Abstract
Purpose
This research aimed to produce biochar from coffee residues (CR) as well as to develop the guidelines for using it as a fuel and a soil amendment.
Methods
Initial properties of coffee residues have been characterized before experimenting with a biomass kiln, designed for use in biochar production. By carbonization method, using biomass kiln at the temperature of 350–550 °C with 45 min of the reaction time.
Results
It was found that the biochar made from coffee residue (BCR) possessed a high heating value and fixed carbon (7360 cal/g and 77.77%) whereas it produced such a low amount of ash, moisture and volatile matter (6.18, 4.16 and 11.89%, respectively). The atomic ratio of H/C (0.71) and O/C (0.28) ratios of BCR were shown that were in the range of coal. Moreover, BCR showed adsorption of iodine (612.28). According to the analysis of the chemical properties of BCR, its pH, EC, Nitrogen, Phosphorus, Potassium, OM and C/N ratio were 9.9, 1.66 dS/m, 3.1, 1, 1.7, 19.5 and 28.9%, respectively.
Conclusions
From the results of this study, it can be concluded that BCR could be potentially used not only as an efficient source of the fuel production but also suitable material for soil amendment due to its direct contribution in nutrient absorption along with retardation of nutrient leaching, thereby helping improve the efficacy of nutrient use and increase crop yields.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the USDA, as of June 2017, (United States Department of Agriculture, June 2017), World coffee production for 2017/18 is estimated at 159 million bags (60 kg each), unchanged from the previous year, as the lower output in Brazil is offset by the higher productions in Vietnam, Mexico, and Indonesia as well as slight increases in most other countries (see Table 1).With global consumption of at a record 158 million bags, there has been an upward trend in its global demand over that period (USDA 2017).
There were large amounts of coffee residues produced from the manufacturing of coffee drinks and instant coffee preparation in the coffee industry worldwide over 6 million tons per year (Tokimoto et al. 2005). The coffee residues have been able to be used for various purposes instead of throwing them as a waste. Coffee residues can be used as manure, insect repellents, exfoliating scrubs, dyes, deodorizers and so forth. Landfill is the most common method for discarding coffee waste, due to mixed municipal waste, resulting in higher strict storage criteria for its high stability and limited biodegradation (Kondamudi et al. 2008). Coffee residues contain several organic compounds such as fatty acids, lignin, cellulose, hemicellulose and other polysaccharides that justify its volatilization (Chinmai et al. 2014). Some researchers had scrutinized the benefit of coffee residues as a biological resource for various valuable compounds. For example, coffee residues were applied as precursors of biomass for the production of activated carbon providing for biodiesel industrial, as well as a source of sugars in the paint removal from the water. There is also study that addressed the use of biochar from coffee residues to help increase pH and absorb heavy metals in water and soil (Kim et al. 2014). Moreover, in terms of chemical and biological assessments,. Coffee residues along with biochar improved the quality of heavy metal-contaminated water and soil. It was indicated that biochar added to compost is advantageous as it helped in reducing ammonia emissions, increasing carbon capture moisture of the substance, and decreasing water-soluble C owing to an increase in the degradation rate and the sorption of labile compounds in the biomass (Sanchez-Monedero 2016; Jindo et al. 2012; Khan et al. 2016). Biochar was initially linked to the exploration and the archeological study of early human settlement and soils. Furthermore, biochar has the potential in improving soil quality and carbon sequestration in the soil. Both charcoal and biochar were products from the pyrolysis process, which is crucially heating of biomass in the near or complete absence of oxygen (Jeff 2010). Pyrolysis of biomass contributed to the charcoal, oil and gas production. The amount of these materials depended on the conditions used in this process; then the oil and gas derived from the pyrolysis process can be further used for energy production. Actually, biochar could be produced from a variety of biomass materials; however, in general we called biochar production only when it was defined as a co-product that could be used for soil improvement. According to various reviews of the studies related to biochar and coffee grounds, we see the following guidelines for handling coffee residues: The aims of this study were to find out the physicochemical properties to reveal an effective way to produce biochar from coffee residue and to determine physical properties, chemical properties as well as to analyse biochar for utilization as a fuel and a soil amendment These results will be able to be used to determine the correct approach for producing biochar from coffee residue (BCR) in the future.
Materials and methods
Coffee residue collection, processing and characterization
Coffee residue was collected from Starbucks coffee shop inside a campus of Srinakharinwirot University in Bangkok, Thailand, before being dried and rehydrated in an oven at 105 °C for 24 h.
Determination of physical properties and Ultimate analysis
Heating values were measured in a bomb calorimeter (Leco, Model AC-350) for coffee residue without pyrolysis (CR and for biochar made from coffee residue (BCR). The values derived from proximate analysis (moisture content, ash content and volatile matter content) were measured using thermogravimetric analysis (TGA) (Mettler Toledo). TGA experiments were conducted using 20 mg. of samples and 100 cm3/min nitrogen flow at the heating
ranges and rates 25–600 °C and 10 °C/min, respectively, then at the heating ranges 600–950 °C; instead of nitrogen, oxygen in the same flow rate was introduced.
The amounts of carbon, hydrogen, nitrogen, oxygen and sulfur (under ultimate analysis) were estimated by Elemental Analyzer, Perkin Elmer, model PE 2400 series II), X-Ray Fluorescence Spectrometer (XRF) and Wavelength Dispersive (Phillips, model PW2400).
Preparation of biochar on biomass kiln
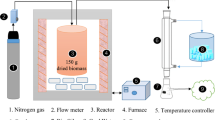
Comprehensive analyses of the results found in the previous topic (physical properties and ultimate analysis) were conducted before the experimentations with a biomass kiln, designed and produced for biochar used in household, were implemented. Three kg of coffee residue (dried and rehydrated in an oven at 105 °C for 24 h) was made as a spherical lump with diameter of 3 cm. To reduce surface area of the coffee residues and increase gaps among the particles of the samples resulting in even spreading heat, the flour paste and the coffee residues were mixed and molded prior to being set on fire. The process was carried out in a stainless-steel biomass kiln. The biomass kiln had the capacity of 25 l and the inside diameter of 20 cm and height 80 cm. The reaction temperature was measured by a K-type thermometer (Sato keiryoki MFG.CO.TH Model SK-1110). The biomass kiln was heated to the desired temperature using the heat from biomass (corn on the cob, wood and charcoal fuels) and a powerful fan circulating air to reach the controlled temperature. Coffee residue was charged into the reactor at room temperature. The biomass was used as a heat generator for the biomass kiln, at the average temperature 350–550 °C which was measured from both the upper part and the bottom part of the kiln. After the 45-min reaction time the biochar characteristics were defined and compared with the results from the previous study in terms of physical and chemical properties.
Characteristic of biochar obtained after carbonization
Scanning electron microscopy (SEM) images of the obtained biochar were taken by a Jeol (JSM-6610LV). The liquid adsorption test, measuring the adsorption quality of the derived biochar, was evaluated in terms of iodine adsorption capacity (Iodine number) reflecting the surface area. From this experiment, ASTM D4607-14 was used to find the iodine numbers. (Annual book ASTM standards 2015) The iodine number is defined as the milligrams of iodine absorbed by 1.0 g of carbon when the iodine concentration of the filtrate is 0.02 mol/l. To determine the iodine number, conical flasks containing 10 ml of 5% by weight of HCl with 0.1 g. of CR and BCR, respectively, were boiled for 1.0 min to eliminate any trace of sulfur and then cooled to room temperature. Next, 50 ml of 1 mol/l iodine solution was added to each flask. The flasks were instantly stopped, vibrated vigorously for 30 s and immediately filtrated. A 25-mL aliquot of individual filtrate was directly titrated with a standardized 0.1 mol/l sodium thiosulfate (Na2S2O3·5H2O) until the solution became pale yellow. The endpoint was regulated by adding 2 ml of starch until the solution turned colorless. Repeating the titration experiment was accomplished and then mean values were calculated. It was confirmed that filtrate normalities (Z) determined from equation (I) did not stay in the range of 0.08–0.04 mol/l, while the equation (II) was applied to calculate the amount iodine adsorbed per gram of carbon (D/C; mg/g). Iodine number is the value of D/C at the residual iodine concentration (Z) of 0.02 mol/l
where Q1 is the normality of sodium thiosulfate (Q); F is the amount of filtrate (ml); and S is the amount of sodium thiosulfate (ml). Furthermore, we have X = 0.1 × 12693.0; Y = Q1 × 126.93; and DF = (I + H)/F [DF = the dilution factor, I = the amount of iodine (ml) and H = the amount of 5% HCl used (ml)] (Hai et al. 2018)
Chemical properties and nutrient content of biochar
One kilogram each of both dried coffee residue sample and biochar produced from coffee residues was measured and analyzed following the Manual on organic Fertilizer Analysis (Horwitz 2000; Horwitz and Latimer 2005) as mentioned below: APSRDO, DOA:4/2551 for pH, APSRDO, DOA:4/2551 (DOA-MBF-017) for electrical conductivity (EC) and APSRDO, DOA:4/2551 (DOA-MBF-011) for amount of organic matter (OM).Then the main nutrient value was measured by the following: In- house method TE-CH-211 based on AOAC (2016) 993.13, In-house method TE-CH-183 based on AOAC (2016) 958.01 and In-house method TE-CH-191 based on official Method of Analysis of Fertilizers JAPAN (1987) at the Central Laboratory (Thailand) Co, Ltd. for Nitrogen (Total N), Phosphorus (Total P2O5) and Potassium (Total K2O) respectively.
Results and discussion
Physical and chemical properties of coffee residue (Proximate, Thermal and ultimate analysis of coffee residue)
CR was being dried and put in the oven at 105 °C for 24 h, after which it was measured using TGA. Figure 1 shows the TGA plot of CR at a heating rate 10 °C/min under nitrogen atmosphere at 200–600 °C and instead nitrogen, oxygen flow by an oxygen flow of 100 cm3/min for the heating range of 600–950 °C in the same flow rate. From Fig. 1, the sample weight change was recorded in the form of temperature and weight loss (%) at the different temperatures as shown in the TGA curve and temperature while the weight loss rate was shown by the DTG curve. There were three phases of decomposition: the first occurred at the temperatures below 200 °C which corresponded to the drying time, where the substance was mostly water that would be released throughout the process. The next phase was a key step, called the Devolatilization process, which is all thermo-chemical conversion process involving in the biomass. This step is represented by the second stage of decomposition, occurring at temperature between 200 and 500 °C, where remarkable slope of the TGA curves were observed, corresponding to significantly decreasing in weight of samples due to liberation of volatile hydrocarbon from rapid thermal decomposition of hemicelluloses, cellulose and some part of lignin (Chen and Kuo 2010).
It is important to consider the temperature in the biochar production from coffee residue on a biomass kiln. Therefore, the temperature range is used for carbonization process in a kiln to obtain BCR. For the last step of the decomposition that took place at temperatures above 500 °C, weight loss is not as significant as in step 2; this is mainly caused by steady decomposition of the remaining heavy components from lignin (Thippa Reddy and Agrawal 2012; Rojith and Bright Singh (2013); Mustapha et al. 2012). Heating value determines the energy content of fuel; it is the property of biomass fuel that depends on its chemical composition and moisture content. The most important fuel property is its heating value. From Table 2, it is evident that the carbonization process raised the heating values of CR. To illustrate, the heating value of CR was 5517 cal/g which is close to heating value in previous studies (Tsai et al. 2012). In addition, Table 2 shows the values derived from proximate analysis (moisture content, ash content and volatile matter) were measured in TGA (Mettler Toledo).
Moisture was one of the main factors that influenced the quality of biomass. The pyrolysis results obtained as reported in weight percent remaining of CR (TG curve) are shown in Fig. 1. For TG curve, the small peak occurring at the temperature below 105 °C corresponds to the removal of water (dehydration) giving the weight loss of around 6.64%. An increase in temperature causes the main pyrolysis to decompose mostly into volatile products, resulting in the occurrence of almost two peaks in the range of 200–400 °C. The TGA curves at the heating rate of 10 °C/min are shown in Fig. 1. It can be seen that the pyrolysis process of coffee residue can be divided into three stages: Taking place below 200 °C, the first stage is regarded as the elimination process of absorbed water in samples, which corresponds to a slight weight loss rate peak shown in TGA curve. Known as the devolatilization process of sample, the second stage (the major stage of pyrolysis) occurs between 200 and 400 °C, primarily generating small molecular gases and large molecular condensable volatile components (Wenjia et al. 2013). Above 400 °C, they enter the third stage, termed as the carbonization process of residues. The weight loss in this phase is very slow, reaching its peak in TGA curve. In general, biomass contains three main cellulosic components, namely hemicelluloses, cellulose and lignin. During the carbonization of biomass, the hemicelluloses and cellulose will decompose mostly into volatile products, while lignin content is more difficult to decompose, generating residue called char, in line with the result conducted by Antal and Michael (1983) in that lignocellulose decomposition usually occurs over a wide range of temperature from 200 to 400 °C. According to Table 2, the fixed carbon was equal to 19.43% with the ash content of 1.78%, and the value of volatile matter of 72.15%. Table 3 illustrates weight fractions among different elements of coffee residue which the values for C, H, O, S (wt%) were in the range of typical biomass and their values were close to previous research (Tsai et al. 2012) except nitrogen content was more than 1 (wt%) that causing the fuel-bound nitrogen to be partly converted to nitrogen oxides (NOx).This discovery can be applied to help design and manufacture fuel from coffee residue. Alternatively, one can take to develop a bio-char or activated carbon from coffee residue to reduce carbon dioxide and nitrogen oxide emissions from biomass combustion (Antal and Michael 1983; Chaney 2010). On the other hand, the molar ratio of hydrogen to carbon (H/C) is in the range of 1.2–1.8 and oxygen–carbon (O/C) is between 0.4 and 0.9 for biomass. In coffee residue, the molar ratio H/C and O/C are 1.74 and 0.57 respectively. Furthermore, the result showed that coffee residue was located in the region of typical biomass.
Biochar production and characterization
The results derived from physical properties and ultimate analysis of CR and the review paper and other related research found that gasification is one methods that can be used for pyrolysis to produce biochar. This method has advantages in processing for the reason that it can be performed in normal atmospheric conditions eliminating the use of inert gas, which responds to scale up. In this research, gasification and carbonization process were easily applied using a top-lit updraft style cook kiln that was used to produce BCR as shown in Fig. 2, which were then compared with biochar generated from a fixed bed reactor using pyrolysis process (Tsai et al. 2012). Table 4 shows the value of proximate analysis, heating value and Iodine NO. of CR (derived from Table 2) and BCR. According to Table 4, in case of coffee residue, fixed carbon rose from 19. 43 to 77.77% (BCR), the heating value increased from 5517 to 7360 cal/g and the ash content also rose from 1.78% (CR) to 6.18% (BCR) since BCR can be derived from used coffee residue in higher amount than CR. Nonetheless, the carbonization process caused sharp declines in volatile contents of waste, which were 72.15% for coffee residue as opposed to 11.89% for BCR. As BCR and C Biochar had high contents of carbon as well as rises in fixed carbon, it can be concluded that that coffee residues could be used as a fuel resource. Table 5 shows that BCR and C Biochar (Tsai et al. 2012) had high contents of carbon as well as increases in the amounts of fixed carbon. Furthermore, the results showed that BCR and C Biochar were located in the region of typical biochar (Van Krevelen 1950; Peter 2002; Didem 2013). Nonetheless, the fuel-bound nitrogen will be partly converted to nitrogen oxides (NOx) during biomass combustion. The findings can be applied to design and manufacture of fuel from CR when it was mixed with other biomass fuels proportionately. Alternatively, one can take to develop biochar or activated carbon from coffee residue to reduce carbon dioxide and nitrogen oxide emissions from biomass combustion. However, Van Krevelen diagram of biomass (Van Krevelen 1950; Peter 2002) tried to classify solid fuel of biomass to correlate between parameter of H/C to parameter of O/C (Peter 2002). Corresponding to Table 5, H/C (0.71) and O/C (0.28) atomic ratio of BCR (were found that were in the range of coal [H/C (0.38–1.0 and O/C (< 0.3)]. The H/C and O/C atomic ratio are inversely proportional to the heating value when the O/C, H/C atomic ratio of the solid fuels decreases, resulting in higher heating values.
SEM analysis of CR and BCR at 250× magnifications are presented as Fig. 3a, b respectively. Figure 3a shows a SEM image of a sample from highly aggregated powder and its shallow cavity. Figure 3b is a SEM image of a sample from a loose agglomeration with a deep cavity, suitable for adsorbing minerals and soil enhancement, in line with the results shown in Table 4 in that the iodine number increased from 297.08 to 618.28. It shows that BCR had smaller pore volume resulting in higher adsorption.
Analysis of physicochemical properties and nutrient content of CR and BCR for utilization as a soil conditioner
Based on the results (Table 6) obtained from the analysis of physicochemical properties and nutrient content for utilization as a soil conditioner (Rayment and Higginson 1992; Soil and Plant Analysis Council 2000; Stevenson 1986; Schroth et al. 2003; Compost and Soil Conditioner Quality Standards 2005), three main points were discussed as follows: First of all, nitrogen, phosphorous and potassium in good and balanced amounts. Those elements are used as key indicators to evaluate the quality of BCR. The moisture content of a fertilizer should be the main consideration at the time of its purchase as moisture content increases its gross weight, resulting in using less fertilizer. The results from Table 6 show that the moisture content of biochar made from coffee residue was 4.16% and it is below 35%, indicating that this biochar has acceptable moisture content. Second, pH and electrical conductivity are considered together to determine if a certain compost needs to be amended before putting into soil. A commonly known standard indicates that its pH should range between 5.5 and 8.5. Table 6 shows that the pH of BCR is equal to 9.9, exceeding the specified limit. The pH determines the survival of microorganisms and each group has optimal pH for growth and multiplication.
Most bacteria activity occurs at pH 6.0–7.5, while most fungi activity occurs at pH 5.5–8.0 and was related to excess of nitrogen in the source material, with poor C:N ratio (Compost and Soil Conditioner Quality Standards 2005). Electrical conductivity is an important variable that indicates the amount of ions of elements dissolved in water. It simply means the amount of salt in water. Water that contains salt in large amounts is not suitable for plant growth due to an increase in osmotic pressure of the solution of the soil, resulting in lacking nutrients essential to the growth and development of plants. Considering the EC ranges of the compost and its application rate, it was claimed that the compost with an EC range of 0–2 dS/m could be used at any rate to the salt-sensitive plants. In most circumstances, applying the compost with EC range of 2–4 at moderate rate would not cause any problem, whereas careful consideration of the application rate should be addressed in the compost with EC above 4. The electrical conductivity (EC) of the BCR was 1.67, which is considered acceptable (John and Kathy 2014).
Finally, A carbon-to-nitrogen ratio (C/N ratio) is often considered one of the most important chemical parameters of soil amendments as it is used to predict mineralization and nitrogen release in soils. Typically, a C/N ratio in the range 15–35 is suitable for soil amendments such as manures and composts. The ratio beyond that will cause immobilization, a process that transforms nitrogen into inorganic substances (\({\text{NH}}_{4}^{ + }\) and NO3) reducing the ability of plants to absorb nutrients. C/N ratio of the biochar was 28.9, which falls within the acceptable range. Therefore, immobilization did not occur in this case. On the other hand, the ratio is less than 15 which will be related causes there is a higher amount of nitrogen-rich material in the mixture when fertilizers mixed with soil, the process may cause odors from the ammonia released (Maria et al. 2015; Sanjai et al. 2015).
Conclusions
From the analysis of the physical and chemical properties of BCR, it can be considered as a potential source for the fuel production because of its high heating value and carbon richness. However, more than 1% Nitrogen in BCR could generate nitrogen oxide during the combustion. To minimize this unfavorable product, biochar produced from coffee residues should be added with other biomass in appropriate proportion so that the content of Nitrogen could be less than 1%. Rich in Nitrogen (3.1%), Phosphorus (1.0%) and Potassium (1.7%) and high pH (9.9), BCR should be mixed with the compost to improve the soil quality in terms of enhancing the effectiveness of the compost together with reducing the acidity of the acidic soil. Additionally, BCR is a good adsorbent (with 618.28 of iodine number), so it can contribute directly to the nutrient adsorption and decrease nutrient leaching at the same time. According to the agricultural production system, BCR additives are expected to enhance soil fertility through improving the nutrient availability, retention and cycling.
To make the study more complete, comprehensive analyses of compost and soil conditioners based on their industry standards should be conducted to discover the proper combinations of BCR and other organic fertilizers in achievement of desirable results
References
Annual book ASTM standards (2015) Volume 15.01- Refractories, Activated Carbon; Advanced Ceramics
Antal MJ, Michael J (1983) Biomass pyrolysis: advances in solar energy. Am Solar Energy Soc Boulder Colo 1:61–111
Chaney J (2010) Combustion Characteristics of Biomass Briquettes. Ph.D. Dissertation, University of Nottingham, Nottingham, United Kingdom. http://eprints.nottingham.ac.uk/11732/. Accessed 20 Dec 2017
Chen WH, Kuo PC (2010) A study on torrefaction of various biomass. Energy 35(12):2580–2586. https://doi.org/10.1016/j.energy.2010.02.054
Chinmai K, Hamsa BC, Kerwyn D (2014) Feasibility studies on spent coffee Grounds Biochar. Int J Innov Eng Res Manag 3(10): 9-13. www.ijaiem.org/Volume3Issue10/IJAIEM-2014-10-07-2.pdf. Accessed 19 Dec 2017
Compost and Soil Conditioner Quality Standards (2005) Hongkong Organic Resource Centre Hong Kong Baptist University, KowloonTong, Hongkong http://www.hkbu.edu.hk/hkorc/. Accessed 17 Nov 2017
Didem O (2013) An approach to the characterization of biochar and bio-oil. Thesis, Bioengineering Department Yildiz Technical Univ. Turkey
Hai N, Tran H, Sheng J (2018) Activated carbons from golden shower upon different chemical activation methods: synthesis and characterizations. Adsorpt Sci Technol 36(1–2):95–113. https://doi.org/10.1177/0263617416684837
Horwitz W (2000) Official method of analysis of AOAC International, 17th edn. AOAC International Inc., Gaithersburg
Horwitz W, Latimer (2005) Official method of analysis of AOAC International, 18th Ed. official method 957.02, 958.01, 18th edn. AOAC International, Gaithersburg
Jeff SC (2010) Biochar and sustainable agriculture. ATTRA-National Sustainable. United States. http://www.attra.ncat.org/attra-pub/biochar.html/. Accessed 3 Jan 2017
Jindo K, Matsumoto K, Suto K et al (2012) Chemical and biochemical characterization of biochar-blended composts. Bioresour Technol 110:396–404. https://doi.org/10.1016/j.biortech.2012.01.120
John P, Kathy P (2014) Plant Nutrient and Carbon Content of Equine Manure. https://www.clemson.edu/extension/camm/manuals/publications/equine_manure_and_stall_mgt.pdf/. Accessed 16 Dec 2017
Khan N, Sánchez-Monedero MA, Clark I et al (2016) Physical and chemical properties of biochars co-composted. Chemosphere 142:14–23. https://doi.org/10.1016/j.chemosphere.2015.05.065
Kim M-S, Koo N, Min H-G et al (2014) The effectiveness of spent coffee grounds and its biochar on the amelioration. J Environ Manag 46:124–130. https://doi.org/10.1016/j.jenvman.2014.07.001
Kondamudi N, Mohapatra S, Misra M (2008) Spent coffee grounds as a versatile source of green energy. J Agric Food Chem 56:11757–11760. https://doi.org/10.1021/jf802487s
Maria M, Alberto P, Pilar R (2015) Farmer’s compost handbook experience in Latin America. Food and Agriculture Organization of the United Nations, Santiago. http://www.fao.org/3/a-i3388e.pdf. Accessed 30 Nov 2017
Mustapha S, Ashhuby B, Abidin Z (2012) Characterization of biomass in up flow anaerobic filters. J Environ Res Dev 7(2):738–745
Peter M (2002) Energy production from biomass. Appl Environ Res Centre Bioresour Technol 83:37–46. https://doi.org/10.1016/S0960-8524(01)00118-3
Rayment GE, Higginson FR (1992) Australian laboratory handbook of soil and water chemical methods. In: Australian soil and land survey handbooks; v. 3
Rojith G, Bright Singh IS (2013) Hydrogen peroxide pretreatment efficiency. J Environ Res Devel 7(4):1333–1339
Sanchez-Monedero MA (2016) Influence of biochar addition on the humic substances. Waste Manag 49:545–552. https://doi.org/10.1016/j.wasman.2016.01.007
Sanjai J, Parikh, Mukome FND (2015) Biochar Production: Chemical, Physical, and Surface Characterization of Biochar, Taylor & Francis Group, New York, pp 68–96
Schroth G, Lehmann J, Barrios E (2003) Soil Nutrient availability and acidity. Wallingford, UK
Soil and Plant Analysis Council (2000) Soil analysis: handbook of reference methods. Soil and Plant Analysis Council, CRC Press, Boca Raton
Stevenson FJ (1986) Cycle of soil: carbon, nitrogen, phosphorus, sulfur and micronutrients. Wiley, New York
Thippa Reddy KS, Agrawal P (2012) Studies on characterizations of agriculture waste. J Environ Res Dev 7(2A):1076–1084
Tokimoto T, Kawasaki N, Nakamura T et al (2005) Removal of lead ions in drinking water coffee grounds. J Colloid Interface Sci 281:56–61. https://doi.org/10.1016/j.jcis.2004.08.083
Tsai Wen-Tien, Liu Sii-Chew, Hsieh Ching-Hsiang (2012) Preparation and fuel properties of biochars. J Anal Appl Pyrol 93:63–67. https://doi.org/10.1016/j.jaap.2011.09.010
United States Department of Agriculture (USDA) (2017) Coffee: World Markets and Trade. https://apps.fas.usda.gov/psdonline/circulars/coffee.pdf/20/01/18. Accessed 17 Dec 2017
Van Krevelen DW (1950) Graphical-statistical method for the study of structure and reaction processes of coal. Fuel 29:269–284 (Survey Handbook, Inkata Press, Melbourne & Sydney)
Wenjia J, Kaushlendra S, John Z (2013) Kinetics of physical components of wood and wood-polymers using isoconversion method. Agriculture 3:12–32. https://doi.org/10.3390/agriculture3010012
Acknowledgements
This work has been funded by The Strategic Wisdom and Research Institute Srinakharinwirot University. The authors acknowledge the help of Scientific and technological Research Equipment Center (STREC) Chulalongkorn University for help in CHNS, SEM, TGA and FTIR analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Tangmankongworakoon, N. An approach to produce biochar from coffee residue for fuel and soil amendment purpose. Int J Recycl Org Waste Agricult 8 (Suppl 1), 37–44 (2019). https://doi.org/10.1007/s40093-019-0267-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40093-019-0267-5