Abstract
Purpose
Litter decomposition is a biological process resulting from enzymatic activities of microorganisms and influenced in a variety of ways by activities of termites in semi-arid regions. We presented a general model of the decomposition process from litter to carbon sequestration and nitrogen. We aimed at building a termite population growth model which could deal with one substrate.
Methods
Our model divides the decomposition/growth process at the population level. We put these changes into equations using an analogy with chemical reactions at equilibrium.
Results
Our findings provide evidence that activities of termites can promote the significant activity of microbial decomposers and increase degradation rates of soil organic matter (SOM). Also, termite activity was probably an additional contributor to the difference between fungus-comb chamber and soil environment, in which the fungus-comb compartment was positively related to carbon and nutrients release. According to the developed, observed differences in decomposition rate, changes were strongly affected by the termite communities’ activities in the two types of compartment.
Conclusion
This functional distinction highlights the importance of termites’ activities on microbial activities stimulation through their development featuring their impacts on soil nutrient cycling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soil organic matter (SOM) governs many physical, chemical and biological characteristics of soil, and is one determinant of soil capacity for fertility and ability for carbone dioxide (CO2) sequestration (Stockmann et al. 2013; Lal 2019). SOM is generated from litter decomposition, which is determined by many factors including litter quality and soil decomposer organisms (Garcıa-Palacios et al. 2015; Bradford et al. 2017). The decomposition of plant material is carried out primarily by termites by their partial digestion and fragmentation of organic matter or dragging litter into the soil (Bender et al. 2016). After gut transit, organic matter may be either readily available or less available for decomposition (Bradford et al. 2017; Cheik et al. 2019). Hence, termites participate actively in the process of bioturbation through their influences on organic matter dynamic and despite their small size, termites can largely contribute to total soil faunal biomass (Jouquet et al. 2011).
Termites create and maintain a humid environment (microclimate) favorable for microorganisms’ activities (Jouquet et al. 2006; Ashton et al. 2019) as well as softening their food material for easy consumption (Khan et al. 2018). As a result, the temperature inside the nest is generally cooler than that of the outside environment (King et al. 2015; Ashton et al. 2019). This environment provides a favorable habitat for a diverse species like termitomyces fungi and directly affects the growth of microorganisms’ community.
In fact, after termites’ activities, the breakdown is marked by a chemical alteration of the soil organic matter by microorganisms (bacteria and fungi) which release the mineral nitrogen (N) and the carbon (C) into the soil for plants reusing. Thus, these nutrients are recycled through termites activities, microbes mineralization and plants absorption. In the process of N mineralization, the carbon part of organic matter is released through respiration which has long been considered as an index of soil metabolism (Han et al. 2018; Hammed et al. 2019; Rajput et al. 2019) and soil carbon sequestration (CO2) (Han et al. 2018).
Given the dynamic and interactive nature of termite–litter interactions, increased understanding of the role of termite traits and of the generality of litter decomposition are critical to the development of a more synthetic understanding of the termite–soil system. Very few studies have modeled the influence of termite growth on litter decomposition and few models include description of all chemical components.
In this paper, the objective is to investigate, with the aid of the model, the contribution of termites’ growth on carbon sequestration and soil productivity to answer the question of how best to model the litter decomposition responses of two different ecosystems. We, therefore, hypothesized that (i) variation in termites’ population within the two ecosystems would impact microorganisms growth and generate distinct microclimates; (ii) which in turn affect litter decomposition rate; and (iii) variation in litter decomposition rates within the two ecosystems would generate different, predicted soil C and N.
Method
We outlined the gradual decomposition of litter taking place in two different compartments of which a comparison was made between a fungus-comb chamber and a soil environment. We set out the different chemical transformation steps of litter and we make explicit equations of kinetics transformation with assumptions as basic equations of the model for the force field. We find it illustrative to describe SOM transformation by kinetic equations. After explaining the modeling framework and the formulation of the model, we simulate the biogeochemical dynamics of litter in a fungus-comb chamber and in soil environment, and evaluate the role of termite in soil productivity and soil carbon sequestration to soil recovery.
Modeling approach
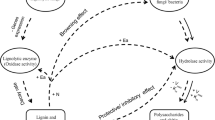
The model developed is supposed to provide an elementary demonstration of how termites’ action work when the change in the SOM increases during the process of physical and chemical transformations of the biomass (Fig. 1).
The model is performed to have two main pathways of decomposition of a given pool of organic matter representing litter (B). α pathway is characterized by microclimate from fungus-comb chamber with termites activities and 1−α which is a microclimate of a degraded soil environment (Fig. 2). Each microclimate system is a couple of ordinary differential equations describing the transfers and transformations of these pools due to growth, maintenance, activation, deactivation and death, according to the conceptual diagram.
The common deSolve package in R version 3.4.3 (R Development Core Team 2018) is implemented to solve differential equations.
Results and discussion
Microbial dynamics
With this model, we assumed that litter incorporation (l) is pushed by termites. Once litter is in one of the compartments, microbial population could deal with the substrate. We assumed that decomposition of substrate is controlled by bacterial (b) communities’ growth, hence:
We studied two possibilities: the first is bacteria have gathered one substrate to form a composite. The second possibility is the use of the complexed substrate to produce new biomass.
A portion of the complex (s) is absorbed across the bacteria and used in active metabolism and growth of the bacteria community. The study (Neill and Gignoux 2006) provides an application in the same context, in which they assumed that the model gives biomass increments through:
We assume that x = b when biomass has complexed enough substrate and it is exponentially growing at specific rate \(\mu\). In reverse, if all the substrate is being complexed by microbial, then x = (s/ν), with ν the stoichiometry coefficient. This substrate is ultimately consumed by microbial. In this case, we have:
The equation is considered to have a certain dependence on b and s; knowing them, it can be expressed like in (Neill and Gignoux 2006) as:
with k an affinity of a given termites population for a given litter. The complex (x) will be different, accordingly it is used in the fungus-comb chamber or in the soil environment.
In the fungus-comb chamber: \(b \gg (l/\nu )\), biomass is not limiting but it is the litter intake that limits decomposition, the Eq. (3) leads to x = (Tl)/((1/k) + \(\nu T\)).
In the soil, (l/\(\nu\)) ≫ T, biomass is limiting, the Eq. (3) leads to x = (Tl)/((1/k) +l). Then, we can express Eq. (1) as:
As hypothesized, when we run, we have a rapid substantial growth of microorganisms during the first 1 month in the fungus-comb chamber compared to the soil environment (Fig. 3). The pic rates of microbial growth have been obtained 6 months later in the fungus-comb chamber. But in the soil, the pic has been attended after 10 months.
Fungus-comb chambers are known to promote the growth of a selected and possibly specialized community of commensal bacteria and fungi (Artursson et al. 2006; Fallah et al. 2017; Vesala et al. 2017) which can in turn influence the decomposition of litter. In the fungus-comb chamber, the decomposition was enhanced by 26.8%, which accounted for 81.7% of the overall process. In contrast to soil, the microbial growth took more time than in the fungus-comb chamber. The microclimate created in the fungus-comb chamber may explain the observed differences in the decomposition rate between the two environments. Thus, changes in decomposition rates are linked to the climate. These findings suggest that a faster decay may be a characteristic of microorganisms’ growth. This result is in agreement with a detailed analysis by Poulsen et al. (2014) on the decomposition of leaves with a fungal community. Another explanation is that fungi are considered the most active decomposers of complex plant biopolymers because of their ability to produce a wide range of extracellular enzymes, allowing them to efficiently break down the recalcitrant lignocelluloses (Wietse et al. 2005; Frouz 2018). In addition, the specific activities of the microbial populations make termite excellent model systems for studying functional interactions within organized microbial communities.
Litter dynamics
To move beyond microbial compartments, the physiological features of individual populations (growth rate and respiration) need to be linked to the food for which they compete. Then, for a microbial community B with maintenance, some of the carbon in the complexed substrate must be allocated to maintenance charges. Let us designate \(y_{c}\) as the carbon yields of microbial population B with regard to litter l, \(m_{x}\) and \(m_{t}\) which are, respectively, the maintenance coefficient and the turnover rate coefficient of biomass.
The overall rate of change in active litter in the fungus-comb chamber and in the soil is given by:
However, to get better point of this, we would like to refer the interested reader more accurately to the Neill and Gignoux’s (2006) paper. Values used are secondary data from the review of literature: (Neill and Gignoux 2006; Dorado et al. 2008), who substituting substrate kinetics for microbial kinetics, which produced a model behavior, very similar to this study.
Microbial populations growing on similar insoluble substrates at different temperatures should display similar different specific growth rates (\(\mu\)). Generally, decomposition models assign to any substrate at a maximum specific decay rate. In the fungus-comb chamber, the decay is at a maximum rate (kmax); while it is at a minimum rate in the soil (Fig. 4).
To determine how microorganism varies in the two compounds, data from continuous cultures of aerobic and anaerobic strains on cellulose was examined from the study of Lynd et al. (2002).
We consider that the most fundamental process is maintenance of the active microbial biomass, which means that the most important requirement of microbes is their nutrients source. As a result, decomposition is primarily driven by carbon accessibility and secondarily by nutrient availability. The secondary limitation of decomposition is soil microclimate (humidity and temperature) (Moncrieff et al. 2013; Iriany et al. 2018). Figure 4 revealed highly rapid rates of decomposition in the fungus-comb chamber and very low decomposition rates in the degraded soil.
Effects of temperature and moisture on decomposition
When we integrated the difference of microclimate in the two compartments, the potential decomposition equation for a single substrate is:
where \(k_{ \hbox{max} }\) is the constant rate applied to the decomposition of SOM, \(T_{S}\) and \(w_{s}\) are functions describing the effects of temperature and moisture on decomposition rate.
The effect of temperature, \(T_{s}\), is a dimensionless number between 0 and 1 and calculated according to the following functional form \(T_{s} = \frac{{T - T_{ \hbox{min} } }}{{T_{ \hbox{max} } - T_{ \hbox{min} } }}\) of Parton et al. (1987).
We derived the soil moisture equation from a comparison between the sensitivity of fungal and bacterial communities to soil moisture variations in Kaisermann et al. (2015). \(W_{s}\) is also calculated according to Parton et al. (1987):
Let \(k_{s}\) be the rate in the soil compartment. In the fungus-comb chamber, it is higher because of the microclimate (soil temperature remains low and high moisture) created by termites, contrary to \(k_{s}\) the degraded soil compound decomposition rate (Table 1).
Soil carbon and nitrogen predictions
Predicted carbon rate
Assume that biomass pool grows by , loses \(D_{b}\) by dilution and also turns over by \(m_{t} b\). Then, we have the following equation:\({\text{d}}b/{\text{d}}t = I - \left( {m_{t} + D} \right)*b\). From this equation, we know that the maximum attainable dilution rate Dmax equals the maximum specific growth rate minus its turnover rate, mt. Let us assume that total carbon consumption flux is equal to the sum of the growth of related carbon consumption flux and the maintenance respiration. Then, we have the following equation:
We assumed those fluxes would determine all the other fluxes through stoichiometric relationships. Dividing by μx the equation by μx and we assumed that the achieved specific growth rate D + mt, equals μxb at the steady state. It gives:
where \(y_{c}\) is the carbon yield.
The amount of soil carbon at steady state (Css) is described by Eq. 12:
In the degraded sites, litter was estimated to be decomposed 2–10 times more slowly than in the fungus-comb chamber (Fig. 5). The decomposition of litter was not related to soil fertility; whereas, it was related to the existence of fungus-comb chamber.
There was a significant correlation between predicted soil carbon and growth of microorganisms. This indicated that the predicted carbon was relatively sensitive to termites’ growth. Poulsen et al. (2014) tested the potential of macro-fauna to be used as a soil fertility index. Moreover, indices or species of soil fauna used for indicating changes in soil fertility offer a promising means for scientists to gauge the effectiveness of promoting termites growth and allow for a better informed response in addressing new issues as they arise (Moorhead and Sinsabaugh 2006).
Predicted nitrogen stock
The nitrogen stock is fed by an influx \(Dn_{0}\) and depleted by both a dilution efflux and a growth consumption flux, \(D_{n}\) and \(I/4y_{n}\), respectively. Part of the nitrogen consumption flux goes into the nitrogen waste pool, and it amounts to ((1 − \(y_{n}\))/4 \(y_{n}\)).I. Nitrogen waste stocks are depleted by a dilution efflux D\(w_{n}\) as well. Finally, the biomass pool grows by I, loses \(D_{b}\) by dilution and also turns over by \(m_{t} b\). We retained the following equation:
After 4 months of mulching, the rate of N is null, similar in the degraded soil compartment as in the fungus-comb chamber but increased significantly (12000 g cm−1) in the fungus-comb chamber at months 5–10 (Fig. 6); while it is still null in the degraded soil.
Predicted nitrogen significantly correlated with microorganisms, regardless of time period. The observed differences in climate would further accentuate these differences. Termite activity was probably an additional contributor to the gap between the fungus-comb chamber and soil environment. Several studies have shown significant enhancement of microbial biomass by termites, while others have found the opposite effect (Adair et al. 2008; Jouquet et al. 2018). This implies that termites stimulate the relatively inactive microbial communities and accelerate soil N recycling (Frouz 2018).
The functions and direct impacts of termites’ growth are more important in organic material decomposition (Jouquet et al. 2014; Dangles and Casas 2019). Thus, to evaluate integrated changes in carbon sequestration and soil fertility, termites should be used combined with microorganisms.
Conclusion
We were able to describe long-term rates of decomposition in a wide range of ecosystems and climates using a relatively simple model based on impacts of termites’ growth. Our analysis suggests that observed differences in decomposition rate changes were strongly affected by the termite communities’ activities, the microclimate and the microbial growth. This functional distinction highlights their importance on microbial activities stimulation through their development featuring their impacts on soil carbon sequestration and nitrogen recycling.
Here, we demonstrated that the inoculation of termite in a restoration context might provide very fruitful opportunities to study their influences on belowground fauna and also consequences for ecosystem services such as crop yield. The model framework may still be useful for modeling efforts, because it accurately described general patterns of long-term decomposition for a wide array of litter.
References
Adair EC, Parton WJ, Del Grosso SJ, Silver WL, Harmon ME, Hall SA, Burke IC, Hart SC (2008) Simple three-pool model accurately describes patterns of long-term litter decomposition in diverse climates. Glob Change Biol 14:2636–2660. https://doi.org/10.1111/j.1365-2486.2008.01674.x
Artursson V, Finlay RD, Jansson JK (2006) Interactions between arbuscular mycorrhizal fungi and bacteria and their potential for stimulating plant growth. Environ Microbiol. https://doi.org/10.1111/j.1462-2920.2005.00942.x
Ashton LA, Griffiths HM, Parr CL, Evans TA, Didham RK, Hasan F, Teh YA, Tin HS, Vairappan CS, Eggleton P (2019) Termites mitigate the effects of drought in tropical rainforest. Science 177:174–177. https://doi.org/10.1126/science.aau9565
Bender SF, Wagg C, van der Heijden MGA (2016) An underground revolution: biodiversity and soil ecological engineering for agricultural sustainability. Trends Ecol Evol 31:440–452. https://doi.org/10.1016/j.tree.2016.02.016
Bradford MA, Veen GFC, Bonis A, Bradford EM, Classen AT, Cornelissen JHC, Crowther TW, De Long JR, Freschet GT, Kardol P, Manrubia-Freixa M, Maynard DS, Newman GS, Logtestijn RSP, Viketoft M, Wardle DA, Wieder WR, Wood SA, van der Putten WH (2017) A test of the hierarchical model of litter decomposition. Nat Ecol Evol. https://doi.org/10.1038/s41559-017-0367-4
Cheik S, Shanbhag R, Harit A, Bottinelli N, Sukumar R, Jouquet P (2019) Linking termite feeding preferences and soil physical functioning in Southern-Indian woodlands. Insects 10:4. https://doi.org/10.3390/insects10010004
Dangles O, Casas J (2019) Ecosystem services provided by insects for achieving sustainable development goals. Ecosyst Serv 35:109–115. https://doi.org/10.1016/j.ecoser.2018.12.002
de Boer Wietse, Richard LB, Summerbell LB (2005) Living in a fungal world: impact of fungi on soil bacterial niche development. Microbiol Rev 29:795–811. https://doi.org/10.1016/j.femsre.2004.11.005
Dorado AD, Baquerizo G, Maestre JP, Gamisans X, Gabriel D, Lafuente J (2008) Modeling of a bacterial and fungal biofilter applied to toluene abatement: kinetic parameters estimation and model validation. Chem Eng J 140:52–61. https://doi.org/10.1016/j.cej.2007.09.004
Fallah M, Farzam M, Hosseini V, Moravej G, Eldridge DJ (2017) Termite effects on soils and plants are generally consistent along a gradient in livestock grazing. Arid Land Res Manag. https://doi.org/10.1080/15324982.2017.1288177
Frouz J (2018) Effects of soil macro- and mesofauna on litter decomposition and soil organic matter stabilization. Geoderma 332:161–172. https://doi.org/10.1016/j.geoderma.2017.08.039
Garcıa-Palacios P, McKie BG, Handa IT, Frainer A, Hattenschwiler S (2015) The importance of litter traits and decomposers forlitter decomposition: a comparison of aquatic andterrestrial ecosystems within and across biomes. Funct Ecol. https://doi.org/10.1111/1365-2435.12589
Hammed TB, Oloruntoba EO, Ana GREE (2019) Enhancing growth and yield of crops with nutrient-enriched organic fertilizer at wet and dry seasons in ensuring climate-smart agriculture. Int J Recycl Org Waste Agric. https://doi.org/10.1007/s40093-019-0274-6
Han CL, Gu YJ, Kong M, Hu LWK, Yu H, Li FM, Sun GJ, Siddique KHM (2018) Responses of soil microorganisms, carbon and nitrogen to freeze–thaw cycles in diverse land-use types. Appl Soil Ecol 124:211–217. https://doi.org/10.1016/j.apsoil.2017.11.012
Iriany A, Chanan M, Djoyowasito G (2018) Organic mulch sheet formulation as an effort to help plants adapt to climate change. Int J Recycl Org Waste Agric 7:41–47. https://doi.org/10.1007/s40093-017-0189-z
Jouquet P, Dauber J, Lagerlöf J, Lavelle P, Lepage M (2006) Soil invertebrates as ecosystem engineers: Intended and accidental effects on soil and feedback loops. Appl Soil Ecol 32:153–164. https://doi.org/10.1016/j.apsoil.2005.07.004
Jouquet P, Traoré S, Choosai C, Hartmann C, Bignell D (2011) Influence of termites on ecosystem functioning. Ecosystem services provided by termites. Eur J Soil Biol 47:215–222. https://doi.org/10.1016/j.ejsobi.2011.05.005
Jouquet P, Blanchart E, Capowiez Y (2014) Utilization of earthworms and termites for the restoration of ecosystem functioning. Appl Soil Ecol 73:34–40. https://doi.org/10.1016/j.apsoil.2013.08.004
Jouquet P, Chaudhary E, Kumar ARV (2018) Sustainable use of termite activity in agro-ecosystems with reference to earthworms. A review. Agron Sustain Dev. https://doi.org/10.1007/s13593-017-0483-1
Kaisermann A, Maron PA, Beaumelle L, Lata JC (2015) Fungal communities are more sensitive indicators to non-extreme soil moisture variations than bacterial communities. Appl Soil Ecol 86:158–164. https://doi.org/10.1016/j.apsoil.2014.10.009
Khan MA, Ahmad W, Paul B (2018) Ecological impacts of termites. In: Khan M, Ahmad W (eds) Termites and sustainable management. Sustainability in plant and crop protection, vol 2. Springer, Cham, Germany, pp 47–68
King H, Ocko S, Mahadevan L (2015) Termite mounds harness diurnal temperature oscillations for ventilation. Proc Natl Acad Sci USA 112:11589–11593. https://doi.org/10.1073/pnas.1423242112
Lal R (2019) Eco-intensification through soil carbon sequestration: Harnessing ecosystem services and advancing sustainable development goals. J Soil Water Conserv 74:55A–61A. https://doi.org/10.2489/jswc.74.3.55A
Lynd LR, Weimer PJ, Willem H, van Zyl ISP (2002) Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev. https://doi.org/10.1128/mmbr.66.3.506-577.2002
Moncrieff GR, Scheiter S, Bond WJ, Higgins SI (2013) Increasing atmospheric CO 2 overrides the historical legacy of multiple stable biome states in Africa. New Phytol. https://doi.org/10.1111/nph.12551
Moorhead DL, Sinsabaugh RL (2006) A Theoretical model of litter decay and mirobial interaction. Ecol monogr 76(2):151–174. https://www.jstor.org/stable/27646035
Neill C, Gignoux J (2006) Soil organic matter decomposition driven by microbial growth: A simple model for a complex network of interactions. Soil Biol Biochem 38:803–811. https://doi.org/10.1016/j.soilbio.2005.07.007
Parton WJ, Schimel DS, Cole CV, Ojima DS (1987) Analysis of factors controlling soil organic matter levels in great plains grasslands. Soil Sci Soc Am J 51:1173–1179. https://doi.org/10.2136/sssaj1987.03615995005100050015x
Poulsen M, Hu H, Li C, Chen Z, Xu L, Otani S, Nygaard S, Nobre T, Klaubauf S, Schindler PM, Hauser F, Pan H, Yang Z, Sonnenberg ASM, de Beer ZW, Zhang Y, Wingfieldi MJ, Grimmelikhuijzen CJP, de Vries RP, Korb J, Aanen DK, Wang J, Boomsma JJ, Zhang G (2014) Complementary symbiont contributions to plant decomposition in a fungus-farming termite. PNAS 111:7. https://doi.org/10.1073/pnas.1319718111
Rajput R, Pokhriya P, Panwar P, Arunachalam A, Arunachalam K (2019) Soil nutrients, microbial biomass, and crop response to organic amendments in rice cropping system in the Shiwaliks of Indian Himalayas. Int J Recycl Org Waste Agric 8:73–85. https://doi.org/10.1007/s40093-018-0230-x
Stockmann U, Adams MA, Crawford JW, Field DJ, Henakaarchchi N, Jenkins M, Minasny B, McBratney AB, de Remy Vivien, de Courcelles Singh K, Wheeler I, Abbott L, Angers DA, Baldock J, Bird M, Brookes PC, Chenu C, Jastrow JD, Lal R, Lehmann J, O’Donnell AG, Parton WJ, Whitehead D, Zimmermann M (2013) The knowns, known unknowns and unknowns of sequestration of soil organic carbon. Agric Ecosyst Environ 164:80–99. https://doi.org/10.1016/j.agee.2012.10.001
Team RC (2018) A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Vesala R, Niskanen T, Liimatainen K, Boga H, Pellikka P, Rikkinen J (2017) Diversity of fungus-growing termites (Macrotermes) and their fungal symbionts (Termitomyces) in the semiarid Tsavo Ecosystem, Kenya. Biotropica. https://doi.org/10.1111/btp.12422
Acknowledgements
Financial support for this research was provided via WASCAL (West African Science service center on Climate change and Adapted Land use). The work would not have been possible without the help of Jacques Gignoux.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest associated with this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Issoufou, A.a., Soumana, I., Maman, G. et al. Effects of termites growth on litter decomposition: a modeling approach. Int J Recycl Org Waste Agricult 8 (Suppl 1), 415–421 (2019). https://doi.org/10.1007/s40093-019-00314-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40093-019-00314-7