Abstract
Purpose
Novel protein sources are urgently needed to meet the increasing protein demand of a continuously growing world population. This study is focused on the production of protein rich mushroom mycelia on industrial side streams.
Methods
Submerged propagation of mushrooms was carried out in shake flasks which contained agro-industrial side streams as the sole carbon source. The biomass obtained was analyzed for its crude protein, ash and fat content as well as for its fatty acid and amino acid profiles. Vitamin D2 production from ergosterol in the biomass was induced by UV-B irradiation and determined by HPLC–DAD. The share of fungal mycelium in the total biomass was determined by extraction and quantitation of ergosterol. Additionally, water and oil binding capacity (WBC and OBC) were evaluated.
Results
A screening of basidiomycetes grown on agro-industrial side streams indicated a fast growth of Pleurotus sapidus on apple pomace. After 4 days of cultivation, the biomass obtained from this mushroom–substrate combination contained 21% true protein in dry matter. In addition to proteins, the amounts of lipids (4%), ash (2%) and carbohydrates (74%) were quantitated. The dominating fatty and amino acids of Pleurotus sapidus grown on apple pomace were linoleic acid and glutamic acid/glutamine, respectively. Concentrations of up to 115 µg (g dry matter)−1 vitamin D2 were formed from ergosterol by UV-B irradiation. Ergosterol was used as a biomarker to monitor the amount of fungal content.
Conclusion
The nutritional value of agro-industrial side streams such as apple pomace can be upcycled by biotransformation with basidiomycetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fungi have been considered as a desirable source of human nourishment for thousands of years. Because of their high nutritional value and pleasant taste, the fruiting bodies of mushrooms are commonly consumed. They are rich in fiber (i.e., chitin and glucan), contain all essential amino acids, vitamin D2, several vitamins of the B group, and have low fat contents (Manzi et al. 1999). Due to their low energy content, they represent an ideal food for low calorie diets. Fruiting bodies of the oyster mushroom Pleurotus ostreatus have been reported to lower the oxidized low density lipoprotein (oxLDL) and triacylglycerol levels in the blood and help maintain a normal blood cholesterol level (Alam et al. 2009; Schneider et al. 2011). In addition to the fruiting bodies, vegetative mycelia of fungi are consumed as food as well. Prominent examples include tempeh, which is produced by fermentation of soy beans with the mucoromycete Rhizopus oligosporus, and Quorn™ which is based on the mycelium of the mold Fusarium venenatum. These mycelia based foods are consumed as alternatives to meat. Currently, there is an increasing demand for meat-analogue products. Microbial proteins offer the advantages of additional health benefits, minimization of animal slaughter and a reduction in the carbon foot print of food production. In addition, mycoprotein is deemed as a high quality protein with a Protein Digestibility-Corrected Amino Acid Score (PDCAAS) of 0.91 (Miller and Dwyer 2001), which is comparable to that of animal proteins such as casein, egg white and beef with PDCAAS values of 1.0, 1.0 and 0.92, respectively (Singh et al. 2008). Fungal mycelia are also considered to provide a high protein to energy ratio. For example, a fillet of beef provides 0.18 g protein per kcal, while P. ostreatus delivers 0.21 g protein per kcal (based on data from Andersen and Soyka 2011).
Apart from valuable proteins, fungi contain ergosterol, the direct precursor of vitamin D2, which is a structural analogue of vitamin D3. Vitamin D3 is formed in the skin from 7-dehydrocholesterol after exposure to sunlight or can be supplied by a few animal sources, such as egg yolk or fish. Vegetarian or vegan diets may cause a vitamin D deficiency, which can lead to severe osteoamalacia and rickets due to insufficient bone mineralization. Ergosterol, located in the cell membrane of fungi, is easily transformed to vitamin D2 by UV-B exposure (Roberts et al. 2008).
Apples belong to the most popular fruits worldwide, and the world production of fresh apples amounted to 89 million tons in 2016 (FAO 2018). Frequently, apples are pressed for production of juice, and apple pomace, consisting of peels, seeds and pulp, accrues as side stream. Currently, apple pomace is either used as feed for ruminants or it is incinerated. Recently, Aghili et al. (2019) analyzed the effects of apple pomace as poultry feed. They found adverse effects regarding growth performance and some blood parameters. In Germany, 200–250 thousand tons of fresh apple pomace are produced every year (Kammerer et al. 2014). This agro-industrial side stream is available throughout the year, thus making it an ideal carbohydrate source for mushroom production. Villas-Bôas et al. (2003) valorized apple pomace using it as a substrate for solid-state fermentation of Candida utilis and Pleurotus ostreatus. The product obtained from this fermentation was fed to ruminants in their study. Another important side stream is molasses. It accrues as one of the major by-products of the sugar industry and is either fed to animals or used as a substrate for yeast production. However, only approximately 60% of the molasses arising in Germany, which amounts to 650 thousand tons, is used. Molasses are rich in carbohydrates and may be used as substrate for mushroom production.
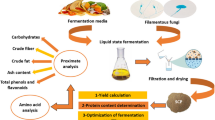
In this study, basidiomycetes (edible mushrooms) were grown in submerged cultures which contained industrial side streams as sole carbon source. This resulted in the conversion of apple pomace into nutritionally valuable mushroom mycelia. Finally, the mycelia were enriched in vitamin D2.
Materials and methods
Cultivation of microorganisms
Agrocybe aegerita, strain 4022 (AAE) was received from Sylvan (NT Horst, The Netherlands), Pleurotus sapidus, strain 8266 (PSA) from DSMZ (Braunschweig, Germany), Lentinula edodes, strain 389.89 (LED), Wolfiporia cocos, strain 279.55 (WCO) from the Dutch Westerdijk Fungal Biodiversity Institute (Utrecht, The Netherlands) and Stropharia rugosoannulata, strain M 5012 (SRU) from Mycelia (Nevele, Belgium). Pleurotus sajor-caju (PSC) and Pleurotus salmoneostramineus were obtained from the institute’s culture collection and were cultivated on apple pomace only. These fungi were selected based on data from previous studies (Bosse et al. 2013; Stephan et al. 2018). All cultivation procedures were performed under sterile conditions. Stock cultures were maintained on malt extract agar plates (20 g L−1 malt extract, 15 g L−1 agar agar) at 24 °C in darkness.
Pre-cultures were prepared as described by Trapp et al. (2018), and were grown for 6–13 days: AAE 11 d, LED 13 d, LSU 13 d, PSA 6 d, SRU 7 d and WCO 7 d. For the main cultures, the carbohydrate content of each side-stream was calculated (see 2.5) and adjusted to 15 g carbohydrate per liter medium as the sole carbon source. In the case of apple pomace 23.7 g DM were used per liter. According to Bosse et al. (2013) a medium containing l-aspartic acid, NH4NO3, KH2PO4, MgSO4·H2O and 1 mL trace element solution was used. The composition of the trace element solution was adapted from Trapp et al. (2018). Prior to sterilization, the pH was adjusted to 6.0 with 1 mol L−1 NaOH. The respective amount of substrate and 200 mL medium were transferred to a 500 mL Erlenmeyer flask and inoculated with 20 mL of the homogenized pre-culture (Ultra Turrax 30 s, 10,000 rpm). Cultivation was performed at 24 °C and 150 rpm in the dark. The fully grown cultures were harvested by centrifugation (10 min, 4 °C, 3300 g), and the mycelium was washed three times with water and freeze-dried for further analysis.
Substrates
As substrates for the submerged cultivation, different pomaces (apple, pomegranate and aronia), leaf spinach and beet molasses were investigated. They were obtained from industrial partners. Leaf spinach was stored at − 20 °C and molasses at 4 °C. The other substrates were stored at room temperature in the dark. The substrates were used untreated except for leaf spinach, which was lyophilized and ground to powder with a particle size of ≤ 2 mm.
Selection criteria
Culture period, obtained dry matter, and protein content of the biomass were chosen as selection criteria.
Chemicals
Standards for vitamin D and ergosterol analyses were purchased from Sigma-Aldrich (Steinheim, Germany): cholecalciferol (vitamin D3) 99.9%, ergocalciferol (vitamin D2) 99.1%, ergosterol and 7-dehydrocholesterol ≥ 95.0%. The amino acid calibration standard was purchased from Sykam (Fuerstenfeldbruck, Germany), and l–tryptophan ≥ 99.0% from Roth (Karlsruhe, Germany). All chemicals were at least of analytical grade and solvents for chromatography of HPLC–grade.
Analysis of substrates and fungal lyophilisates
Moisture content was determined by a Moisture Analyzer MA35 (Sartorius, Göttingen, Germany). Total nitrogen was analyzed based on the method of Kjeldahl (1883) and Matissek et al. (2010). The amount of ash was determined by difference in weight after total incineration at 550 °C. The fat content was determined after acid treatment and extraction with petroleum ether (Soxtherm rapid extraction system, Gerhardt, Koenigswinter, Germany). The amount of carbohydrates was calculated as difference to 100%.
Fatty acid profile
The fatty acid profile was analyzed by gas chromatography (Agilent Technologies 7890 A, Varian Optima FFAP 30 m × 0.25 mm ID, 0.25 µm film thickness) after transesterification with boron triflouride.
Glucan analysis
The Mushroom and Yeast Beta-Glucan, (Megazyme Inc., Ireland) enzyme assay kit was used for glucan analysis. Total glucans and α-glucans were determined according to the manufacturer’s protocol, and the amount of β-glucans was calculated as the difference.
Chitin analysis
Chitin was quantitated based on a method of Smith and Gilkerson (1979) which employed a colourimetric assay of 3-methyl-2-benzothiazolinone hydrazone hydrochloride (MBTH) at 650 nm, with slight modifications. Briefly, 20 mg sample was hydrolyzed with 2.5 mL 6 M HCl at 105 °C. After 24 h, the pH was adjusted to 7.0 using NaOH (6 M, 1 M and 0.1 M). The volume was made up to 10 mL using ultra-pure water. 100 µL of the filtered solution was diluted 1:10 by adding 500 µL 0.5 M HCl and 400 µL water. This mixture was incubated for another 2 h at 105 °C. Next, 2 mL sodium nitrite (2.5%) was added and incubated for 15 min following which 1 mL ammonium sulphamate (12.5%) was added to the samples. After another 5 min, 1 mL MBTH solution (0.25%) was added, mixed and incubated for 30 min at 37 °C. Finally, 1 mL FeCl3∙6 H2O (0.5%) was added and the sample was incubated for 5 min at 37 °C. Standards containing N-acetyl-d-glucosamine (6.18–61.8 µg mL−1) were analyzed in the same way as the samples. This resulted in a calibration curve with a regression equation, y = 0.0172x + 0.0119, and a R2 = 0.9981.
Amino acid analysis
For identification and quantitation of amino acids in the lyophilized biomass after total hydrolysis, an amino acid analyzer S433 with columns LCA K13/Na and LCA K04/Na (Sykam) and Chromstar software (version 7) was used. According to the manufacturer’s protocol, a gradient of two sodium citrate buffer solutions (A: 0.17 M, pH 3.4; B: 0.20 M, pH 10.85; Sykam) and a regenerating solution (RegSol Na 0.5 M, Sykam) were used. Ninhydrin (0.2 M, pH 10.85, Sykam) was used for derivatization. The amino acids were separated at a flow rate of 0.45 mL min−1 and quantified by single-point calibration (n = 3); the injection volume was 50 µL.
Acid hydrolysis for total amino acids and oxidative acid hydrolysis for cysteine and methionine were performed, as described in Commission Directive 98/64/EC of the European Unions. Tryptophan analysis was carried out analogous to the acid hydrolysis with slight modifications. Approximately 250 mg sample was mixed with 25 mL NaOH (5 M with 0.1% phenol) instead of HCl and heated at 110 °C for 24 h. After hydrolysis, the pH was adjusted to 2.2 with 10 mL 0.5 M phosphoric acid, followed by 3.75 M and 1 M HCl.
The biological value (BV) was calculated using the amino acid score (AAS), the chemical score (CS) and the essential amino acid index (EAAI). Equations are shown in the supplementary material.
Calculation of the Kjeldahl factor
Summing up all of the analyzed single amino acids (AA) would cause overestimation of the protein content. Thus, it was necessary to calculate amino acid residues (AAres) which took into account the loss of water that takes place during the formation of peptide bonds. Nitrogen-to-protein conversion factors (N) found in literature mostly do not consider this. To calculate the true protein content, Nnet was calculated as AAres divided by the total nitrogen content, which was analyzed by Kjeldahl’s method.
Quantitation of vitamin D2 by HPLC–DAD
Two gram lyophilized biomass of Pleurotus sapidus grown on apple pomace was spread onto a crystallizing dish with a diameter of 19.5 cm and exposed to UV-B light at room temperature. Three UV-B Medical Hg lamps, Arimed B12 9 W (compact) from Philips (Amsterdam, NL) with an emission maximum of 290–310 nm were used as UV source. Lamps were mounted at a distance of 13 cm from each other and 10 cm to the working surface. Irradiance was measured by Newport Power Meter model 1918R with sensor 818P-015-19 and amounted to 4.44 mW cm−2. After irradiation for up to 45 min, the lyophilised biomass was ground to a fine powder in liquid nitrogen, and the liquid–liquid extraction of vitamin D was performed as described by Ahlborn et al. (2018). Vitamin D3 was used as the internal standard. HPLC–DAD was used for detection of vitamin D2 at a wavelength of 265 nm (Ahlborn et al. 2018).
Quantitation of fungal mycelium using ergosterol as biomarker
0.15–0.2 g of lyophilised biomass was extracted with the method described for vitamin D, using 7-dehydrocholesterol (7-DHC, 0.5 mL of 1 mg mL−1 in methanol) as the internal standard. Quantitation was performed by HPLC–DAD at a detection wavelength of 282 nm. Limits of detection (LOD) and of quantitation (LOQ) were calculated based on DIN 32645 resulting in a LOD of 7.06 µg mL−1 and a LOQ of 25.10 µg mL−1, corresponding to 53.2 and 188.3 µg (g DM)−1, respectively.
For determining the ergosterol amount of pure fungal biomass, the basidiomycete was cultivated in malt extract medium (20 g L−1), and the mycelium was harvested and lyophilized as described above. As this medium did not contain insoluble components, the lyophilisate was assumed to contain 100% fungal biomass.
Functional characteristics
The functional properties of the mycelium of P. sapidus grown on apple pomace were analyzed by measuring the water and oil binding capacity (WBC and OBC) as well as the influence of various salts (food additives) on the water binding capacity. WBC and OBC were determined according to Stephan (2018). The determination was carried out in five replications. The fungal biomass was compared to other commercially available plant proteins, soy protein isolate (Gushen, China, 90.2% protein content), soy protein concentrate (Gushen, China, 69.8% protein content) and pea protein isolate (Cosucra, Belgium, 90.1% protein content), which were used in meat analogues. To evaluate the influence of various salts on the WBC, 15 g kg−1 sodium chloride (esco, Germany), potassium chloride (Jaeklechemie GmbH&Co. KG, Germany), magnesium chloride (Sapho GmbH, Germany) or calcium chloride (Chemische Fabrik Kalk, Germany) were added.
Statistical analysis
Average values and standard deviations were calculated for WBC and OBC, and one-way Analysis of Variance (ANOVA) with pairwise PostHoc Test (Tukey Test) was conducted. A significance level of p < 0.05 was selected. The Grubbs outlier test with a significance level of p < 0.05 was also performed. For other values half range was calculated in case of duplicates and in case of multiples standard deviation was calculated.
Results and discussion
Screening of basidiomycete–substrate combinations
No satisfying growth was achieved with pomegranate and aronia pomace as well as with leaf spinach and beet molasses as carbon sources (Supplementary Fig. 1). All seven basidiomycetes grew well on apple pomace (Table 1). Pleurotus sajor-caju showed the highest biomass but only 14.6% protein. Pleurotus sapidus showed the second highest formation of biomass on apple pomace but the highest protein content (25.4%). Therefore, this combination was chosen for further analysis. The ability of mushrooms to grow on different substrates depends on the presence of nutrients in these substrates. Apple pomace is a versatile substrate for biotechnological applications, such as enzyme, chitosan, and aroma production as well as nutritional enrichment (Vendruscolo et al. 2008). Villas-Bôas et al. (2003) studied the solid-state fermentation of apple pomace by Candida utilis and Pleurotus ostreatus to increase the digestibility for use as ruminant feed. They found that P. ostreatus and C. utilis increased the crude protein contents. Worrall and Yang (1992) produced shiitake and oyster mushrooms on a mixture of apple pomace and sawdust. Both mushrooms grew faster and more densely when apple pomace was added. They suggested that the nitrogen level provided by the apple pomace contributed to a better growth. To calculate the protein content, different nitrogen-to-protein-conversion factors are needed due to the differences in chitin contents of the mushroom species (Crisan and Sands 1978). To compare the protein content within the study, the nitrogen content of all Pleurotus species was multiplied by a factor of 4.97 according to Mattila et al. (2002). For Lentinula edodes a factor of 4.50 (Mattila et al. 2002) was applied, and for the remaining mushrooms a factor of 4.38 was used (Crisan and Sands 1978). With Pleurotus sapidus grown on apple pomace, a crude protein content of 25.4% was obtained. Guo et al. (2007) reported 20.4% (N = 4.38) protein for fruiting bodies of P. sapidus, which is in good accordance with the results for mycelia in this study.
Analysis of the substrate
Apple pomace was analyzed for its chemical composition: true protein AAres (4.3 ± 0.0)% (Nnet = 4.15, analyzed by amino acid analysis), ash (1.5 ± 0.0)% and fat (3.0 ± 0.3)%. The total carbohydrate contents of (91.2 ± 0.1)% were calculated. The fatty acid profile was dominated by linoleic acid (51%), followed by oleic acid (30%) and palmitic acid (12%). These results are comparable to those of Ravn-Haren et al. (2018), who determined 4.6% fat and Givens and Barber (1987), who determined 6.7% proteins and 2.3% ash. The composition may vary due to stage of ripeness and the variety of apples. The amino acids (AA) analyzed by total hydrolysis (Supplementary Fig. 1) summed up to (4.98 ± 0.06) g (100 g DM)−1, with tryptophan as the first limiting amino acid.
Composition of Pleurotus sapidus mycelium grown on apple pomace
The analysis of lyophilised mycelia of P. sapidus revealed ~ 4% lipids (45% linoleic acid, 29% oleic acid, 16% palmitic acid), 2% ash and a true protein content (AAres) of 21%. Dimou et al. (2002) reported a mycelial fatty acid profile for P. sapidus grown on potato dextrose broth as follows: 14.9% C16:0, 9.6% C18:0, 21.4% C18:1 and 52.6% C18:2. Comparable profiles have been reported for other Pleurotus species (Hadar and Cohen-Azari 1986; Kavishree et al. 2008; Yilmaz et al. 2006). For a healthy diet, an intake of polyunsaturated fatty acids is essential. With 45% linoleic acid P. sapidus can contribute to a healthy and balanced diet.
The calculated carbohydrate content was 74%. Guo et al. (2007) reported a crude protein content of 20%, and 5% of both ash and fat for fruiting bodies of P. sapidus. For other Pleurotus species, crude protein contents of 10–25%, 2–4% fat and 6–8% ash have been reported (Alam et al. 2008; Crisan and Sands 1978). These values are similar to the data determined for submerged cultivated mycelia of P. sapidus in this study.
The amounts of total glucans, β- and α-glucans of P. sapidus cultivated on apple pomace were (9.2 ± 0.2)%, (5.6 ± 0.2)% and (3.6 ± 0.1)%, respectively. In apple pomace (4.4 ± 1.0)% total glucans, (3.6 ± 1.0)% β-glucans and (0.8 ± 0.0)% α-glucans were analyzed. As expected, chitin was not detected in apple pomace, whereas (6.3 ± 0.4)% chitin was determined for mycelia of PSA grown on apple pomace. The dietary fibers reported here may show beneficial health effects, such as reduction of blood cholesterol and stimulation of the immune system (Manzi and Pizzoferrato 2000). β-Glucans from Pleurotus spp. are called “pleuran” and are considered to be the most biologically effective glucans, especially with respect to their anticarcinogenic properties (Rop et al. 2009).
Amino acid profile
Based on the sum of amino acids determined after protein hydrolysis, a protein content of (24.01 ± 0.35) g (100 g DM)−1 was calculated for P. sapidus grown on apple pomace after 4 days (Table 2). AAres resulted in (20.70 ± 0.31)% for PSA ATD and (4.27 ± 0.05) for ATD resulting in Kjeldahl factors Nnet of 4.11 (PSA ATD) and 4.15 (ATD), respectively.
The amino acid profile was in good agreement with those of other Pleurotus spp. reported in the literature (Manzi et al. 1999; Table 2). Compared to the mycoprotein derived from Fusarium venenatum (Rodger 2001; Table 2) a significantly higher percentage of histidine was determined for P. sapidus, while that of tryptophan was lower. Methionine and cysteine represented the limiting amino acids in the lyophilised mycelia of P. sapidus. The total protein content of F. venenatum (48% in DM; Rodger 2001) was higher compared to the protein content of P. sapidus (25.4%).
The comparison of the sum of amino acids (AA) of apple pomace and of P. sapidus grown on apple pomace showed a significant enrichment of proteins. Apple pomace contained only about 5% amino acids, whereas the fermented pomace resulted in 24%. Fermentation of apple pomace by basidiomycetes thus delivers a vegan food rich in proteins. The biological value (86) of the fermented pomace is high and indicates a good nutritional value for humans.
Vitamin D
By irradiation of P. sapidus grown on apple pomace with UV-B light, an enrichment of vitamin D2 up to 115 µg (g DM)−1 was achieved. A maximum was observed after 20 min of irradiation (Fig. 1), which has already been found in previous studies (Ahlborn et al. 2018; Wittig et al. 2013). The vitamin D content of the final product may be adjusted by mixing with non-illuminated lyophilisate. For adults, the daily reference intake of vitamin D is 5 µg (Regulation (EU) No 1169/2011). In 2015, the Deutsche Gesellschaft für Ernaehrung (DGE) recommended a daily intake of 10 µg for infants (0–1 year) and of 20 µg for children and adults.
Quantitation of fungal biomass in lyophilisate
Using biomass dry weight as a parameter to monitor cell growth is inappropriate for cultures containing insoluble substrates and microbial biomass. In both solid-state and submerged fermentation using solids as substrates, a fermentation process might not lead to complete degradation of the substrate. Therefore, an appropriate biomarker is needed. Fungi contain the mycosterol ergosterol, located almost exclusively in the fungal cell membrane. Ergosterol has been widely used as a biomarker and indicator for fungal growth, e.g., in soil, compost, ectomycorrhizal communities and in other applications since the late 1970s (Kim et al. 2005; Klamer and Bååth 2004; Martin et al. 1990; Porep et al. 2014; Seitz et al. 1977, 1979; Zelles et al. 1987).
The growth of P. sapidus on apple pomace (Fig. 2) was monitored by measuring the ergosterol content over a period of 6 days. The fungal dry matter was calculated using mycelium cultivated on malt extract medium, a completely soluble substrate, as reference. The biomass obtained from this culture was assumed to contain 100% fungal mycelium. P. sapidus grown on malt extract medium contained (7148 ± 354) µg (g DM)−1 ergosterol (n = 6).
The experiments described above were carried out with P. sapidus mycelium grown on apple pomace harvested on the 4th culture day. At this point of time, the share of the fungal mycelium in the total biomass was about 51%. An extended cultivation period did not lead to significantly different amounts. Therefore, various attempts were made to increase the share of fungal mycelium in the lyophilisate. Reducing the amount of apple pomace in the culture medium by 50% and 75% resulted in 55% and 90% mycelium, respectively. However, this came along with decreased amounts of 7.3 g L−1 and 3.2 g L−1 lyophilisate, respectively. Finally, a fed-batch fermentation led to 89% mycelium in 5.4 g L−1 DM. To achieve this, the starting concentration of apple pomace was 5.92 g DM L−1, which was added again after 4 days and the fermentation was continued for another 2 days. The share of the protein content in the mycelium decreases slightly with increasing cultivation time. At the 2nd day of cultivation the protein share was 44% after subtracting the blank value, followed by 38%, 33%, 35% and 32% from 3rd to 6th day of cultivation. A decrease in protein content was also observed by Steuder and Bley (2015) and Ooijkaas et al. (1998).
Functional characteristics
Water and oil binding capacities
According to Stephan (2018) the average value of WBC was (6.7 ± 0.1) mL g−1 for P. sapidus grown on apple pomace, which was significantly higher than those of the reference proteins pea protein isolate (3.0 ± 0.1 mg L−1) and soy protein concentrate (4.0 ± 0.1 mg L−1). The WBC of the reference protein soy protein isolate (6.7 ± 0.2 mg L−1) was comparable to that of P. sapidus.
The OBC of P. sapidus grown on apple pomace and of the plant proteins differed significantly. Soy and pea protein isolates showed OBC of (0.9 ± 0.1) mL g−1 and (1.0 ± 0.1) mL g−1, respectively, and soy protein concentrate showed (1.0 ± 0.1) mL g−1, while the OBC of P. sapidus was (6.7 ± 0.1) mL g−1 (Stephan 2018). The high OBC seems to be mainly due to the presence of β-glucan in the fungal mycelium and the drying process applied. In a study of Petravić-Tominac et al. (2011), lyophilized β-glucan extracted from the ascomycetous yeast Saccharomyces cerevisiae revealed a 10 times higher OBC than air-dried or spray-dried β-glucan, whereas the WBC only varied marginally between the drying methods.
Influence of various salts on WBC
The influence of various chlorides on the WBC of P. sapidus grown on apple pomace was tested (Fig. 3). For the unheated system, a minor decrease of the WBC was observed after addition of MgCl2 (5.00 ± 0.39 mL g−1) compared to the control batch which contained no additives (5.31 ± 0.42 mL g−1). After heating at 80 °C for 1 h, the WBC of all samples increased. A significant difference in comparison to the control (5.78 ± 0.52 mL g−1) was detected for the use of CaCl2 (6.37 ± 0.25 mL g−1), MgCl2 (6.75 ± 0.22 mL g−1), and NaCl (7.20 ± 0.11 mL g−1). Similar increases in WBC have been reported for soy proteins (Nishinari et al. 2014), meat protein (Desmond 2006) and other plant protein systems (Ragab et al. 2004; Ahmed 2016).
Conclusions
Vegetative mycelia of mushrooms, produced by submerged cultivation on industrial side streams such as apple pomace showed beneficial nutritional properties. The substrate, apple pomace, can be valorized by fermentation with basidiomycetes and the resultant biomass appears to be suitable for use as an alternative protein source. The mycelia contain all essential amino acids and polyunsaturated fatty acids, especially linoleic acid (45%). Vitamin D2 may easily be produced from the ergosterol present in the mycelium by exposure to UV-B light. In addition, ergosterol could be used as an indicator for fungal growth in cultures containing debris from by-products of the agro industry. The water binding capacity of P. sapidus grown on apple pomace was similar to that of the reference protein soy protein isolate, whereas the oil binding capacity was significantly higher than that of vegetable proteins.
References
Aghili AH, Toghyani M, Tabeidian SA (2019) Effect of incremental levels of apple pomace and multi enzyme on performance, immune response, gut development and blood biochemical parameters of broiler chickens. Int J Recycl Org Waste Agricult. https://doi.org/10.1007/s40093-019-00305-8
Ahlborn J, Calzolari N, Spielmeyer A, Avci SS, Zimmer M, Rühl M (2018) Enrichment of vitamin D2 in mycelium from submerged cultures of the agaric mushroom Pleurotus sapidus. J Food Sci Technol 55:3833–3839. https://doi.org/10.1007/s13197-018-3290-z
Ahmed SMO (2016) Isolation of the protein concentrate from the leaves of Moringa oleifera and study of its functional properties. Dissertation, Sudan University of Science and Technology
Alam N, Amin R, Khan A, Ara I, Shim MJ, Lee MW, Lee TS (2008) Nutritional analysis of cultivated mushrooms in Bangladesh—Pleurotus ostreatus, Pleurotus sajor-caju, Pleurotus florida and Calocybe indica. Mycobiology 36:228–232. https://doi.org/10.4489/MYCO.2008.36.4.228
Alam N, Amin R, Khan A, Ara I, Shim MJ, Lee MW, Lee UY, Lee TS (2009) Comparative effects of oyster mushrooms on lipid profile, liver and kidney function in hypercholesterolemic rats. Mycobiology 37:37–42. https://doi.org/10.4489/MYCO.2009.37.1.037
Andersen G, Soyka K (2011) Lebensmitteltabelle für die Praxis, vol 5 and 9, 5th edn. Wissenschaftliche Verlagsgesellschaft, Stuttgart (Part B)
Bosse AK, Fraatz MA, Zorn H (2013) Formation of complex natural flavours by biotransformation of apple pomace with basidiomycetes. Food Chem 141:2952–2959. https://doi.org/10.1016/j.foodchem.2013.05.116Commission(Directive 98/64/EC of 3 September 1998 establishing Community methods of analysis for the determination of amino-acids, crude oils and fats, and olaquindox in feedingstuffs and amending Directive 71/393/EEC. Official Journal of the European Communities, L257/14)
Crisan EV, Sands A (1978) Nutritional value. In: Chang ST, Hayes WA (eds) The biology and cultivation of edible mushrooms. Academic Press, London, pp 137–168
Desmond E (2006) Reducing salt: a challenge for the meat industry. Meat Sci 74:188–196. https://doi.org/10.1016/j.meatsci.2006.04.014
Dimou DM, Georgala A, Komaitis M, Aggelis G (2002) Mycelial fatty acid composition of Pleurotus spp. and its application in the intrageneric differentiation. Mycol Res 106:925–929. https://doi.org/10.1017/S0953756202006184
Food and Agriculture Organization of the United Nations (FAO) (2018) http://www.fao.org/faostat/en/#data/QC. Accessed 14 Feb 2019
Givens DI, Barber WP (1987) Nutritive value of apple pomace for ruminants. Anim Feed Sci Tech 16:311–315. https://doi.org/10.1016/0377-8401(87)90020-4
Guo LQ, Lin JY, Lin JF (2007) Non-volatile components of several novel species of edible fungi in China. Food Chem 100:643–649. https://doi.org/10.1016/j.foodchem.2005.09.087
Hadar Y, Cohen-Azari E (1986) Chemical composition of the edible mushroom Pleurotus ostreatus produced by fermentation. Appl Environ Microb 51:1352–1354
Kammerer DR, Kammerer J, Valet R, Carle R (2014) Recovery of polyphenols from the by-products of plant food processing and application as valuable food ingredients. Food Res Int 65:2–12. https://doi.org/10.1016/j.foodres.2014.06.012
Kavishree S, Hemavathy J, Lokesh BR, Shashirekha MN, Rajarathnam S (2008) Fat and fatty acids of Indian edible mushrooms. Food Chem 106:597–602. https://doi.org/10.1016/j.foodchem.2007.06.018
Kim YD, Kim BK, Park HK (2005) Ergosterol content of basidiomycetes culture in rice. Int Rice Res Notes 30:43–44
Kjeldahl J (1883) Neue Methoden zur Bestimmung des Stickstoffs in organischen Körpern. Z Anal Chem 22:366–382. https://doi.org/10.1007/bf01338151
Klamer M, Bååth E (2004) Estimation of conversion factors for fungal biomass determination in compost using ergosterol and PLFA 18:2ω6,9. Soil Biol Biochem 36:57–65. https://doi.org/10.1016/j.soilbio.2003.08.019
Manzi P, Pizzoferrato L (2000) Beta-glucans in edible mushrooms. Food Chem 68:315–318. https://doi.org/10.1016/S0308-8146(99)00197-1
Manzi P, Gambelli L, Marconi S, Vivanti V, Pizzoferrato L (1999) Nutrients in edible mushrooms: an inter-species comparative study. Food Chem 65:477–482. https://doi.org/10.1016/S0308-8146(98)00212-X
Martin F, Delaruelle C, Hilbert JL (1990) An improved ergosterol assay to estimate fungal biomass in ectomycorrhizas. Mycol Res 94:1059–1064. https://doi.org/10.1016/S0953-7562(09)81333-6
Matissek R, Steiner G, Fischer M (2010) Aminosäuren, peptide, proteine, nucleinsäuren. In: Matissek R, Steiner G, Fischer M (eds) Lebensmittelanalytik, 5th edn. Springer, Berlin, pp 271–279
Mattila P, Salo-Väänänen P, Könkö K, Aro H, Jalava T (2002) Basic composition and amino acid contents of mushrooms cultivated in Finland. J Agr Food Chem 50:6419–6422. https://doi.org/10.1021/jf020608m
Miller SA, Dwyer JT (2001) Evaluating the safety and nutritional value of myco-protein. Food Technol 55:42–47
Nishinari K, Fang Y, Guo S, Phillips GO (2014) Soy proteins: a review on composition, aggregation and emulsification. Food Hydrocoll 39:301–318. https://doi.org/10.1016/j.foodhyd.2014.01.013
Ooijkaas LP, Tramper J, Buitelaar RM (1998) Biomass estimation of Coniothyrium Minitans in solid-state fermentation. Enzyme Microb Technol 22:480–486. https://doi.org/10.1016/S0141-0229(97)00246-9
Petravić-Tominac V, Zechner-Krpan V, Berković K, Galović P, Herceg Z, Srečec S, Špoljarić I (2011) Rheological properties, water-holding and oil-binding capacities of particulate β-glucans isolated from spent brewer’s yeast by three different procedures. Food Technol Biotech 49:56–64
Porep JU, Walter R, Kortekamp A, Carle R (2014) Ergosterol as an objective indicator for grape rot and fungal biomass in grapes. Food Control 37:77–84. https://doi.org/10.1016/j.foodcont.2013.09.012
Ragab DM, Babiker EE, Eltinay AH (2004) Fractionation, solubility and functional properties of cowpea (Vigna unguiculata) proteins as affected by pH and/or salt concentration. Food Chem 84:207–212. https://doi.org/10.1016/S0308-8146(03)00203-6
Ravn-Haren G, Krath BN, Markowski J, Poulsen M, Hansen M, Kotodziejczyk K, Kosmala M, Dragsted LO (2018) Apple pomace improves gut health in fisher rats independent of seed content. Food Funct 9:2931–2941. https://doi.org/10.1039/c7fo01932g
Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the provision of food information to consumers, amending Regulations (EC) No 1924/2006 and (EC) No 1925/2006 of the European Parliament and of the Council, and repealing Commission Directive 87/250/EEC, Council Directive 90/496/EEC, Commission Directive 1999/10/EC, Directive 2000/13/EC of the European Parliament and of the Council, Commission Directives 2002/67/EC and 2008/5/EC and Commission Regulation (EC) No 608/2004
Roberts JS, Teichert A, McHugh TH (2008) Vitamin D2 formation from post-harvest UV-B treatment of mushrooms (Agaricus bisporus) and retention during storage. J Agric Food Chem 56:4541–4544. https://doi.org/10.1021/jf0732511
Rodger G (2001) Mycoprotein—a meat alternative new to the US. Food Technol 55:36–41
Rop O, Mlcek J, Jurikova T (2009) Beta-glucans in higher fungi and their health effects. Nutr Rev 67:624–631. https://doi.org/10.1111/j.1753-4887.2009.00230.x
Schneider I, Kressel G, Meyer A, Krings U, Berger RG, Hahn A (2011) Lipid lowering effects of oyster mushroom (Pleurotus ostreatus) in humans. J Funct Food 3:17–24. https://doi.org/10.1016/j.jff.2010.11.004
Seitz LM, Mohr HE, Burroughs R, Sauer DB (1977) Ergosterol as an indicator of fungal invasion in grains. Cereal Chem 54:1207–1217
Seitz LM, Sauer DB, Burroughs R, Mohr HE, Hubbard JD (1979) Ergosterol as a measure of fungal growth. Phytopathology 69:1202–1203
Singh P, Kumar R, Sabapathy SN, Bawa AS (2008) Functional and edible uses of soy protein products. Compr Rev Food Sci F 7:14–28. https://doi.org/10.1111/j.1541-4337.2007.00025.x
Smith RL, Gilkerson E (1979) Quantitation of glycosaminoglycan hexosamine using 3–methyl–2–benzothiazolone hydrazone hydrochloride. Anal Biochem 98:478–480. https://doi.org/10.1016/0003-2697(79)90170-2
Stephan A (2018) Produktentwicklung von innovativen Lebensmitteln auf Basis einer alternativen Proteinquelle aus Basidiomyceten. Dissertation, Justus-Liebig-University Giessen
Stephan A, Ahlborn J, Zajul M, Zorn H (2018) Edible mushroom mycelia of Pleurotus sapidus as novel protein sources in a vegan boiled sausage analog system: functionality and sensory tests in comparison to commercial proteins and meat sausages. Eur Food Res Technol 244:913–924. https://doi.org/10.1007/s00217-017-3012-1
Steudler S, Bley T (2015) Biomass estimation during macro-scale solid-state fermentation of basidiomycetes using established and novel approaches. Bioproc Biosyst Eng 38:1313–1323. https://doi.org/10.1007/s00449-015-1372-0
Trapp T, Zajul M, Ahlborn J, Stephan A, Zorn H, Fraatz MA (2018) Submerged cultivation of Pleurotus sapidus with molasses: aroma dilution analyses by means of solid phase microextraction and stir bar sorptive extraction. J Agric Food Chem 66:2393–2402. https://doi.org/10.1021/acs.jafc.6b05292
Vendruscolo F, Albuquerque PM, Streit F, Esposito E, Ninow JL (2008) Apple pomace: a versatile substrate for biotechnological applications. Crit Rev Biotechnol 28:1–12. https://doi.org/10.1080/07388550801913840
Villas-Bôas SG, Esposito E, Mendoça MM (2003) Bioconversion of apple pomace into a nutritionally enriched substrate by Candida utilis and Pleurotus ostreatus. World J Micro Biot 19:461–467. https://doi.org/10.1023/A:1025105506004
Wittig M, Krings U, Berger RG (2013) Single-run analysis of vitamin D photoproducts in oyster mushroom (Pleurotus ostreatus) after UV-B treatment. J Food Compos Anal 31:266–274. https://doi.org/10.1016/j.jfca.2013.05.017
Worrall JJ, Yang CS (1992) Shiitake and oyster mushroom production on apple pomace and sawdust. Hort Sci 27:1131–1133
Yilmaz N, Solmaz M, Türkekul I, Elmastaş M (2006) Fatty acid composition in some wild edible mushrooms growing in the middle Black Sea region of Turkey. Food Chem 99:168–174. https://doi.org/10.1016/j.foodchem.2005.08.017
Zelles L, Hund K, Stepper K (1987) Methoden zur relativen Quantifizierung der pilzlichen Biomasse im Boden. Zeitschrift für Pflanzenernährung und Bodenkunde 150:249–252. https://doi.org/10.1002/jpln.19871500410
Acknowledgements
The authors thank the Bundesministerium für Wirtschaft und Energie for funding the ZIM-cooperation project KF3310001CS4. The study has partially been financed with funds of LOEWE—Landes-Offensive zur Entwicklung Wissenschaftlich-ökonomischer Exzellenz-AromaPlus (State Offensive for the Development of Scientific and Economic Excellence).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ahlborn, J., Stephan, A., Meckel, T. et al. Upcycling of food industry side streams by basidiomycetes for production of a vegan protein source. Int J Recycl Org Waste Agricult 8 (Suppl 1), 447–455 (2019). https://doi.org/10.1007/s40093-019-00317-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40093-019-00317-4