respectively [14]. For the same magnetic field of 1.5 T, these values are larger than that of Gd, \(\left| {\Delta S_{\text{M}} } \right|\) = 4.2 J/(kg K) [2]. As other significant information, the magnitude of \(\left| {\Delta S_{\text{M}} } \right|\) was found to be inversely proportional to the grain size [16]. This paper is devoted to seeking for new perovskite manganites with broad refrigerant capacity and large MCE demanding only low applied magnetic fields close to the room temperature. Precisely, we report the effect of Fe doping on the magnetic and magnetocaloric properties of La0.8Ca0.2Mn1−x Fe x O3 (x = 0, 0.01, 0.15, 0.2). They present large magnetic entropy change values and high relative cooling power (RCP) factors.
Experimental
The nano-sized La0.8Ca0.2Mn1−x Fe x O3 (x = 0, 0.01, 0.15, 0.2) manganites were synthesized using the sol–gel method. The starting precursor MnO2 was mixed in appropriate proportion and dissolved in the concentrated nitric acid. Suitable amounts of citric acid and ethylene glycol, as a coordinate agent, were added. After the addition of the other precursors La2O3, CaCO3, and Fe2O3, a clear black stained solution was obtained. Then the solution is allowed to dry to form a dried gel, followed by baking at 170° C to obtain black precursor powder. Finally, the resulting powder was heated in air at 950 °C for 24 h. The X-ray diffraction patterns at room temperature were obtained using SIEMENS D8 X-ray diffractometer with Cu Kα radiation. The FULLPROF program based on the Rietveld method [17] was used for phase analysis. The magnetic isotherms were recorded in the magnetic field of up to 5 T and at the temperature ranging from 4 to 400 K. The magnetocaloric effects (MCE) were estimated via the Maxwell relation [2].
Results and discussion
X-ray diffraction
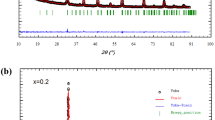
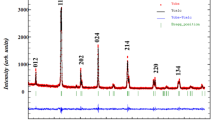
Powder X-ray diffraction patterns (Fig. 1) show that the samples show single phase and indexed in the orthorhombic structure with Pnma group space (Fig. 2). Refined cell parameters such as unit cell parameters, unit cell volume, R factor, and the goodness-of-fit indicator (χ 2) are listed in Table 1. We can deduce that the substitution of Mn3+ by Fe3+ ions induces an increase of the unit cell volume. The linear increase is unexpected because we substitute Mn3+ having 0.0645 nm as ionic radius by Fe3+ with the same ionic radius (0.0645 nm). Consequently, no change induced by this substitution is expected. Therefore, the increase could be attributed to the lattice disorder arising from the random occupancy of Fe and Mn ions on the B-site. Indeed, in the pure perovskite La0.8Ca0.2MnO3 system (LCMO), Mn shows a mixed valence with Mn3+/Mn4+ ratio close to 4 ([Mn4+] = 0.2 and [Mn3+] = 0.8) with a valence of +3 for La. The partial substitution of the Mn ions by transition metal ions (Fe) in La0.8Ca0.2Mn1−x Fe x O3 manganites gives rise to changes in the Mn3+/Mn4+ rate, and some Mn3+–O2−–Mn4+ networks are substituted by Fe3+–O2−–Mn4+. This causes a disorder of the charge transfer mechanism. Such disorder causes a change in the Mn–O distances and Mn–O–Mn angles. Consequently, the distortion of the ideal perovskite structure in which the Mn–O–Mn angle is equal to 180° undergoes a modification. These results are similar to those obtained by Othmani et al. [18].
Magnetic properties
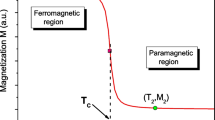
To study the effect of substitution of iron in manganese sites on the magnetic properties, we have analyzed the magnetization variation versus temperature of La0.8Ca0.2Mn1−x Fe x O3 (x = 0, 0.01, 0.15, 0.2) samples under an applied magnetic field of H = 0.05 T (Fig. 3 ). The M (T) curves reveal that all samples exhibit a ferromagnetic (FM)–paramagnetic (PM) transition at Curie temperature, T C = 223, 205, 114, and 70 K, for x = 0, 0.01, 0.15, 0.2, respectively. The Curie temperature T C, defined as the peak of dM/dT in the M (T) curves, is reported for all compositions in Table 3. This table gives evidence that T C and the magnetization are sensitive to Fe content. Indeed, the increase in Fe content causes an increase in T C accompanied by a reduction of the magnetization. Probably, both changes are attributed to the competition between the superexchange (Mn4+–O–Mn4+) and double-exchange (Mn3+–O–Mn4+) interactions. The Fe takes place at the Mn site as Fe3+ (replacement of some Mn3+–O–Mn4+ bonds by Mn4+–O–Fe3+ bonds), giving rise to an antiferromagnetic coupling between Mn and Fe ions that favors the superexchange mechanism. The evolution of magnetization (M) versus the applied magnetic field (µ 0 H) for x = 0, 0.01, 0.15, and 0.2 samples, obtained at different temperatures and measured under applied magnetic field ranging from 1 to 5 T, is shown in Fig. 4. These curves show that, below the Curie temperature, the magnetization greatly increases with the magnetic field and the saturated M is reached at H = 1 T. For T > T C, the variation of M (T, µ 0 H) does not reach the saturation and a linear behavior appears. This result confirms that all samples present a typical ferromagnetic behavior.
Figure 5 presents the magnetization measurements performed at 4 K under applied magnetic fields of up to 6 T, for La0.8Ca0.2Mn1−x Fe x O3 (x = 0, 0.01, 0.15, 0.2) samples. Table 2 lists the experimental and the calculated magnetic moments per Mn ion, denoted by \(M_{\text{Sat}}^{\text{Exp}}\) and \(M_{\text{Sat}}^{\text{Theo}}\), respectively. The values of \(M_{\text{Sat}}^{\text{Theo}}\) have been calculated by considering that the spins of all Mn and Fe ions are aligned. The magnetic moment of \({\text{La}}_{0.8}^{3 + } {\text{Ca}}_{0.2}^{2 + } \left( {{\text{Mn}}_{1 - x} {\text{Fe}}_{x} } \right)_{0.8}^{3 + } {\text{Mn}}_{0.2 }^{4 + } {\text{O}}_{3}\) is expressed as
The magnetic moments of Mn3+, Mn4+, and Fe3+ ions are µ 3+Mn = 4µ B, µ 4+Mn = 3µ B, and µ 3+Fe = 5µ B, respectively. The x is the iron concentration and µ B is the Bohr magneton. We note that the magnetization saturation values \(M_{\text{Sat}}\) decrease with increasing Fe content, especially for x = 0.2. It is worth noting that similar results are reported in [19]. The difference between the measured and the calculated values should be explained by the presence of a magnetic inhomogeneity or by spin-canted state at low temperature.
Arrott curve
To determine the nature of magnetic transition type (first or second order), we have considered the experimental criterion given by Banerjee [20]. It consists in inspecting the slope of isotherm plots of µ 0 H/M versus M 2. According to this criterion, magnetic transition is of second order if all the curves have positive slopes, while, if some of these curves show a negative slope, the transition is first order. Figure 6 shows the isotherm M 2 versus µ 0 H/M above and below T C for La0.8Ca0.2Mn1−x Fe x O3 (x = 0, 0.01, 0.15) samples. Based on the of Banerjee’s criterion, the LCM and LCMF0.01 systems exhibit a first-order ferromagnetic-to-paramagnetic phase transition, whereas a second-order transition is confirmed for LCMF0.15 and LCMF0.2.
Magnetocaloric study
The MCE is defined as the heating or cooling of a magnetic material due to the application or suppression of a magnetic field, respectively. To estimate the magnetocaloric effect, the change of magnetic entropy (\(\Delta S_{\text{M}}\)) was calculated numerically using the equation [21]:
The M i and M i+1 are the experimental values of magnetization measured at temperatures T i and T i+1, respectively. The H i is the applied magnetic field. The magnetic entropy change (\(\Delta S_{\text{M}}\)) determined numerically using Eq. (2) and the M (T, µ 0 H) curves are shown in Fig. 7. The (\(\Delta S_{\text{M}}\)) value increases with temperature increase to reach a maximum near T C and lowers above T c. To compare our results with previously published data for other perovskite manganites, we listed in Table 3 the data of several magnetic materials that could be used as magnetic refrigerants. Also, the maximum magnetic entropy change of Fe-doped manganites increases gradually with increasing applied magnetic field for such materials. We noted that the maximum entropy change \(\left| {\Delta S_{\text{M}}^{ \hbox{max} } } \right|\) corresponding to a magnetic field variation of 5 T for La0.8Ca0.2MnO3 and La0.8Ca0.2Mn0.99Fe0.01O3 is about 4.42 and 4.32 J/(kg K), respectively.
In Table 3, we compared our performances of MCE with those of Gd [2]-based materials as well as rare earth manganites. The highest value of the magnetic entropy change for La0.8Ca0.2MnO3 and La0.8Ca0.2Mn0.99Fe0.01O3 samples is observed with x = 0 content and is equal to 1.96 and 4.42 J/(kg K) under magnetic fields of 1 and 5 T, respectively. In addition, similar results were observed by Shaobo Xi et al. for La0.8Ca0.2MnO3 [25] and by S. Ghodhbane et al. for Pr0.8Ba0.2MnO3 compounds under applied magnetic fields of 3 and 1 T, respectively. These values are lower than that of pure Gd [2.8 J/(kg K)] in a magnetic field change of 1 T [2] ) and Gd5 (SixGe1−x )4 system [9] which have been considered as good magnetic refrigerants. For LCMFe0.15 and LCMFe0.2, the maximum value of magnetic entropy change, \(\left| {\Delta S_{\text{M}}^{ \hbox{max} } } \right|,\) is 1.6 and 0.54 K/(kg K) under a magnetic field of 5 T, respectively. Similar results have been reported for La0.7Ca0.15Sr0.15Mn0.9Fe0.1O3 [18] and La0.63Ca0.33Mn0.8Fe0.2O3 [30].
The temperature dependence of the \(\Delta S_{\text{M}}\) upon the magnetic applied field changes of 5 T is shown in Fig. 8. These curves reveal that the La0.8Ca0.2Mn1−x Fe x O3 (x = 0, 0.01) samples present large magnetic entropy change and that \(\Delta S_{\text{M}}\) decreases when increasing the Fe content (x). This behavior is understood as the reduction of the double-exchange mechanism between Mn3+ and Mn4+ ions for La0.8Ca0.2Mn1−x Fe x O3 samples when x increases.
Relative cooling power (RCP)
Another useful parameter which examines the efficiency of a magnetocaloric material is the RCP or the refrigerant capacity. It expresses the heat transfer between the hot and the cold reservoirs during an ideal refrigeration cycle. This is defined as the product of peak value of change in the magnetic entropy and the full width at half maximum (FWHM) of ΔS M versus T curve (Fig. 8, inset) [31].
We have represented in Fig. 9 the variation of the RCP factor as a function of the applied magnetic field. The RCP values exhibit a linear rise with increasing field for LCMO and LCMO0.01 samples. Under the influence of an applied field of 5 T, the RCP values are found to be 146 and 116 J/kg for the samples x = 0 and 0.01, respectively. Similar RCP values at 5 T (RCP = 140 J/kg) have been reported for La0.8Ca0.2MnO3 [26] and La0.7Ca0.15Sr0.15Mn0.9Fe0.1O3 samples [24]. Another interesting feature in the MCE plot is its asymmetric shape, especially under high field. Similar behavior is observed in Fe-substituted lanthanum calcium manganite [30]. For comparison, the maximum magnetic entropy change, the Curie temperature, and the relative magnetic cooling efficiency of several manganese perovskites considered useful for room-temperature magnetic refrigerators are summarized in Table 3. Thus, due to the high ΔS M and RCP values, our compounds with x = 0 and 0.01 could be considered as active magnetic refrigerants for near-room-temperature magnetic refrigeration.
Conclusion
We have studied the structural, magnetic, and magnetocaloric properties of the Fe-doped manganite perovskite La0.8Ca0.2Mn1−x Fe x O3 compounds with 0 ≤ x ≤ 0.2. The results show that the samples crystallize in the orthorhombic structure with Pnma space group. The magnetic properties reveal that all samples exhibit a paramagnetic–ferromagnetic transition when temperature decreases. From magnetocaloric study, the LCMO and LCMF0.01O samples have a large magnetic entropy change. The maximum values of the magnetic entropy changes decrease with the increase of Fe concentration. In addition, due to the high ΔS M and RCP values, Fe-doped manganite perovskite samples can be considered a potential refrigerant for use in near-room-temperature magnetic refrigeration.
References
Warburg, E.: Magnetische untersuchung. Ann. Phys. 13, 141–164 (1881)
Gschneidner, K.A., Pecharsky, V.K., Tsokol, A.O.: Recent developments in magnetocaloric materials. Rep. Prog. Phys. 68, 1479–1539 (2005)
Bruck, E.: Developments in magnetocaloric refrigeration. J. Phys. D Appl. Phys. 38, R381–R391 (2005)
Vitalij, K., Pecharsky, V.K., Gschneidner Jr, K.A.: Magnetocaloric effect and magnetic refrigeration. J. Magn. Magn. Mater. 200, 4456 (1999)
Phan, M.H., Yu, S.C.: Review of the magnetocaloric effect in manganite materials. J. Magn. Magn. Mater. 308, 325–340 (2007)
Tishin, A.M., Spichkin, I.: The Magnetocaloric Effect and Its Applications. Institute of Physics Publishing, Bristol (2003)
Gschneidner Jr, K.A., Pecharsky, V.K.: Advanced magnetocaloric materials: what does the future hold? Int. J. Refrig 29, 1239–1249 (2006)
Atalay, S., Gencer, H., Kolat, V.S.: Magnetic entropy change in Fe74−xCrxCu1Nb3Si13B9 (x= 14 and 17) amorphous alloys. J. Non-Cryst. Solids 351(30), 2373–2377 (2005)
Pecharsky, V.K., Gschneidner Jr, K.A.: Giant magnetocaloric effect in Gd5(Si2Ge2). Appl. Phys. Lett. 70, 3299 (1997)
Fujieda, S., Fujita, A., Fukamichi, K.: Large magnetocaloric effect in La(FexSi1−x)13 itinerant-electron metamagnetic compounds. Appl. Phys. Lett. 81, 1276 (2002)
Tegus, Q., Bruck, E., Buschow, K.H.: Boer, F. R.: Transition-metal-based magnetic refrigerants for room-temperature applications. Nature 415, 150 (2002)
Wang, F.W., Zhang, X.X., Hu, F.X.: Large magnetic entropy change in TbAl2 and (Tb0.4Gd0.6)Al2. Appl. Phys. Lett. 77, 1360 (2000)
Morelli, D.T., Mance, A.M., Mantese, J.V., Micheli, A.L.: Magnetocaloric properties of doped lanthanum manganite films. J. Appl. Phys. 79, 373–375 (1996)
Guo, Z.B., Du, Y.W., Zhu, J.S., Huang, H., Ding, W.P., Feng, D.: Large magnetic entropy change in perovskite-type manganese oxides. Phys. Rev. Lett. 78, 1142 (1997)
Guo, Z.B., Zhang, J.R., Huang, H., Ding, W.P., Du, Y.W.: Lattice effect in Pr doped La Sr Mn O perovskite. Solid State Commun. 100, 769–771 (1996)
Hueso, L.E., Sande, P., Miguens, D.R., Rivas, J., Rivadulla, F., Lopez-Quintela, M.A.: Tuning of the magneto-caloric effect in La0.67Ca0.33MnO3-δ nanoparticles synthesized by sol-gel techniques. J. Appl. Phys. 91, 9943 (2002)
Rodrigez-Carjaval, J.: XVth congess of the international union of crystallography. In: Proceedings of the satellite meeting on powder diffraction, vol. 127.Toulouse (1990)
Othmani, S., Blel, R., Bejar, M., Sajieddine, M., Dhahri, E., Hlil, E.K.: New complex magnetic materials for an application in Ericsson refrigerator. J. Solid State Commun. 149, 969–972 (2009)
Issaoui, F., Tlili, M.T., Bejar, M., Dhahri, E., Hlil, E.K.: Structural and magnetic studies of Ca2−xSmxMnO compounds (x = 0–0.4). J. Supercond. Novel. Mag. 25(4), 1169–1175 (2012)
Banerjee, S.K.: On a generalised approach to first and second order magnetic transitions. Phys. Lett. 12, 16–17 (1964)
Foldeaki, M., Chahine, R., Gopal, B.R., Bose, T.K.: Investigation of the magnetic properties of the Gd1-xErx alloy system in the x < 0.62 composition range. J. Magn. Magn. Mater. 150(3), 421–429 (1995)
Nisha, P.: Pilla, i S.S., Varma, M.R., Surech, K.G.: Critical behavior and magnetocaloric effect in La0.67Ca0.33Mn1−xCrxO3 (x = 0.1, 0.25). Solid State Sci. 14, 40–47 (2012)
Khlifi, M., Bejar, M., Sadek, O.E.L., Dhahri, E., Ahmed, M.A., Hlil, E.K.: Structural, magnetic and magnetocaloric properties of the lanthanum deficient in La0.8Ca0.2−x·xMnO3 (x = 0–0.20) manganites oxides. J. Alloy. Compd. 509, 7410–7415 (2011)
Phan, M.H., Phan, V.T., Yu, S.C., Rhee, J.R., Hur, N.H.: Excellent magnetocaloric properties of La0.7Ca0.3−xSrxMnO3 (0.05-x-0.25) single crystals. Appl. Phys. Lett. 86, 072504 (2005)
Xi, S., Lu, W., Sun, Y.: Magnetic properties and magnetocaloric effect of La0·8Ca0·2MnO3 nanoparticles tuned by particle size. J. Appl. Phys. (2012). doi:10.1063/1.3699037
Ghodhbane, S., Dhahri, A., Dhahri, N., Hlil, E.K., Dhahri, J.: Structural, magnetic and magnetocaloric properties of La0.8Ba0.2Mn1−x Fe x O3 compounds with 0 ⩽ x ⩽ 0.1. J. Alloy. Compd. 550, 358–364 (2013)
Gschneidner, K.A., Pecharsky, V.K.: Magnetocaloric materials. Annu. Rev. Mater. Sci. 30, 387 (2000)
Phan, M.H., Peng, H.X., Yu, S.C., Hanh, D.T., Tho, N.D., Chau, N.: Structure, magnetic, magnetocaloric and magnetoresistance properties of Pr1-xPbxMnO3 perovskites. J. Appl. Phys. Q108, 9908 (2006)
Nisha, P., Pillai, S.S., Vacma, M.R., Surech, K.G.: Influence of cobalt on the structural, magnetic and magnetocaloric properties of La0.67Ca0.33MnO3. J. Magn. Magn. Mater. 327, 189–195 (2013)
Nisha, P., Pillai, S.S., Darbandi, A., Varma, M.R., Suresh, K.G., Hahn, H.: Critical behavior and magnetocaloric effect in nano crystalline La0.67Ca0.33Mn1-xFexO3 (x = 0.05, 0.2) synthesized nebulized spray pyrolysis. Mater. Chem. Phys. 136(1), 66–74 (2012)
Kamilov, I.K., Gamzatov, A.G., Aliev, A.M., Batdalov, A.B., Aliverdiev, A.A., Abdulvagidov, ShB, Melnikov, O.V., Gorbenko, OYu., Kaul, A.R.: Magnetocaloric effect in La1−x Ag y MnO3 (y ≤ x): direct and indirect measurements. J. Phys. D 40, 4413 (2007)
Acknowledgments
This study has been supported by the Tunisian Ministry of Scientific Research and Technology and the Neel Institute at Grenoble, France.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Fatnassi, D., Sbissi, K., Hlil, E.K. et al. Magnetic and magnetocaloric properties of nano-sized La0.8Ca0.2Mn1−x Fe x O3 manganites prepared by sol–gel method. J Nanostruct Chem 5, 375–382 (2015). https://doi.org/10.1007/s40097-015-0169-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40097-015-0169-7