Abstract
Purpose
In the almond industry, the major by-product is the shell, the woody outer layer of the almond fruits. The goal of this research was to study the consequence of the incorporation of almond shell to cultivation substrates on green bean plant grown in a growth chamber.
Methods
Almond shell was mixed with peat (20%:80%) (AS), or used as mulch (AM) on top of the control (C) substrate (33.3%:66.6% vermiculite and peat). Evaluated parameters included green bean pod production and characteristics and their biochemical parameters, namely pigments, total phenolics and antioxidant activity, and soluble sugars and proteins, but also leaf gas exchange parameters.
Results
The use of almond shell as a mulch resulted in unexpected higher crop growth rate, relative leaf growth rate, and leaf area index, with similar production and dimension of pods, when compared to the control assay, with comparable amounts of phenolic compounds, antioxidant capacity, and soluble sugars and proteins, even if gas exchange parameters were negatively affected. By other hand, the data from the almond shell:peat mixture indicate important increase of carotenoid content, contrasting to the control substrate.
Conclusion
These results indicate that almond shell has some potential as growing medium for green bean cultivation, when mixed with peat or used as a mulch.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to FAO (FAOstat 2016), production of almond [Prunus dulcis (Mill.) D.A. Webb] with shell, in 2013, was above 2.9 million tonnes. The shell can represent as much as 70% of the weight, and indicates a large quantity of by-products that is produced by this industry (Ledbetter 2008), being primarily made of cellulose, hemicellulose, and lignin (Valverde et al. 2013). This large amount of material has no economically and straight-forward important use, being either incinerated or discarded or using more complex approaches, used as substrate for xylose production (Pou-Ilinas et al. 1990), furfural (Quesada et al. 2002), or cellulose, pentosans, and lignin (Martinez et al. 1995b). Other uses include its ability to absorb heavy metals or dyes, the presence of characteristics that allow its conversion into activated carbons, extracted to yield antioxidants, or to be used as a growing medium for plants (Esfahlan et al. 2010). Regarding this specific use, some works are available concerning the use of almond shell for pot cultivation of ornamental or horticultural plants (Lao and Jiménez 2004a; Urrestarazu et al. 2008), but, little or no reports concerning the applicability of this by-product as a substrate for green bean cultivation are available. This culture—green bean (Phaseolus vulgaris L.)—is a key greenhouse crop worldwide and is widely cultivated in the Mediterranean countries. However, studies lack regarding soilless (either hydroponic culture or substrate culture) growing conditions (Bouchaaba et al. 2015). In a previous work by our group, we found some detrimental outcomes of the presence of almond shell in substrates used in green bean cultivation (Oliveira et al. 2017), but, at a leaf level, but few is known about its effects on plant and pod characteristics. Hence, the goal of this study was to appraise the possibility of use of almond shell incorporated as substrate or mulch for the growth of green bean in soilless conditions. To perform this evaluation, several parameters were measured, including leaf gas exchange, production, pod characteristics, and biochemical parameters of green bean cultivated on several almond shell-based substrates.
Materials and methods
Substrates
Three different substrates were prepared, in different volumetric ratios, to analyze the effect of almond shell for green bean cultivation. The control (C) substrate was a mixture of 33.3%: 66.6% of vermiculite and peat. The almond shell (AS) substrate was made of 20% shell and 80% peat, which has proved to increase height, dry and fresh weight of roots and shoots, and nitrogen foliar level of other plants (Lao and Jiménez 2004b). Almond shells were also used for mulching (AM), and in this case, the C substrate was covered with 1 cm of mulch from almond shell. Cracked shell size ranged from 0.5 to 2 cm, after cracking, by hand, using a conventional hammer. Almonds, in a mixture in equivalent volumetric ratios, were from the traditional Portuguese cultivars, namely Amendoão, Bonita, Casanova, Pegarinhos, and Refêgo.
Growth conditions
The trial was designed as a complete randomized design of five replications for each treatment. Pots were randomly placed in the growth chamber, and each treatment was arbitrarily assigned to a pot. Green bean (Phaseolus vulgaris cultivar “Saxa”) seeds, uniform and pre-germinated (one per pot) were sowed in plastic pots (13 cm diameter, 12 cm height) and the respective substrate. The assay was carried out in a walk-in growth chamber (FitoClima 10000 EHHF, Aralab), using controlled climate conditions: 16 h and 23 °C for light period and 8 h and 18 °C of temperature for dark period, and photosynthetic photon flux density of 300 µmol/m2 s−1. Relative humidity was kept at 75% during the light period and 80% during the night. Watering was performed once a week, at field capacity of tap water, or using standard Hoagland solution (Hoagland and Arnon 1938) at 15 days, 30 days, and 45 days after sowing. At 60 days after sowing, total number of pod per plants was evaluated, and a harvest of pods was performed. Only pods visually considered suitable for consumption were collected, measured, and weighted, while immature pods were not collected. For each substrate, pods were collected from five homogeneous plants. The pods from each substrate were pooled together, deep-frozen in liquid nitrogen, and stored at − 80 °C waiting analysis. Plants were also analyzed as previously referred, and several physiological plant growth indexes were calculated: crop growth rate (CGR), leaf area index (LAI), leaf area ratio (LAR), leaf mass ratio (LMR), net assimilation rate (NAR), relative leaf growth rate (RLGR), and specific leaf area (SLA).
Leaf gas exchange
The photosynthetic rate (A), stomatal conductance (gs), intercellular CO2 concentration (Ci), and transpiration rate (E) were calculated using data collected using a Infrared Gas Analyser System LCA-3 (ADC, England) and the equations of von Caemmerer and Farquhar (1981). Intrinsic water-use efficiency was according to Düring (1994). Measurements were performed 60 days after sowing, and under an average photosynthetic photon flux (PPFD) of 1434 µmol photons m−2 s−1 and external CO2 concentration of 411.1 ppm, with mean ambient temperature of 19.79 °C, using an Infrared Gas Analyzer System LCPro-SD (ADC Bioscientific Ltd., UK).
Photosynthetic pigments
Samples for the analysis of photosynthetic pigments were prepared by grounding pods (including the seeds) in liquid nitrogen. Extraction was performed with 80% (v/v) acetone (distilled water), and using the methods of Sesták et al. (1971) and Lichtenthaler (1987), respectively, for chlorophyll (Cla and Clb) and total carotenoids.
Soluble proteins
Samples for the quantification of soluble proteins were homogenized in a grinding medium containing 50 mM phosphate buffer (pH 7.5), 0.1 mM ethylenediaminetetraacetic acid (EDTA), 100 mM phenylmethylsulfonyl fluoride (PMSF), and 2% (w/v) polyvinylpyrrolidone (PVP), with centrifugation at 22,000g for 30 min, at 4 °C. Reading was recorded at 595 nm, using bovine serum albumin (BSA) standard (Bradford 1976).
Soluble sugars
Carbohydrate content was measured using the methodology of Irigoyen et al. (1992), through heating (80 °C) samples in 80% (v/v) ethanol/distilled water solution, for 1 h. Afterwards, 0.2 mL of previous extract and 3 mL of anthrone were mixed and putted in a water bath at 100 °C, during 10 min. The liquid fraction was used for soluble sugar quantification, using glucose as standard.
Total phenolics
The procedure of the Folin–Ciocalteu method, modified by Tsao et al. (2003) was employed to quantify the phenolic compounds, using the same extracts obtained for the quantification of photosynthetic pigments. Gallic acid was used as a standard.
Antioxidant activity
The same extracts obtained for the quantification of photosynthetic pigments were used for the evaluation of the antioxidant activity. Three methodologies were used, namely the DPPH (2.2-diphenyl-1-picrylhydrazyl) radical scavenging activity, ABTS·+ [2,2-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid], and the FRAP (ferric reducing/antioxidant power) assays, according to Huang et al. (2005) and references therein. All results are expressed as µmoles of Trolox equivalent (TE) per gram of fresh sample.
Statistical analysis
Results are presented as mean ± standard deviation of five replicates, presented by fresh weight (FW) of green bean pods. Analysis of variance (ANOVA) was used to detect differences among means, using SPSS (Statistical Package for Social Sciences) software, version 19.0 (IBM Corporation, New York, U.S.A.) software. The ANOVA requirements, namely the normal distribution of the residuals, by means of the Shapiro–Wilk’s test, and the homogeneity of variance, using the Levene’s tests, were evaluated. Dependent variables were analyzed using ANOVA with or without Welch correction, depending if homogeneity of variances was observed or not. If statistical significant effect was found, comparison of means was performed using Tukey’s honestly significant difference multiple comparison test if equal variances could be assumed or Dunnett T3 test depending if not. All statistical tests were performed at a 5% significance level.
Results and discussion
Plant growth parameters, production, and pod dimensions
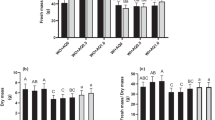
After the assay, no significant differences were found for the productivity of plants. Using the AS substrate, a lower productivity compared was registered, but without significant statistical differences for the other substrates (Table 1).
Other works with mulching (although not with almond shells) also show some beneficial effects of mulches in green bean pod production and dimensions, although linked to water levels inputs (Onder et al. 2006). Similarly, the number of leaves was statistically comparable in all the treatments. Plants were considerably smaller when grown in AS substrate, while the use of AM led to an increase of height regarding the control substrate. This is further noticed on the data regarding plant biomass, considerably higher in plant grown in the AM substrate. After collecting the pods that were visually considered suitable for consumption, significant differences caused by the use of different substrates were observed, for pod weight, length, and in the number of bean in each pod (Table 1). For pod dimension (weight and length), significant differences were observed only between the AS and the other substrates. In fact, in the AS substrate, pods presented, at least, 50% less weight and were 25% smaller in length. When analyzing the results for the number of beans per pod, the AS substrate presented again the lowest value. However, for this parameter, the statistically significant differences were only observed when comparing with the C. These results are most likely due to the effect caused by the incorporation of the almond shell on the physical properties of the substrate. In fact, Lao and Jiménez (2004a) have showed that the use of a similar substrate (20% shell of the same approximate size as the one used in the present work—0.5–2 cm, and 80% peat), when compared to a 33.3% expanded clay and 66.6% peat substrate, reduced the substrate air content, that can lead to problems for the plant to get the oxygen necessary for metabolic activity. These same authors indicate that the AS substrate increases the electric conductivity, reducing the cation exchange capacity, to values, of both parameters, that can be adverse for plants with sensitivity to salinity, as green bean is or outside the recommended limits for cultivation in substrate (Pessarakli 1993). Regarding the plant growth indexes (Table 2), although LAR, LMR, and SLA did not show significant differences between plants grown in different substrates, variations were found for CGR, LAI, and RLGR.
In these parameters, plants grown in AM presented higher values. For RLGR, statistical differences were only recorded between plants grown in AM and C substrate. In fact, in the AM substrate, plants presented around or over two times the values for CGR, compared to C substrate, which may indicate, according to Bergamaschi et al. (1988), a higher availability of water in the substrate, but also resulting from increased ability to intercept light for photosynthesis. This latter factor is most likely to be the one responsible for this increase of CGR, as calculated LAI is also higher in plants grown in the AM substrate. Again, values for those plants are, at least, two times higher to those recorded in plant grown in the other substrates, as a result of higher leaf area (data not shown), which could lead to a higher ability to intercept light and to perform photosynthesis. Furthermore, high CGR results in the allocation of more nutrients to plant structural and protective functions, reducing those available for photosynthetic roles which, as a result, leads to higher LAI, to compensate those lower leaf photosynthetic capacity (Hirons and Thomas 2017) and, for green bean, a strong relationship between LAI and intercepted photosynthetically active radiation (PAR) has been recorded (Kellman 2008). As referred before, RLGR only differed significantly from plant grown in AM and C substrate, showing that, for the control substrate, leaf expansion was lower, while the other substrates presented a similar level. For all these parameters, the mineral composition of almond shell can also be responsible, to some extent, for some of the recorded results. When in non-saline stress conditions, green bean plants use NO3-N rather than NH4-N for synthesis of proteins, and, ultimately, for plant development and growth. Almond shell has been reported to contain both NO3-N and NH4-N (Valverde et al. 2013), and the increase of plant and pod biomass recorded for AM could indicate that NO3–N from shells may have been available for plant absorption in higher concentration than in AS substrate, (although keeping in mind that mineral composition of the shell used in the present work was not known). The previous works show that high concentrations of NH4+ led to a reduction of growth and yield when compared to the presence of NO3 (Harada et al. 1968). Similarly, the results recorded for AS appear to indicate that plant are under salinity stress, as it has been reported that this factor can cause the reduction of growth and dry-matter accumulation (Taïbi et al. 2016), linked to cell elongation inhibition (Bandeoglu et al. 2004). However, and considering the results of leaf gas exchange measurements, other factors rather than salinity issues may be responsible for this lower growth and yield of AS. Furthermore, the AS substrate may present a higher C/N ratio than C or AM substrates, a fact that will also affect plant growth. Indeed, the C/N ratio of a similar growth substrate as AM (Lao and Jiménez 2004a) was high, and increases of this value result in plant growth reduction.
Leaf gas exchange, photosynthetic pigments, soluble sugars, and proteins
Leaf gas exchange parameters recorded in plants showed significant differences in A and E, (Table 3), while the other parameters (gs, A/gs, and Ci) were similar between substrates.
For the statistically different parameters, lower values were recorded in plants grown in the AM substrate, especially when compared to those of the AS substrate, which registered higher values. As referred before, for plant growth and production, it appears that salinity issues may not be responsible for the observed results. In fact, if such was true, data from leaf gas exchange should show a reduction in A and E in the AM substrate. This would be due to the fact that salinity can result in stomatal closure and reduction in photosynthesis (Bayuelo-Jiménez et al. 2003), which, actually, did not occur in this substrate, but rather in the AM substrate. These results are most likely linked, to some extent, to the water availability in each of the substrates and its connection to leaf gas exchange. The AS substrate is likely to have a much higher water content and easily available water, as previously reported (Lao and Jiménez 2004a) than the C substrate, as well as in the AM, where some of the water will be retained in the almond shell layer (Jafari et al. 2012) the reduction of A and E due to some water stress cannot be discarded.
As the green colour has been recognized as quality parameter of bean, it is of great importance its evaluation, namely chlorophyll content appraisal. The use of different substrates resulted in significant variations on the content of photosynthetic pigments present in the green bean pods (Table 4).
The content of chlorophyll can be considered similar to those previously reported in green bean pods (Gross 1991). For chlorophyll a, the AS substrate presented highest values, while, for chlorophyll b and total chlorophyll, this substrate had similar amount than those recorded for AM. Although the substrate conditions cannot be discarded as a factor that caused these differences, there may be other explanations for this fact: pods were collected when visually ready for harvest, but minor differences between developmental stage may have occur (not detected at harvest), which have been reported to cause differences in the chlorophyll content of green bean pods (Martinez et al. 1995a). Although few is known regarding chlorophyll content of green bean pods, the other studies show that it decreases, in leaves, with salt stress (Taïbi et al. 2016), while the use of foliar application of salt antioxidants increases pod content of chlorophylls (Shokr et al. 2014). For carotenoid content, the pods collected from control plants presented the lower values, with AM presenting a statistically similar content. By other hand, there was a considerable increase on the content of carotenoids in the pods collected from the plants grown in AS. The values recorded in these samples are similar to those that have been found in the other works (Oruña-Concha et al. 1997). This is a very interesting result, since carotenoids are compounds of interest as vitamin A precursors. Furthermore, they are also antioxidants, protecting cells and tissues from damaging effects of free radicals and singlet oxygen (Maiani et al. 2009). The increase of the carotenoid content in pods from plant grown in substrates containing almond shell may be related to a higher availability of nutrients. In fact, López et al. (2014) have shown a higher content of N and K in soil with mulching, using almond shell, and the presence of these nutrients has been linked to an increase on the carotenoid content of fruits (Fanasca et al. 2006). In addition, the presence of K, Ca, Mg, or Zn in foliar sprays also led to an increase of carotenoid content in green bean pods (Shokr et al. 2014).
Furthermore, the use of almond shell has been proved to increase the electric conductivity of substrates and nutrient solutions (De Lucia et al. 2011; Valverde et al. 2013; López et al. 2014), which may also lead to an increase on carotenoid content, as previously reported (Krauss et al. 2006).
The evaluation of the content on soluble sugars and proteins of green bean pods grown in the different substrates did not result in significant differences between them (Table 4). These values can be considered analogous to those formerly recorded with the other green bean varieties (Singer et al. 2002; Sánchez-Mata et al. 2003; Sanchez et al. 2004; Proulx et al. 2010; Ramírez et al. 2013).
Total phenolic content and antioxidant activity
The quantification of total phenolic content and the evaluation of the antioxidant activity of the green bean pods grown in different substrates did not show significant variations (Table 5), with the exception of the FRAP assay.
For the total phenolic content, values ranged from 0.84 ± 0.12 mg GAE/g of fresh weight, in the pods from the C substrate, to 1.00 ± 0.24 mg GAE/g, in the pods form the AM substrate. These values are comparable to those reported by the other works (Zhou and Yu 2006; Turkmen et al. 2005). These authors indicate a content of around 6–8 mg GAE/g, and 3.5 mg GAE/g, but expressed by dry weight. Considering an average amount of water in green bean pods of 90% (data not shown), our values will be ranging from 7.7 to 10 mg/g dry weight. The antioxidant activity measured using the FRAP radical scavenging activity assay was affected by the substrate used (Table 5). Values ranged from 3.39 ± 1.33 µmol Trolox/g fw to 5.32 ± 1.36 µmol Trolox/g fw, in pods from the AS and C substrates, respectively. From this study, although difficult to put in perspective, due to the few works currently available for this specific parameter in green bean, appear to be higher than previously reported. In fact, some works detected lower antioxidant activities for green bean pods (Wolosiak et al. 2011; Baardseth et al. 2010), that can be related to the different analyzed cultivar, or due to variations on the extraction and quantification methodology between works, rather than linked to the tested substrates. Regarding the ABTS and the DPPH antioxidant assays, no significant variations were observed between the tested substrates. As for FRAP, lower results for ABTS and DPPH were found in the previous works (Ou et al. 2002; Wolosiak et al. 2011), which can be also associated with the different extraction and quantification methodologies.
Conclusions
The present results indicate that the use of almond shell, without further processing, as an alternative substrate for the growth of green bean is a viable approach. By one hand, the use of a 1 cm mulch of almond shell does not significantly change production and pod dimension, as well as total phenolic content and antioxidant activity, while increasing plant growth. However, it reduced significantly photosynthetic rate of plants. In contrast, the use of almond shell mixed with peat, although leading to smaller plant, does not change yield, but reduced pod dimensions. However, leaf gas exchange parameters are improved, as was the amount of carotenoids present in green bean pods. These results indicate some positive effects of the use of almond shell as an alternative substrate for the growth of green bean that must be further explored.
References
Baardseth P, Bjerke F, Martinsen B, Skrede G (2010) Vitamin C, total phenolics and antioxidative activity in tip-cut green beans (Phaseolus vulgaris) and swede rods (Brassica napus var. napobrassica) processed by methods used in catering. J Sci Food Agric 90:1245–1255. https://doi.org/10.1002/jsfa.3967
Bandeoglu E, Eyidogan F, Yucel M, Oktem H (2004) Antioxidant responses of shoots and roots of lentil to NaCl-salinity stress. Plant Growth Regul 42:69–77. https://doi.org/10.1023/B:GROW.0000014891.35427.7b
Bayuelo-Jiménez J, Debouck D, Lynch J (2003) Growth, gas exchange, water relations, and ion composition of Phaseolus species grown under saline conditions. Field Crops Res 80:207–222. https://doi.org/10.1016/S0378-4290(02)00179-X
Bergamaschi H, Vieira H, Ometto J, Angelocci L, Libardi P (1988) Deficiência hídrica em feijoeiro. I. Análise de crescimento e fenologia. Pesqui Agropecu Bras 23:733–743
Bouchaaba Z, Santamaria P, Choukr-Allah R, Lamaddalena N, Montesano F (2015) Open-cycle drip vs closed-cycle subirrigation: effects on growth and yield of greenhouse soilless green bean. Sci Hortic Amsterdam 182:77–85. https://doi.org/10.1016/j.scienta.2014.11.007
Bradford M (1976) A rapid and sensitive method for the quantification of microgram quantities of protein using the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
De Lucia B, Vecchietti L, Leone A (2011) Italian buckthorn response to compost based substrates. Acta Hortic 891:231–236. https://doi.org/10.17660/ActaHortic.2011.891.27
Düring H (1994) Photosynthesis of ungrafted and grafted grapevines: effects of rootstock genotype and plant age. Am J Enol Viticult 45:297–299
Esfahlan A, Jamei R, Esfahlan R (2010) The importance of almond (Prunus amygdalus L.) and its by-products. Food Chem 120:349–360. https://doi.org/10.1016/j.foodchem.2009.09.063
Fanasca S, Colla G, Maiani G, Venneria E, Rouphael Y, Azzini E, Saccardo F (2006) Changes in antioxidant content of tomato fruits in response to cultivar and nutrient solution composition. J Agric Food Chem 54:4319–4325. https://doi.org/10.1021/jf0602572
FAOstat (2016) Agriculture data. http://faostat3.fao.org/faostat-gateway/go/to/home/E. Accessed 09 Oct 2016
Gross J (1991) Pigments in vegetables: chlorophylls and carotenoids. Van Nostrand, Reinhold, New York
Harada T, Takaki H, Yamada Y (1968) Effect of nitrogen sources on the chemical components of young plants. Soil Sci Plant Anal 14:47–55
Hirons AD, Thomas PA (2017) Tree carbon relations. In: Hirons AD, Thomas PA (eds) Applied tree biology. https://doi.org/10.1002/9781118296387.ch7
Hoagland DR, Arnon DI (1938) The water-culture method for growing plants without soil. Calif Agric Exp Stn Bull 347:36–39
Huang D, Ou B, Prior R (2005) The chemistry behind antioxidant capacity assays. J Agric Food Chem 53:1841–1856. https://doi.org/10.1021/jf030723c
Irigoyen J, Emerich D, Sánchez-Díaz M (1992) Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiol Plant 84:55–60. https://doi.org/10.1111/j.1399-3054.1992.tb08764.x
Jafari M, Haghighi J, Zare H (2012) Mulching impact on plant growth and production of rainfed fig orchards under drought conditions. J Food Agric Environ 10:428–433
Kellman A (2008) Rhizobium inoculation, cultivar and management effects on the growth, development and yield of common bean (Phaseolus vulgaris L.) (Doctoral dissertation, Lincoln University)
Krauss S, Schnitzler W, Woitke J, Woitke M (2006) The influence of different electrical conductivity values in a simplified recirculating soilless system on inner and outer fruit quality characteristics of tomato. J Agric Food Chem 54:441–448. https://doi.org/10.1021/jf051930a
Lao M, Jiménez S (2004a) Evaluation of almond shell as a culture substrate for ornamental plants. I. Phyton 73:69–78
Lao M, Jiménez S (2004b) Evaluation of almond shell as a culture substrate for ornamental plants. II. Ficus Benjamina Phyton 73:79–84
Ledbetter C (2008) Shell cracking strength in almond (Prunus dulcis Mill. D.A. Webb.) and its implication in uses as a value-added product. Bioresource Technol 99:5567–5573. https://doi.org/10.1016/j.biortech.2007.10.059
Lichtenthaler H (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382. https://doi.org/10.1016/0076-6879(87)48036-1
López R, Burgos P, Hermoso J, Hormaza J, González-Fernández J (2014) Long term changes in soil properties and enzyme activities after almond shell mulching in avocado organic production. Soil Tillage Res 143:155–163. https://doi.org/10.1016/j.still.2014.06.004
Maiani G, Periago Castón M, Catasta G, Toti E, Cambrodón I, Bysted A, Granado-Lorencio F, Olmedilla-Alonso B, Knuthsen P, Valoti M, Böhm V, Mayer-Miebach E, Behsnilian D, Böhm V (2009) Carotenoids: actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol Nutr Food Res 53:S194–S218. https://doi.org/10.1002/mnfr.200800053
Martinez C, Ros G, Periago M, Lopez G, Ortuno J, Rincon F (1995a) Physico-chemical and sensory quality criteria of green beans (Phaseolus vulgaris L.). Lebensmittel Wissenschaft Technol 28:515–520. https://doi.org/10.1006/fstl.1995.0086
Martinez J, Granado J, Montane D, Salvado J, Farriol X (1995b) Fractionation of residual lignocellulosics by dilute-acid prehydrolysis and alkaline extraction: application to almond shells. Bioresour Technol 52:59–67. https://doi.org/10.1016/0960-8524(95)00005-Y
Oliveira I, Meyer A, Aires A, Afonso S, Gonçalves B (2017) Enzymatic activity and biochemical composition in leaves of green bean (Phaseolus vulgaris L. cv. Saxa) grown in almond shell substrates. Waste Biomass Valoriz 1:1–7. https://doi.org/10.1007/s12649-017-0141-5
Onder S, Bozkurt S, Sayilikan G, Onder D, Kara M (2006) Effects of water stress and mulch on green bean yield and yield components in greenhouse condition. Asian J Plant Sci 5:127–132. https://doi.org/10.3923/ajps.2006.127.132
Oruña-Concha M, González-Castro M, López-Hernández J, Simal-Lozano J (1997) Effects of freezing on the pigment content in green beans and padrón peppers. Z Lebensm Unters Forsch A 205:148–152. https://doi.org/10.1007/s002170050143
Ou B, Huang D, Hampsch-Woodil M, Flanagan J, Deemer E (2002) Analysis of antioxidant activities of common vegetables employing oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) assays: a comparative study. J Agric Food Chem 50:3122–3128. https://doi.org/10.1021/jf0116606
Pessarakli M (1993) Response of green beans (Phaseolus vulgaris L.) to salt stress. In: Pessarakli M (ed) Handbook of plant and crop stress. Marcel Dekker, New York, pp 827–842
Pou-Ilinas J, Canellas J, Driguez H, Excoffier G, Vignon M (1990) Steam pretreatment of almond shells for xylose production. Carbohydr Res 207:126–130. https://doi.org/10.1016/0008-6215(90)80011-Q
Proulx E, Yagiz Y, Cecilia M, Nunes N, Emond J (2010) Quality attributes limiting snap bean (Phaseolus vulgaris L.) postharvest life at chilling and non-chilling temperatures. HortScience 45:1238–1249
Quesada J, Teffo-Bertaud F, Croue J, Rubio M (2002) Ozone oxidation and structural features of an almond shell lignin remaining after furfural manufacture. Holzforschung 56:32–38. https://doi.org/10.1515/HF.2002.006
Ramírez N, Estrada J, González M, Montes E (2013) Rendimiento, calidad nutrimental y rentabilidad del frijol ejotero de temporal en San Pablo Ixayoc, México. Rev Chapingo Ser Hortic 19:333–342. https://doi.org/10.5154/r.rchsh.2010.08.031
Sanchez E, Rivero R, Ruiz J, Romero L (2004) Yield and biosynthesis of nitrogenous compounds in fruits of green bean (Phaseolus vulgaris L cv. Strike) in response to increasing N fertilisation. J Sci Food Agric 84:575–580. https://doi.org/10.1002/jsfa.1700
Sánchez-Mata M, Camara M, Dıez-Marques C (2003) Extending shelf-life and nutritive value of green beans (Phaseolus vulgaris L.), by controlled atmosphere storage: macronutrients. Food Chem 80:309–315. https://doi.org/10.1016/S0308-8146(02)00265-0
Sesták Z, Castky J, Jarvis P (1971) Plant photosynthetic production. Manual of methods. Dr. W. Junk Publishers, Hague
Shokr M, Elsaid M, Shafeek M (2014) Effect of some stimulative substances as foliar applications on snap bean (Phaseolus vulgaris L.) productivity under milder thermo-stress of local summer season. Middle East J Appl Sci 4:175–180
Singer S, Helmy Y, Karas A, Abou-Hadid A (2002) Influences of different water–stress treatments on growth, development and production of snap bean (Phaseolus vulgaris L.). Acta Hortic 614:605–611. https://doi.org/10.17660/actahortic.2003.614.90
Taïbi K, Taïbi F, Abderrahim L, Ennajah A, Belkhodja M, Mulet J (2016) Effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidant defence systems in Phaseolus vulgaris L. S Afr J Bot 105:306–312. https://doi.org/10.1016/j.sajb.2016.03.011
Tsao R, Yang R, Young J, Zhu H (2003) Polyphenolic profiles in eight apple cultivars using high-performance liquid chromatography (HPLC). J Agric Food Chem 51:6347–6353. https://doi.org/10.1021/jf0346298
Turkmen N, Sari F, Velioglu Y (2005) The effect of cooking methods on total phenolics and antioxidant activity of selected green vegetables. Food Chem 93:713–718. https://doi.org/10.1016/j.foodchem.2004.12.038
Urrestarazu M, Mazuela P, Martínez G (2008) Effect of substrate reutilization on yield and properties of melon and tomato crops. J Plant Nutr 31:2031–2043. https://doi.org/10.1080/01904160802405420
Valverde M, Madrid R, García A, Del Amor F, Rincón L (2013) Use of almond shell and almond hull as substrates for sweet pepper cultivation. effects on fruit yield and mineral content. Span J Agric Res 11:164–172. https://doi.org/10.5424/sjar/2013111-3566
von Caemmerer S, Farquhar G (1981) Some relationships between the biochemistry of photosynthesis and gas exchange of leaves. Planta 153:376–387. https://doi.org/10.1007/BF00384257
Wolosiak R, Druzynska B, Piecyk M, Worobiej E, Majewska E, Lewicki P (2011) Influence of industrial sterilisation, freezing and steam cooking on antioxidant properties of green peas and string beans. Int J Food Sci Technol 46:93–100. https://doi.org/10.1111/j.1365-2621.2010.02456.x
Zhou K, Yu L (2006) Total phenolic contents and antioxidant properties of commonly consumed vegetables grown in Colorado. Food Sci Technol 39:1155–1162. https://doi.org/10.1016/j.lwt.2005.07.015
Acknowledgements
Ivo Oliveira is grateful to FCT—Fundação para a Ciência e a Tecnologia (FCT), POPH-QREN, and FSE for the Post-doctoral Fellowship SFRH/BPD/111005/2015. This work was supported by: European Investment Funds by FEDER/COMPETE/POCI-Operacional Competitiveness and Internacionalization Programme, under Project POCI-01-0145-FEDER-006958 and National Funds by FCT-Portuguese Foundation for Science and Technology, under the project UID/AGR/04033/2013.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Oliveira, I., Meyer, A., Silva, R. et al. Effect of almond shell addition to substrates in Phaseolus vulgaris L. (cv. Saxa) growth, and physiological and biochemical characteristics. Int J Recycl Org Waste Agricult 8, 179–186 (2019). https://doi.org/10.1007/s40093-019-0249-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40093-019-0249-7