Abstract
Purpose
Soil fertility management requires a precise assessment of nutrient release from organic amendments to decide the rates and frequency of application.
Methods
Time-dependent release of some macro- and micro-nutrients from farmyard manure, mushroom compost, poultry manure, vermi-compost, biogas slurry and biochar of Lantana sp. was studied during 120 days incubation through entrapment of released nutrients on ion exchange resins.
Results
Highest total concentration of K, Ca, Mg and S was in farmyard manure, Fe and Mn in mushroom compost, P, Zn and Cu in biogas slurry and B in biochar. Farmyard manure had the highest C:N ratio (34.2), while other organic amendment had C:N ratio < 20. The highest release of P, Ca, Mg, Zn and Cu was recorded from biogas slurry, of K and S from farmyard manure, of B from poultry manure and of Fe and Mn from mushroom compost. Mean percentage of total nutrient released was 30.5% P, 71.8% K, 23.1% Ca, 24.4% Mg, 29.3% S, 47.2% Zn, 22.9% Cu, 38.6% Fe, 46.6% Mn, and 70.9% B. Nutrient release from different organic amendments conformed to zero-order kinetics. The percent of total released nutrient, release kinetic constants and half-life of nutrients was related to the properties of organic amendments.
Conclusions
Based on the released amount of nutrients from different organic amendments, the best source seemed to be farmyard manure for S and K, mushroom compost for Fe and Mn, biogas slurry for P, Ca, Mg, Zn and Cu and poultry manure for B.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Modern agriculture mainly involves the use of chemical fertilizers for supplying essential nutrients to crop plants. However, an excessive use of chemical fertilizers often results in poor soil physical conditions and low microbial activity ultimately degrading soil quality which in turn has deleterious effects on sustenance of soil productivity. The use of organic manures in addition to chemical fertilizers is, therefore, recommended in many developing countries for achieving sustainable food production without endangering soil quality and environment. A sound soil fertility management program requires a precise assessment of nutrient supply from each component of nutrient cycle for developing an efficient integrated nutrient supply system. Mineralization is a process of conversion of unavailable organic forms of nutrient to the plant available inorganic form (Tisdale et al. 1997). Soil organic matter releases substantial quantities of nitrogen, phosphorus, sulfur and little amount of micro-nutrients upon mineralization (Rahman et al. 2013; Amanullah 2007). Mineralization of nutrients from the applied manure depends on the type of soil, soil moisture and temperature regimes, manure characteristics and microbial activity in the soil, etc. (Moorhead et al. 1996). Since the effect of these factors cannot be accurately quantified, the mineralization of nutrients from the organic amendments can only be approximated. To efficiently utilize the macro- and micro-nutrients from organic manures, the mineralization potential of each organic amendment needs to be assessed and considered while deciding their application rates (Eghball et al. 2002).
Manures are known to have residual effect on nutrient supply for two or more crop seasons. Mineralization of N from organic manures has been studied and modeled by several researchers (Pratt et al. 1973; Klausner et al. 1994; Chambers and Richardson 1993; Abbasi et al. 2007). However, very limited information exists about the release of other plant nutrients from different organic amendments. The availability of P, K, S and micro-nutrients from organic manures needs to be assessed for effective utilization of these organic nutrient sources in crop production (Villegas-Pangga et al. 2000). The information is important for farmers to reduce their sole dependence on costly chemical fertilizers and may help them to adopt integrated plant nutrient supply system approach using the most efficient organic manure/amendment for supplying critical nutrient(s) to achieve the sustainability of the production system and also to ensure good soil health. The farmers practicing organic mode of cultivation can suitably opt for a combination of varying doses of different organic amendments to maintain the soil fertility.
In the light of the above-mentioned facts, the present investigation was carried out to study the time-dependent release of some macro- and micro-nutrients from different organic amendments under soil microbial inoculation performed in quartz sand.
Materials and methods
A bulk (10 kg) sample of quartz sand was screened through 0.5 mm sieve and washed several times in tap water to remove dirt. Quartz sand was then soaked into 1 N HNO3 solution for 48 h and repeatedly washed by water, 1 N Ca(NO3)2 solution and finally with deionized water till the pH of effluent water reached around 6–7. After washing, the quartz sand was finally air dried and stored in a plastic bag until use.
Farmyard manure (FYM) and vermi-compost (VC) were collected from Instructional Dairy Farm (IDF) of the university located at Nagla. Mushroom compost (MC) was collected from Mushroom Research and Training Centre, Pantnagar. Poultry manure (PM) was collected from poultry farm, Nagla. Biogas slurry (BS) was collected from Integrated Farming System model farm, N.E. Borlaug Crop Research Centre. Biochar (BC) was prepared by incineration of Lantana camara (a bush weed) at 350 °C under limited O2 supply. All organic amendments were initially sampled in two lots. Each lot was air dried and crushed with pestle and mortar to make it fine and homogeneous and subdivided into two subsamples for chemical analysis. Thus, there were four samples for each organic amendment.
All samples of organic amendments were analyzed for pH, electrical conductivity in 1:10 solid water suspension and for total C and N concentration by CHN analyzer (Elementar model: vario EL cube). All samples of each organic amendments were digested in di-acid (HNO3: HClO4, 9:4 v/v) and analyzed for P by colorimetry following ascorbic acid method, K by flame photometry, Ca and Mg by sodium versenate titrimetry, S by turbidimetry, Zn; Cu; Fe and Mn by atomic absorption spectrophotometry (GBC Avanta M model). These organic amendment samples were also dry ashed at 550 °C in an electric muffle furnace and residues were dissolved in 2 N HCl and then analyzed for B by colorimetry using azomethine H method. All the procedures followed were as per the details described by Page et al. (1982). All samples of these organic amendments were characterized for organic C speciation including water soluble, fulvic acid, humic acid and humin fractions following the procedure outlined by Yagi et al. (2003). The physico-chemical properties and organic C speciation of all organic amendments are presented in Table 1.
Incubation study
Three hundred grams of acid washed quartz sand on oven dry weight basis were treated separately with 2 g each of FYM, MC, PM, VC, BS and BC in duplicate and filled in 12 plastic containers (8 cm diameter × 14 cm length) which were provided with a basal 1 cm glass wool pad over the drainage pores. To assess the time-dependent release of mineralized ionic forms of nutrients from different organic amendments during incubation, a battery of cation (IRC 86, H+ form, 4 milliequivalent) and anion exchange resins (IRA 96, NO3− form, 4 milliequivalent) spread over basal glass wool pad in a separate similar plastic container was fitted at the base of quartz sand filled plastic pot. Montgomery et al. (2005) also assessed P availability in soils amended with different types of biosolids and manures using anion exchange resin membrane test and reported that P uptake by ryegrass (Lolium perenne L.) related well with in situ exchange resin P soil test method. A basal plastic container without any drainage pore was also provided below ion exchange resin container in each unit to collect the drained solution. Treated quartz sand in each plastic container was first moistened at 50% void volume using 5 ml of clear supernatant of freshly prepared soil (0–15 cm sandy loam soil, Typic Haplludol) water suspension (1:2) and requisite volume of distilled water. The initial weight of whole assembly was recorded and used to maintain moisture regime at 50% void volume twice in a week during incubation period. The incubation was done in the laboratory (maximum temperature 26–27.5 °C and minimum temperature 22.0–24 °C, R.H. 40–56%).
Treated quartz sand was subjected to leaching by adding 20 ml distilled water after 10, 20, 30, 50, 70, 90 and 120 days of incubation. The leachate was passed through beds of cation and anion exchange resins. Next day, 12.5 ml of 0.01 M HNO3 was allowed to gradually pass through cation and anion exchange resins twice and the extract was pooled and total volume of the extract was also measured. The extracts from each replication were analyzed in duplicate for the concentration of total P, K, Ca, Mg, S, Zn, Cu, Fe, Mn and B.
The data on physico-chemical properties, organic C speciation and nutrient composition of organic amendments and computed values of kinetic constants were statistically compared using Duncan’s multiple range test, while the data on nutrient release from different organic amendments at different time intervals were statistically analyzed by completely randomized block design under two (days of incubation and organic amendment) factorial arrangement. Statistical analysis of the data, simple correlation and regression analyses were performed using statistical procedure as described by Snedecor and Cochran (1967) with the help of Standard Computer Programs (STPR) software (GBPUAT 2004). The test of significance was conducted at 5 and 1% level of significance (p ≤ 0.05 and p ≤ 0.01).
Results and discussion
The pH of VC, FYM, MC and PM (6.97–7.60) was nearly neutral while that of BS (8.92) and BC (8.29) was slightly alkaline (Table 1). Higher pH value in BS could be ascribed to the formation of ammonium carbonate and removal of CO2 (Mӧller and Müller 2012) during anaerobic decomposition of BS while slightly alkaline pH in BC could be related to the formation of oxides of alkali and alkaline earth metals during the incineration of plant tissues under limited oxygen supply. Higher pH values in BS could promote more solubility of organic matter and formation of complexes/chelates with micro-nutrient cations (Sanders and Bloomfield 1980) while higher pH of BC could adversely affect the solubilization and release of P and micro-nutrient cations but would favor higher solubilization and release of B. Sulliman and Miller (2001) also reported that the pH for most finished composts ranged from 6.0 to 8.0. In a study, Rout et al. (2012) observed that cattle manure (pH 8.6) and poultry manure (pH 8.7) had more alkaline pH than vermi-compost (7.8), which had a pH very close to standard range (6.5–7.5) earlier reported by Manna (2004). Malav et al. (2015) reported that biogas slurry had a pH of 7.9. The variations between the observed pH values of organic amendments in the present investigation and the values reported in the earlier literature could be ascribed to the age of these organic amendments, preparation method and storage conditions. The net effect of organic amendments on the resultant pH of soil amended with them and on the availability of different nutrients was likely to depend on further mineralization of these organic amendments vis-a-vis buffering capacity of soil. Higher alkalinity might result in volatilization of ammonical-N, reduction in availability of P and micro-nutrient cations. However, depending on the nature of organic amendment a higher pH in the organic matrix might also promote more solubilization of native- and added organic matter and more ionization of function groups in organic molecules for the formation of complexes/chelates of micro-nutrient cations to improve their availability besides indirectly promoting the availability of P and B in soil by blocking the potential sites for their irreversible fixation (Stevenson 1991; Srivastava and Gupta 1996). The electrical conductivity (EC), an index of the concentration of soluble salts in these organic amendments, was the highest in FYM (0.553 dS m−1) followed by PM (0.344 dS m−1), BS (0.340 dS m−1), VC (0.231 dS m−1), MC (0.189 dS m−1) and BC (0.0.097 dS m−1). Uz et al. (2016) also observed higher EC value for FYM (4.325 dS m−1) as compared to VC (1.558 dS m−1). Considering the upper threshold value of EC as 4 dS m−1 for a good quality manure (Manna 2004), all these organic amendments were suitable for application.
With regard to the total C concentration in the organic amendments, the highest total C was noted in BC (475.8 g C kg−1), while the lowest value of C was recorded in MC (179.4 g C kg−1). Carbon speciation revealed that the highest water soluble C content was recorded in PM (32.7 g C kg−1) followed by BS (12.5 g C kg−1), while the lowest water soluble C (2.6 g C kg−1) was noted in BC. The differences among FYM, MC, and VC for water soluble C (3.5–9.0 g C kg−1) were statistically not significant. The highest fulvic acid C concentration was recorded in PM (104.3 g C kg−1) followed by BS (90.4 g C kg−1) and FYM (83.4 g C kg−1), while the lowest fulvic acid concentration was found in BC (13.0 g C kg−1); the difference in fulvic acid concentration of MC and VC was statistically not significant. The highest humic acid C concentration (16.7 g C kg−1) was recorded in BS followed by MC (15.3 g C kg−1) and PM (12.5 g C kg−1), while the lowest humic acid concentration was found in BC (1.5 g C kg−1); the difference in humic acid concentration of FYM and VC was statistically not significant. The highest insoluble humin concentration (458.7 g C kg−1) was observed in BC followed by BS (292.0 C kg−1) and VC (209.2 g C kg−1) while the lowest concentration of humin was recorded in PM (76.8 g C kg−1). The highest N concentration was recorded in BC (68.4 g N kg−1), while the lowest value was recorded in FYM (8.1 g N kg−1). The highest C: N ratio was noted in FYM (34.2), while the lowest C: N ratio was recorded in PM (6.3). In their study, Roy and Kashem (2014) also concluded that organic C and total nitrogen contents of chicken manure were higher in comparison to cow dung. The optimum C: N ratio of a good manure has been suggested as < 20:1 (Manna 2004). In the present study, except FYM all other organic amendments had C:N ratio below 20 which indicated the dominance of N mineralization upon their incorporation in the soil. A higher C:N ratio in FYM indicated a slower mineralization activity during decomposition. A lower C/N ratio associated with slightly alkaline pH of BS might result in the volatilization loss of N in the form of ammonia, if it was surface broadcasted on soils (Rout et al. 2012).
Composition of some nutrients in organic amendments
The composition of some important plant nutrients in different organic amendments is shown in Table 2. Among the different organic amendments, FYM had the highest content of Ca (11,457.8 mg kg−1), Mg (5069.1 mg kg−1) and S (3861.2 mg kg−1). Mushroom compost (MC) had the highest content of Fe (1985.5 mg kg−1), Mn (244.9 mg kg−1) and K (5293.4 mg kg−1) but the lowest content of B (14.9 mg kg−1). Poultry manure (PM) had the lowest content of Ca (7433.6 mg kg−1) and Mg (1152.8 mg kg−1), while VC had the lowest content of K (4538.2 mg kg−1) due to leaching of K as a result of regular watering in preparation of VC. Biogas slurry (BS) had the highest content of Zn (193.0 mg kg−1), Cu (145.6 mg kg−1) and P (5832.3 mg kg−1), but the lowest content of S (1254.8 mg kg−1). Biochar (BC) prepared from Lantana weed had the highest content of B (33.4 mg kg−1), but the lowest content of Zn (24.7 mg kg−1), Fe (968.7 mg kg−1), Mn (61.9 mg kg−1), S (1254.8 mg kg−1) and P (2265.3 mg kg−1).
Uz et al. (2016) also observed that FYM had more total K, exchangeable Ca and micro-nutrients like Zn and Cu, while VC had more total N, available P, exchangeable Mg, Fe and Mn as compared to FYM. In a study, Rout et al. (2012) arranged the organic manures in the following order: poultry manure > cattle manure > vermi-compost > goat manure for total N concentration, poultry manure > goat manure > vermi-compost > cattle manure for total P concentration and goat manure > poultry manure > vermi-compost for total K concentration. The variations between the nutrient composition of different organic amendments observed in the present study and those reported by other workers (Rout et al. 2012; Uz et al. 2016) could be ascribed to differences in their origin and production conditions.
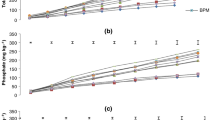
Periodic release of some macro-nutrients from organic amendments
The data on periodic release of some macro-nutrients from different organic amendments during incubation and percent of total macro-nutrient released at the end of incubation are depicted in Fig. 1 . On an average, there was a consistent increase in P release from organic amendments throughout the incubation period. Different organic amendments could be arranged in the following decreasing order of total release of P: BS = PM > FYM = VC > MC > BC. Islas-Espinoza et al. (2014) also observed lesser release of P in vermi-compost as compared to wastewater biosolids. The percent of total P in organic amendments released during incubation period varied between 23.6 (BC) and 36.1% (FYM). A significant interaction effect of incubation time and organic amendment revealed that there was a significant and consistent increase in released P content during the incubation period from all organic amendments except in BC in which a statistically significant increase was recorded after 30 days of incubation possibly due to the lowest P concentration in it. As compared to other macro-nutrients, relatively smaller magnitude of percent release of total P could be related to the formation of some stable Ca bound compounds of P (Li et al. 2014) in these organic amendments.
Release of some macro-nutrients at different time intervals from different organic amendments and percent release of total nutrient concentration in organic amendments. The vertical bars are critical differences at p ≤ 0.05. Dissimilar letter(s) over the histograms indicate statistically significant difference at p ≤ 0.05
In the case of K, there was a regular average increase from organic amendments during incubation. Different organic amendments could be arranged in the following decreasing order of total release of K: FYM = BC > BS > PM ≫ MC > VC. The percent of total K in organic amendments released during 120 days period varied between 52.8 (VC) and 82.3% (BC). A significant interaction effect of incubation time and organic amendment revealed that there was a significant and consistent increase in released K during the incubation period from all organic amendments except in FYM in which the successive increase noted between 20 and 30 days of incubation was statistically not significant. Ragheb et al. (2017) reported peak release of P at 45 days and of K at 60 days from organic wastes. The variations observed in the present study as compared to those reported in earlier work could be ascribed to the differences in experimental conditions.
A consistent average increase in Ca release occurred from organic amendments only after 30 days of incubation. Different organic amendments could be arranged in the following decreasing order of total release of Ca: BS > MC = VC = FYM = PM > BC. The percent of total Ca in organic amendments released during incubation period varied between 9.6 (BC) and 40.9% (BS). A significant interaction effect of incubation time and organic amendment revealed that there was a significant and consistent increase in released Ca content from BS only after 30 days of incubation. Similar trend was also recorded in the case of MC and FYM; however, in both cases a statistically significant increase was recorded up to 90 and 50 days of incubation, respectively. Though the highest concentration of Ca was noted in FYM, yet the highest release of Ca under BS could be ascribed to the acidogenesis during the decomposition of BS (Schnurer and Jarvis 2010) which might affect higher release of Ca from BS.
With regard to Mg, there was a regular average increase from organic amendments throughout the incubation period. Different organic amendments could be arranged in the following decreasing order of total release of Mg: BS > VC = FYM = MC = BC > PM. The percent of total Mg in organic amendments released during incubation period varied between 16.0 (MC) and 48.9% (PM). A significant interaction effect of incubation time and organic amendment revealed that there was a significant and consistent increase in released Mg content during the incubation period from BS only and from rest of the organic amendments a consistent significant increase was recorded only after 30 days of incubation. Relatively higher release of Mg from BS and PM could be ascribed to the same reason as mentioned in the case of Ca (Table 1).
The findings of the present investigation were partly in corroboration with those of Kolahchi and Jalali (2012), who examined the percent release of some macro-nutrients from sheep manure, beet, waste material, rape, poultry manure, sunflower, potato and garlic and reported that the average percentage of nutrients released was in the order K > P > Ca > Mg.
In the case of S, there was a consistent average increase from all organic amendments during incubation. Different organic amendments could be arranged in the following decreasing order of total release of S: FYM >=MC = PM > BS = VC > BC. The highest release of S under FYM could be ascribed to the highest concentration of S in FYM (Table 2). The percent of total S in organic amendments released during incubation period varied between 14.4 (VC) and 39.2% (BS) percent. Higher percent release of total S under BS or PM could be ascribed to the presence of higher amounts of water soluble- and fulvic acid organosulfur compounds in these organic amendments which could be subjected to early mineralization due to their lower molecular weights (Qualls 2004). A significant interaction effect of incubation time and organic amendment revealed that there was a consistent significant increase in released S content during the incubation period from FYM only and under MC and PM a significant increase in S release was only recorded in the beginning possibly due to an early mineralization of organic S in materials of lower C:N ratio (6.3–9.2) (Barrow 1960).
Periodic release of some micro-nutrients from organic amendments
The periodic releases of some micro-nutrients from different organic amendments during incubation and percent of total micro-nutrients released at the end of incubation are depicted in Fig. 2.
Release of some micro-nutrients at different time intervals from different organic amendments and percent release of total nutrient concentration in organic amendments. The vertical bars are critical differences at p ≤ 0.05. Dissimilar letter(s) over the histograms indicate statistically significant difference at p ≤ 0.05
With regard to Zn, there was a consistent average increase from organic amendments from 20 days of incubation time onward. Different organic amendments could be arranged in the following decreasing order of total release of Zn: BS > PM = VC = FYM > MC > BC. Higher release observed under BS and PM could be related to their higher content of water soluble (low molecular weight organic acids)- and fulvic acid-C as compared to other organic amendments (Hattab et al. 2014). The percent of total Zn in organic amendments released during incubation period varied between 38.2 (PM) and 62.3% (BC). Higher percent release of total Zn under BC possibly indicated lesser irreversible incorporation of Zn in humic acid- and humin fractions of C in BC. A significant interaction effect of incubation time and organic amendment revealed that there was a consistent significant increase in released Zn content after 20 days of incubation period from BS only due to the formation of low-molecular weight organic acid during the course of decomposition of biogas sludge (Kalle and Menon 1984). In rest of the organic amendments except BC, a consistent significant increase was recorded only after 30 days of incubation. In the case of BC, the successive increase in release of Zn at different time intervals was found to be statistically not significant.
In the case of Cu, there was a consistent average release from organic amendments from 30 days of incubation time onward. Different organic amendments could be arranged in the following decreasing order of total release of Cu: BS > PM > FYM = BC = VC = MC. Higher release of Cu under BS and PM could be attributed to the same reason as mentioned for Zn. Motaghian et al. (2016) also observed that the manure released higher content of Cu than VC and the amount of released Cu in soil amended with 0.5 and 1% manure or VC was 2.92, 2.72, 2.41, and 2.40 mg kg−1, respectively. The percent of total Cu in organic amendments released during incubation period varied between 8.6 (BS) and 49.9% (PM). A significant interaction effect of incubation time and organic amendment revealed that there was a consistent significant increase in release of Cu after 70 days of incubation period from FYM, MC and VC and in case of PM and BC a significant increase in Cu release was recorded after 50 days of incubation. In the case of BS, a significant successive increase in Cu release occurred earlier, i.e., 30 days of incubation. These variations could be attributed to the formation of stable complexes of Cu with C fractions present in different organic amendments (Stevenson 1991).
There was a consistent average increase in Fe release from organic amendments only after 50 days of incubation. Delayed consistent release of Fe from organic amendments could be ascribed to the formation of organic complexes of higher stability with Fe (Barber 2014) which were likely to be decomposed at slower rate. Different organic amendments did not statistically differ in total release of Fe. The percent of total Fe in organic amendments released during incubation period varied between 26.3 (FYM) and 47.5% (BC) and a higher release of Fe under BC could be attributed to the presence of some potential reducing agents like quinine and condensed aromatic structures in this organic amendment (Klüpfel et al. 2014). The interaction effect of incubation time and organic amendment had no statistically significant effect on release of Fe.
In the case of Mn, there was a consistent average release from organic amendments from 20 days of incubation time onward. Different organic amendments could be arranged in the following decreasing order of total release of Mn: MC > VC = BS > PM = FYM > BC. The highest release of Mn from MC could be attributed to the highest content of Mn in this organic amendment (Table 2). The percent of total Mn in organic amendments released during incubation period varied between 41.9 (MC) and 51.2% (BC). The release of a higher proportion of total Mn from BC could be attributed to the same reason as mentioned in the case of Fe. A significant interaction effect of incubation time and organic amendment revealed that there was a significant and consistent increase in released Mn content after 20 days of incubation period from MC, VC and BS, while in case of FYM and PM a significant increase in Mn release was delayed and recorded after 30 days of incubation. Early release of Mn from MC and VC indicated that in this organic amendment Mn possibly existed in easily displaceable form while early release of Mn from BS despite its alkaline pH could be attributed to the higher concentration of water soluble- and fulvic acid-C in it (Table 1) which could form soluble complexes with Mn. In BC, a significant successive release of Mn was most delayed and occurred only after 70 days of incubation and further increase registered at 120 days of incubation was statistically not significant. This behavior of BC could be related to its alkaline pH (8.29) and the lowest content of water soluble- and fulvic acid-C in this organic amendment.
In the case of B, there was a consistent average release from all organic amendments during incubation period. Different organic amendments could be arranged in the following decreasing order of total release of B: PM = BC > FYM = BS > MC = VC. The higher release of B from PM and BC could be ascribed to a higher concentration of B in them as compared to other organic amendments. The percent of total B in organic amendments released during incubation period varied between 56.6 (BC) and 97.7% (PM). Despite the highest concentration of B in BC, relatively a lower percent release of B from BC indicated possibly the formation of some insoluble B compounds in BC. A significant interaction effect of incubation time and organic amendment revealed that there was a significant and consistent increase in B release from PM and BC only throughout the incubation period. Under MC treatment, a significant increase in released B was recorded after 10 days of incubation only and further significant increase in B release was recorded after 50 days of incubation. In the case of FYM, VC and BS a consistent significant increase in released B content was recorded after 30 days of incubation only possibly due to slower mineralization rate.
Order of nutrient release kinetics
The data on nutrient release from different organic amendments at different time intervals were fitted to the following kinetic equations:
where q0 and qt were the amount of nutrient present in different organic amendments (mg kg−1) initially and at time (t, in days), respectively. The notations k0, k1 and k2 were the release kinetic constants of nutrients from different organic amendments as per zero-, first- and second-order kinetics, respectively. The purpose of the kinetic analysis of the nutrient release data was to know whether the release of different nutrients from organic amendments under study was independent of their initial concentration (zero order) or it was a concentration-dependent process where a constant proportion of nutrient in question was mineralized (first order) or the release of a nutrient in question was dependent on the square of its own concentration or on the concentration of the nutrient in question and of some other nutrient also (second order).
Based on the computed values of coefficient of determination (R2) and standard error of estimate (SE) for different organic amendments, it was noted that the release of all nutrients could be satisfactorily accounted (R2 values varied from 0.896 to 0.997, all significant at p ≤ 0.01) by the zero-order kinetic equation. Earlier study by Ghafoor et al. (2011) also showed that both zero- and first-order equations adequately described K release kinetics. In the present investigation, it was noted that only release of Zn from VC and BC and that of Mg from FYM conformed slightly better to first-order kinetics while the release of S from all organic amendments conformed better to a second-order kinetics (data not presented here). A better fit of the observed data on release of S to second-order kinetic equation could be attributed to the possibility that the release of S might depend on the concentration of S as well that of N in organic amendment. However, considering a satisfactory validity of zero-order kinetics for the release of all investigated macro- and micro-nutrients from organic amendments, the computed values of the constants for only zero-order kinetics and half-life (t1/2, calculated as q0/2k0 in days) are presented in Tables 3 and 4, respectively, for rational comparisons.
The estimated initial value (qo) for P showed that organic amendments could be arranged in the following order of decreasing abundance: BS > PM > VC ≫ FYM > MC > BC (Table 3). The highest value of k0 for P was recorded in BS (15.88 mg kg−1 day−1) in comparison to PM (14.95 mg kg−1 day−1). The values observed for FYM (13.03 mg kg−1 day−1) and VC (12.56 mg kg−1 day−1) did not significantly differ from each other but were significantly higher than the value observed for MC. Significantly lowest k0 value of P was observed for BC (4.84 mg kg−1 day−1). The t1/2 values of P in organic amendments varied from 167 (FYM) to 234 (BC) days.
The estimated initial value (qo) for K showed that organic amendments could be arranged in the following order of decreasing abundance: FYM = MC = BC = PM = BS > VC. The highest value of k0 for K was recorded in BC (37.33 mg kg−1 day−1) which was statistically at par with the values observed for FYM, BS and PM. The value observed for MC (29.39 mg kg−1 day−1) was significantly higher than that of VC (20.88 mg kg−1 day−1). The t1/2 values of K in organic amendments varied from 68 (BS) to 109 (VC) days.
The estimated initial value (qo) for Ca showed that organic amendments could be arranged in the following order of decreasing abundance: FYM > MC = BS > BC > VC > PM. The highest value of k0 for Ca was recorded in BS (39.06 mg kg−1 day−1) followed by MC (25.82 mg kg−1 day−1). The values of k0 for MC and VC were statistically at par with each other. The lowest value of k0 was observed for BC (7.94 mg kg−1 day−1). The k0 value observed for FYM was at par with that of PM. The t1/2 values of Ca in organic amendments varied from 131 (BS) to 590 (BC) days.
The estimated initial value (qo) for Mg showed that organic amendments could be arranged in the following order of decreasing abundance: MC = FYM > BS = VC > BC > PM. The highest value of k0 for Mg was recorded in BS (10.83 mg kg−1 day−1) followed by VC (7.52 mg kg−1 day−1). The lowest value of k0 was observed for PM (4.82 mg kg−1 day−1). The values of k0 for Mg from VC, MC, FYM and BC were statistically at par. The t1/2 values of Mg in organic amendments varied from 120 (PM) to 373 (FYM) days.
The estimated initial value (qo) for S showed that organic amendments could be arranged in the following order of decreasing abundance: FYM > VC > MC > PM > BC = BS. The highest value of k0 for S was recorded in FYM (8.79 mg kg−1 day−1) which was significantly higher over the values observed for MC and PM, followed by VC (3.37 mg kg−1 day−1). The lowest value of k0 was observed for BC (2.87 mg kg−1 day−1). The values of k0 for S among BS, VC, BC were statistically at par and similarly k0 values for MC and PM were also statistically at par with each other. The t1/2 values of S in organic amendments varied from 158 (BS) to 455 (VC) days.
The estimated initial value (qo) for Zn showed that organic amendments could be arranged in the following order of decreasing abundance: BS > PM > VC ≫ FYM > MC > BC (Table 4). The highest value of k0 for Zn was recorded in BS (0.98 mg kg−1 day−1) in comparison to PM (0.61 mg kg−1 day−1), VC (0.56 mg kg−1 day−1) and FYM (0.51 mg kg−1 day−1). The k0 values of PM, VC and FYM did not differ significantly among them. Relatively lower k0 value was observed in MC (0.44 mg kg−1day−1) but significantly lowest value was recorded for BC (0.13 mg kg−1 day−1). The t1/2 values of Zn in organic amendments varied from 92 (BC) to 148 (PM) days.
The estimated initial value (qo) for Cu showed that organic amendments could be arranged in the following order of decreasing abundance: BS > BC = FYM = MC > VC > PM. The highest value of k0 for Cu was recorded in BS (0.11 mg kg−1 day−1) in comparison to PM (0.07 mg kg−1 day−1), while VC, BC and FYM had significantly lower rate constant (0.04 mg kg−1 day−1). A significantly lowest k0 for Cu was recorded in MC (0.03 mg kg−1 day−1). The t1/2 values of Cu in organic amendments varied from 118 (PM) to 636 (BS) days.
The estimated initial value (qo) for Fe showed that organic amendments could be arranged in the following order of decreasing abundance: MC > VC > BS = PM = FYM > BC. The highest value of k0 for Fe was recorded in MC (6.11 mg kg−1 day−1) followed by PM (4.99 mg kg−1 day−1) and BS (4.76 mg kg−1 day−1), while VC and BC had significantly lower rate constant (4.04–4.10 mg kg−1 day−1). A significantly lowest k0 for Fe was recorded in FYM (2.64 mg kg−1 day−1). The t1/2 values of Fe in organic amendments varied from 119 (PM) to 214 (FYM) days.
The estimated initial value (qo) for Mn showed that organic amendments could be arranged in the following order of decreasing abundance: MC > VC > BS > FYM = PM > BC. The highest value of k0 for Mn was recorded in MC (0.94 mg kg−1 day−1) which was significantly higher than those for PM and FYM, while k0 values for VC and BS were statistically similar to that of MC. The trend could be related to Mn concentration in different organic amendments. A significantly lowest k0 for Mn was recorded in BC (0.30 mg kg−1 day−1). The t1/2 values of Mn in organic amendments varied from 104 (BC) to 130 (MC) days.
The estimated initial value (qo) for B showed that organic amendments could be arranged in the following order of decreasing abundance: BC > PM > BS = FYM > VC = MC. The highest value of k0 for B was recorded in BC (0.16 mg kg−1 day−1) which was at par with the value observed for PM, while BS and FYM had significantly lower k0 value. Like Mn, the trend in k0 could be related to total B concentration in these organic amendments. Significantly lower k0 values were recorded in MC (0.07 mg kg−1 day−1) and VC (0.08 mg kg−1 day−1). The t1/2 values of B in organic amendments varied from 63 (PM) to 107 (VC) days.
In general, the values of t1/2 were less than 120 days for K and B from all organic amendments, for Zn and Mn from BS and BC and for Mn, Cu and Fe from PM. The values of t1/2 were in between 120 and 240 days for P from organic amendments, for S from all organic amendments except VC, for Zn from FYM; MC; PM and VC, for Fe from FYM, MC, VC, BS and BC, for Mn from FYM and MC, for Ca from MC, VC and BS and for Mg from PM only. The values of t1/2 were in between 240 and 360 days for the release of Cu from FYM, VC and BC, for Ca from FYM and PM indicating their plausible residual effect in third crop season. The values of t1/2 were in between 360 and 480 days for the release of Cu from MC, Mg from FYM and MC and of S from VC. The values of t1/2 were in between 480 and 600 days for the release of Cu from BC, while t1/2 of Cu from BS was 636 days. Thus, the residual effect of these organic amendments could be expected for varying length of time/season and help in regulating the frequency of their use as potential sources in agriculture.
Relationships of total concentration, release constant, half-life of nutrients and released nutrients as percent of total nutrient concentration with properties of different organic amendments
Macro-nutrients
Simple correlation coefficients (r) computed among total concentration, k0, t1/2 values for macro-nutrients and released macro-nutrients as percent of total macro-nutrient concentration with some properties of different organic manures are presented in Table 5. It was noted that the total concentration of P in organic amendments was significantly and positively correlated to fulvic and humic acid concentrations present in organic amendment. The k0 for P was also significantly and linearly related to fulvic acid concentration and total P concentration in organic amendments. The t1/2 for P was linearly related to total concentration of N but released P as percent of total P in organic amendments was inversely related to the total N concentration possibly due to the association of P with N in some organic compounds resistant to further decomposition. The t1/2 for P was inversely related to electrical conductivity of organic amendments showing that a lower electrical conductance of organic amendment possibly indicated lesser mineralization activity in the organic amendment. Total concentration, k0, t1/2 for K and released K as percent of total K were not significantly related to any properties of organic amendments as K is likely to be easily released from the organic matrices. Total concentration of Ca was not related to any property of organic amendment but k0 for Ca and released Ca as percent of total Ca present in organic amendments was significantly and positively correlated to humic acid concentration. The t1/2 for Ca was significantly and inversely related to humic acid concentration in organic amendments. This clearly showed that Ca present as humate in organic amendments was liable to ultimate release. Both total Mg concentration and t1/2 for Mg were significantly and positively correlated with each other; however, both these parameters had a significant and negative correlation with water-soluble C concentration of organic amendments while released Mg as percent of total Mg in organic amendments was significantly and positively correlated to the concentration of water soluble C in organic amendments. This indicated that water-soluble organic compound could readily release Mg by complexation during decomposition. Total S concentration in organic amendments was significantly and negatively correlated with total N concentration and pH of organic amendments. Neither the t1/2 nor the released S as percent of total S present in organic amendments showed any significant correlation with properties of organic amendments.
Micro-nutrients
Simple correlation coefficients (r) computed among total concentration, k0, t1/2 and released micro-nutrients as percent of total micro-nutrient concentration with some properties of different organic manures are presented in Table 6. Total concentration of Zn in organic amendments was significantly and positively correlated to fulvic acid concentration present in organic amendment. The k0 for Zn was also significantly and linearly related to total Zn concentration in organic amendments. The t1/2 for Zn was inversely related to humin and total C concentration and pH of organic amendments; however, released Zn as percent of total Zn in organic amendments was significantly and positively correlated to the concentrations of both humin and total C in organic amendments. This indicated that possibly Zn existing in combination with humin in these organic amendments was liable to decomposition under basic conditions. Though total Cu concentration in organic amendments was not significantly correlated to any of the monitored property of organic amendments yet, both k0 and t1/2 for Cu were significantly and positively correlated to total Cu concentration in organic amendments. Released Cu as percent of total Cu concentration in organic amendments showed a significant and positive correlation with the concentration of water-soluble organic C in organic amendments as water-soluble organic C compounds such as organic acids form soluble complexes with Cu (Barber 2014). No significant correlation was recorded between total Fe concentration, k0 or t1/2 for Fe in organic amendments and monitored properties of organic amendments; however, released Fe as percent of total Fe in organic amendments showed a significant and positive correlation with total N concentration present in organic amendments. This possibly indicated that organic amendments with higher N content which could sustain faster decomposition rate might also release higher amounts of Fe as compared to organic amendments of lower N concentration. Total Mn in organic amendments was significantly and positively correlated to humic acid concentration present in organic amendment. The k0 for Mn was also significantly and linearly related to total Mn and humic acid-C concentrations in organic amendments, while t1/2 for Mn in organic amendments was significantly and positively correlated to total Mn concentration but inversely related to total C and N concentrations in organic amendments. Released Mn as percent of total Mn in organic amendments was significantly and inversely related to total Mn concentration. This indicated that possibly humic acid bound Mn in organic amendments was liable to ready decomposition and high N content in organic amendments might help an early release of Mn. Total concentration of B in organic amendments was significantly and positively correlated to total N concentration in organic amendment. The k0 for B was not significantly related to any property of organic amendments, while t1/2 for B was significantly and inversely correlated to water-soluble and fulvic acid-C concentration in organic amendments. Released B as percent of total B in organic amendments showed a significant and positive correlation with the concentrations of water soluble- and fulvic acid-C in organic amendments; this clearly indicated that readily mineralizable B fraction in organic amendments possibly existed in association of water-soluble and fulvic acid-C compounds.
Thus, it appeared that both macro-and micro-nutrients in organic amendments were readily subjected to mineralization for ultimate release for subsequent plant uptake or chemical transformations, if these organic amendments were rich in water-soluble, fulvic acid- or humic acid-C.
Conclusions
The study revealed that different organic amendments vary in their nutrient composition and the rate of nutrient release from these organic amendments could be accounted well by the zero-order kinetics. The release of nutrients from organic amendments depends on the nature of nutrient and also the amount of different species of C in these organic amendments. Based on the released amount of nutrients from different organic amendments, the best source seemed to be farmyard manure for S and K, mushroom compost for Fe and Mn, biogas slurry for P, Ca, Mg, Zn and Cu and poultry manure for B. Thus, according to the need of the farm the most suitable source or a combination of organic amendments could be opted to maintain soil fertility.
References
Abbasi MK, Hina M, Khalique A, Khan SR (2007) Mineralization of three organic manures used as nitrogen source in a soil incubated under laboratory conditions. Commun Soil Sci Plant Anal 38:1691–1711. https://doi.org/10.1080/00103620701435464
Amanullah MM (2007) N release pattern in poultry manured soil. J Appl Sci Res 3:1094–1096
Barber SA (2014) Soil nutrient bioavailability, Ist edn. Wiley, New Delhi
Barrow NJ (1960) A comparison of the mineralization of nitrogen and of sulfur from decomposing organic materials. Aust J Agric Res 11:960–969. https://doi.org/10.1071/AR9600960
Chambers BJ, Richardson SJ (1993) Relying on soil mineral reserves. J Sci Food Agric 63:128–131
Eghball B, Wienhold BJ, Gilley JE, Eigenberg RA (2002) Mineralization of manure nutrients. J Soil Water Conserv 57:470–473. http://digitalcommons.unl.edu/biosysengfacpub/139. Accessed 6 May 2018
Ghafoor A, Mohamad MR, Al-Obaidi AJ (2011) Kinetics of potassium desorption from entisol, verisol and mollisol using miscible displacement technique in Sulamani governorate. Mesopotamia J Agric 39:32–40
Govind Ballabh Pant University of Agriculture and Technology (2004) Standard computer programs. Department of Mathematics, Statistics and Computer Science, College of Basic Sciences and Humanities, Pantnagar
Hattab N, Soubrand-Colin M, Guégan R, Motelica-Heino M, Bourrat X (2014) Effect of organic amendments on the mobility of trace elements in phytoremediated techno-soils: role of the humic substances. Environ Sci Pollut Res 21:10470–10480. https://doi.org/10.1007/s11356-014-2959-8
Islas-Espinoza M, Solıs-Mejıa L, Esteller MV (2014) Phosphorus release kinetics in a soil amended with biosolids and vermicompost. Environ Earth Sci 71:1441–1451. https://doi.org/10.1007/s12665-013-2549-y
Kalle GP, Menon KKG (1984) Inhibition of methanogenesis and its reversal during biogas formation from cattle manure. J Biosci 6:315–324. https://doi.org/10.1007/BF02716746
Klausner SD, Kanneganti VR, Bouldin DR (1994) An approach for estimating a decay series for organic nitrogen in animal manures. Agron J 86:897–903. https://doi.org/10.2134/agronj1994.00021962008600050026x
Klüpfel L, Keiluweit M, Kleber M, Sander M (2014) Redox properties of plant biomass-derived black carbon (biochar). Environ Sci Technol 48:5601–5611. https://doi.org/10.1021/es500906d
Kolahchi Z, Jalali M (2012) Kinetics of nutrient release from different organic residues using a laboratory system. Arch Agron Soil Sci 58:1013–1031. https://doi.org/10.1080/03650340.2011.559227
Li G, Li H, Leffelaar PA, Shen J, Zhang F (2014) Characterization of phosphorus in animal manures collected from three (Dairy, Swine, and Broiler) farms in China. PLoS One 9:e102698
Malav LC, Khan SA, Gupta N (2015) Impacts of biogas slurry application on soil environment, yield and nutritional quality of baby corn. Int J Plant Res 28:194–202. https://doi.org/10.5958/2229-4473.2015.00055.5
Manna MC (2004) Evaluation of compost maturity, stability and quality. Short course on Training compendium on assessment of compost quality for agricultural crop production. Indian Institute of Soil Science, Bhopal, pp 20–35
Montgomery MB, Ohno T, Griffin TS, Honeycutt CW, Fernanadez IJ (2005) Phosphorus mineralization and availability in Soil amended with biosolids and animal manures. Biol Agric Hort 22:321–334. https://doi.org/10.1080/01448765.2005.9755295
Moorhead DL, Sinsabaugh RL, Linkins AE, Reynolds JF (1996) Decomposition processes: modeling approaches and applications. Sci Total Environ 183:137–149. https://doi.org/10.1016/0048-9697(95)04974-6
Motaghian HR, Hosseinpur A, Kiani S (2016) Zinc and copper release kinetics in a calcareous soil amended with manure and vermicompost. J Water Soil 30:581–593. http://jsw.um.ac.ir/…/42565. Accessed 8 May 2018
Mӧller K, Müller Y (2012) Effects of anaerobic digestion and digestate nutrient availability and crop growth: a review. Eng Life Sci 12:242–257. https://doi.org/10.1002/elsc.201100085
Page AL, Miller RH, Keeney DR (eds) (1982) Methods of soil analysis. Part 1 and 2. ASA Inc. & SSSA Inc., Madison
Pratt PF, Broadbent FE, Martin JP (1973) Using organic wastes as nitrogen fertilizers. Calif Agric 27:10–13
Qualls RG (2004) Biodegradability of humic substances and other fractions in decomposing leaf litter. Soil Sci Soc Am J 65:1704–1712. https://doi.org/10.2136/sssaj2004.1705
Ragheb HMA, Gomah HH, Basha AAAB, Bakr AAA (2017) Kinetics of N, P and K release and CO2 evolution in organic wastes treated sandy soils. Egypt J Soil Sci 57:125–136. https://doi.org/10.21608/ejss.2017.3611
Rahman MH, Islam MR, Jahiruddin M, Puteh AB, Mondal MMA (2013) Influence of organic matter on nitrogen mineralization pattern in soils under different moisture regimes. Int J Agric Biol 15:55–61
Rout KK, Sahoo S, Mukhi SK, Mohanty GP (2012) Assessment of quality of different organic manures used by the farmers of Khurda district in Orissa and their effect on microbial activity of an acid soil. J Indian Soc Soil Sci 60: 30–37. http://www.isss-india.org. Accessed 5 Apr 2018 (Record Number: 20123388062)
Roy S, Kashem MA (2014) Effects of organic manures in changes of some soil properties at different incubation periods. Open J Soil Sci 4:81–86. https://doi.org/10.4236/ojss.2014.43011
Sanders JR, Bloomfield C (1980) The influence of pH, ionic strength, and reactant concentration on copper complexing by humified organic matter. J Soil Sci 31:53–63. https://doi.org/10.1111/j.1365-2389.1980.tb02064.x
Schnurer A, Jarvis A (2010) Microbiological handbook for biogas plants. Swedish Waste Management U2009:03. Swedish Gas Centre Report 207:1–74
Snedecor GW, Cochran WG (1967) Statistical methods, 6th edn. Oxford and IBH Publishing Corp, Kolkata
Srivastava PC, Gupta UC (1996) Trace elements in crop production. Science Publishers, New Hampshire
Stevenson FJ (1991) Organic matter-micronutrient reactions in soil. In: Mortvedt JJ, Cox FR, Shuman LM, Welch RM (eds) Micronutrients in agriculture. Soil Science Society of America Inc, Wisconsin, pp 145–186
Sulliman DM, Miller RO (2001) Compost quality attributes, measurements and variability. In: Stofella PJ, Kahn BA (eds) Compost utilization in horticultural cropping systems. Lewis Publishers, Washington, pp 95–119. https://doi.org/10.1201/9781420026221.ch4
Tisdale SL, Nelson WL, Beaton JD, Havlin JL (1997) Soil fertility and fertilizer, 5th edn. Prentice hall, New Delhi
Uz I, Sonmez S, Tavali IE, Citak S, Uras DS, Citak S (2016) Effect of vermicompost on chemical and biological properties of an alkaline soil with high lime content during celery (Apium graveolens L. var. dulce Mill.) production. Not Bot Hortic Agrobo 44: 280–290. https://doi.org/10.15835/nbha44110157
Villegas-Pangga G, Blair G, Lefroy R (2000) Measurement of decomposition and associated nutrient release from straw (Oryza sativa L.) of different rice varieties using a perfusion system. Plant Soil 223:1–11. https://doi.org/10.1023/A:1004777911428
Yagi R, Ferreira ME, da Cruz MCP, Barbosa JC (2003) Organic matter fractions and soil fertility under the influence of liming, vermin-compost and cattle manure. Scientia Agricola 60:549–557. https://doi.org/10.1590/S0103-90162003000300021
Acknowledgements
The authors are grateful to Indian Council of Agricultural Research, New Delhi, for supporting the research work under All India Coordinated Research Scheme on Micro- and Secondary-Nutrients and Pollutant Elements in Soil and Plants.
Author information
Authors and Affiliations
Contributions
The work is part of the M.Sc. thesis of AD who performed laboratory experiment and drafted a part of the manuscript. PCS designed the experiment, supervised the work and participated in the chemical analysis of micro-nutrients in samples and compilation of the manuscript. SPP supervised the laboratory analysis of macro-nutrients in samples. AKS helped in statistical analysis and corrections of the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Dey, A., Srivastava, P.C., Pachauri, S.P. et al. Time-dependent release of some plant nutrients from different organic amendments in a laboratory study. Int J Recycl Org Waste Agricult 8 (Suppl 1), 173–188 (2019). https://doi.org/10.1007/s40093-019-0287-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40093-019-0287-1