Abstract
Purpose
In the industrialized production of mushrooms usually only one flush of fruitbody is harvested, so that nutrients and energy in the substrate is not fully exploited. In this study, the spent Pleurotus eryngii substrate was recycled for the cultivation of Agrocybe chaxingu under ambient temperature.
Method
Six formulae were tested: (1) Control: 98% spent substrate, 1% sucrose, 1% lime; (2) Control + 10% wheat bran; (3) Control + 20% wheat bran; (4) Control + 10% T. molitor feces; (5) Control + 20% T. molitor feces; (6) Control + 10% wheat bran + 10% T. molitor feces.
Results
Two flushes of fruitbody were harvested, the control substrate resulted in a biological efficiency of 40.42%; the formulae with supplementation of 10% wheat bran, 20% wheat bran and 10% T. molitor feces significantly increased biological efficiency to 52.50, 54.61 and 51.56%, respectively, and supplementation of 20% T. molitor feces, or 10% wheat bran plus 10% feces further significantly increased biological efficiency to 62.95 and 61.10%, respectively. All supplemented substrates had significantly higher cellulose and laccase activity than the Control (cellulase 0.10 U/g; laccase 41.00 U/g), which were 10% wheat bran (0.15 U/g; 72.67 U/g), 10% T. molitor feces (0.17 U/g; 98.33 U/g), 20% wheat bran (0.22 U/g; 76.00 U/g), 20% T. molitor feces (0.27 U/g; 87.00 U/g), 10% wheat bran plus 10% T. molitor feces (0.25 U/g; 97.67 U/g), respectively.
Conclusion
Spent Pleurotus eryngii substrate was promising for cultivation of Agrocybe chaxingu, especially when supplemented with 20% T. molitor feces, or with 10% T. molitor feces plus 10% wheat bran.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
In the industrialized production of low temperature fruiting type mushrooms like Pleurotus eryngi and Flammulina velutipes, usually only one flush of fruitbodies is harvested (biological efficiency 60–65%) because the biological efficiency of the successive flushes are not high enough (approximately 30%) to make a profit where facilities and air cooling systems are expensive. Currently, most of the spent substrate is burnt to generate steam for substrate sterilization and heating of mushroom farms, some spent substrate is used as organic fertilizers in orchards. Nutrients and energy in the substrate is not fully exploited, as evidenced by over 100% total biological efficiency in non-industrialized mushroom production under natural environmental conditions where 3–4 flushes were harvested (Philippoussis et al. 2001; Mandeel et al. 2005). In recent years, many experiments have been conducted to recycle the spent substrate for cultivation of other mushrooms (usually high temperature fruiting types) (Royse 1992; Li 2013).
Agrocybe chaxingu (in some previous cases mistakenly termed A. cylindracea or A. aegerita) (Callac et al. 2011) is a popular mushroom with a sweet aroma and many medicinal benefits. It is an antioxidant (Choi et al. 2009), possessing properties that aid the curing of cancers (Hyun et al. 1996), diabetes (Lee et al. 2010), etc. With a view to recycle spent P. eryngii substrate for cultivation of other mushrooms in a low cost way, in the present study, A. chaxingu was chosen because it is a moderate temperature fruiting type mushroom suitable for cultivation at a broad range of ambient temperatures for a period from late spring to autumn in south China.
To formulate recycled substrate for A. chaxingu cultivation, the nutrient composition of spent P. erygnii substrate was analyzed and compared with the unused substrate. Tenebrio molitor rearing has expanded rapidly in China, mainly as animal and pet feed, and to a lesser degree for human consumption. The sand-like feces of T. molitor larvae contain digested fiber, crude protein (14–18%), crude lipid (15–18%) and minerals (Wang et al. 2012; Lee and Rho 2014), therefore, T. molitor feces could be an ideal ingredient used for mushroom cultivation, but very few such studies were reported (Gan et al. 2008). Currently T. molitor feces is used mainly as garden fertilizer or livestock feed.
The purpose of the present study was to test if the spent substrate of P. eryngii in the industrialized production setting was a good substrate for production of A. chaxingu; and the effect of supplementing 10–20% T. molitor feces or/and wheat bran in improving fruiting body yield.
Material and methods
A. chaxingu and spent P. eryngii substrate
The experimental A. chaxingu strain was purchased from Xue Shan Er Precious Edible Mushroom Institute, Gutian county, Fujian province. Spent P. eryngii substrate was provided by Guangdong Lantian Agricultural Co., Ltd., Fengshun county, Guangdong province. The formula of the unused substrate for P. eryngii production was: 50% sugarcane bagasse, 20% cottonseed hulls, 20% wheat bran, 5% cornmeal, 3% soymeal, 1% lime, 1% gypsum. T. molitor feces was purchased via Taobao.com from Hong Chang Feed Rearing Farm in Binzhou city, Shandong province. Wheat bran and other ingredients and materials were purchased from a local market.
Determination of composition of P. eryngii substrate
Total carbon content of the spent and unused P. eryngii substrate was determined by potassium dichromate method (ISO 14235 1998). Total nitrogen content was determined by the Kjeldahl method (Bremner and Breitenbeck 1983). Soluble sugar content was determined by the anthrone–sulfuric acid method (Spiro 1966). Starch content was determined with the method described by Holm et al. (1986). Cellulose, hemicellulose, lignin and ash content were determined according to Goering and Van Soest (1970).
Agrocybe chaxingu cultivation experiment
Preparation of substrate
Mushroom cultivation was carried out in a chamber in our laboratory. The following 6 formulae were adopted, representing without or with supplementation of 10–20% T. molitor feces or/and wheat bran. For each formula 30 bags were inoculated.
-
1.
98% Spent substrate, 1% sucrose, 1% lime.
-
2.
88% Spent substrate, 1% sucrose, 1% lime,10% wheat bran.
-
3.
78% spent substrate, 1% sucrose, 1% lime, 20% wheat bran.
-
4.
88% Spent substrate, 1% sucrose, 1% lime, 10% T. molitor feces.
-
5.
78% Spent substrate, 1% sucrose, 1% lime, 20% T. molitor feces.
-
6.
78% Spent substrate, 1% sucrose, 1% lime, 10% wheat bran,10% T. molitor feces.
The spent P. eryngii substrate was fragmented by hand and sun dried for use, and after mixing thoroughly with other ingredients, the 1% sucrose was solved in required water to make sure the wet substrate contain 65% water. Then the wet substrate was filled into 33 × 17 cm HDPE plastic bags with each bag containing 857 g (equals to 300 g dry substrate). The substrate was pressed to a compactness so that the bag side was slightly tensioned to leave no free space for primordial occurrence during cropping stage. The bags were not fully filled so that an empty space was left in the bag to maintain moisture during cropping. After sealing with neck rings and cotton-free (sponge) caps, the bags of substrate were autoclaved (Tomy SS325) at 121 °C for 2 h.
Inoculation and mycelial culture
As the temperature in the autoclave dropped to 60–70 °C, the bags of substrate were moved to a biosafety cabinet for further cooling and UV light sterilization of surface microorganisms (2–3 h). When the substrate was cooled thoroughly each bag was inoculated with about 20 g solid spawn previously cultured in bags with spent P. eryngii substrate. Then the mycelium bags were cultured in the dark (with a closed curtain) at room temperature (18–23 °C), with air relative humidity maintained at 65–70% by occasionally adding moisture with a spray humidifier.
Cropping management
As most of the bags of all six formulae were fully colonized (upon spawn run completion), after 10 additional days of mycelial growth, the neck rings and caps were removed from the bags, and natural light was supplied to induce primordial formation. At the same time the air relative humidity was maintained at 80–90% with a humidifier.
Determination of mycelial cellulase and laccase activity during spawn run
Five samples were taken of each formula for enzyme determination upon completion of substrate colonization. Crude enzyme was extracted by placing 2.0 g fresh mycelial culture into a 250 mL flask added with 20 mL 0.1 mol/L citrate buffer (pH 5.0) which was shaken in a rotary shaker at 28 °C, 200 rpm for 2 h. The extracted solution together with substrate was centrifuged at 4000 rpm for 10 min and the supernatant was used as crude enzyme for activity assay.
The carboxymethyl cellulase (CMCase) activity assay followed the method of Ghose (1987). 1 unit CMCase activity was defined as the enzyme amount required to transfer substrate into 1 μmol glucose and expressed in U/g fresh mycelial culture. Laccase activity was measured by following the method described by Heinzkill et al. (1998), with modification of doubling both the sample volume and reagent volume to suit a 1 cm cuvette. Laccase activity was expressed as U/g substrate (fresh weight), where 1 U was defined as 1 μmol of substrate oxidized per min.
Spawn run period, fruiting body yield and biological efficiency
The spawn run period (the number of days from inoculation to colonization completion of the substrate by the mycelium) was recorded. Two flushes of fruiting bodies were harvested. The fresh weights of fruiting body were recorded and biological efficiency (BE %) was calculated by dividing fresh weight of fruiting body by dry substrate weight per bag.
Data statistical analysis
Original data were processed using EXCEL (Microsoft, WA, USA) and Scheffe’s tests were performed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA).
Results and discussion
Composition of unused and spent P. eryngii substrate
As indicated in Table 1, the content of total carbon, total nitrogen and C/N ratio in the spent P. eryngii substrate was merely slightly reduced as compared with unused substrate. The more easily digestible ingredients (soluble sugar, starch and hemicellulose) are significantly reduced to 40.14, 11.14 and 54.28% of the unused substrate values. The cellulose content in the spent substrate was 91.55% that of the unused substrate, which was slightly reduced, and lignin in the spent substrate was 81.88% that of the unused substrate, reducing to a larger degree than cellulose. The ash in the spent substrate was more than twice that of the unused substrate, reflecting the dry matter loss through respiration by P. eryngii. The data indicated that to obtain good fruitbody yield the spent substrate should be replenished with the easily digestible ingredients like soluble sugar, starch and hemicellulose, but extra nitrogen source (e.g., soymeal), lignocellulosic ingredients were not required to be supplemented, hence wheat bran, cornmeal and T. molitor feces could satisfy this purpose. In fact, too much nitrogen in the substrate can lead to ammonia accumulation during storage or preparation which inhibited mycelial growth (Choi 2004; Mohamed et al. 2016).
Mycelial cellulase and laccase activity and spawn run period
During vegetative growth edible fungi produce a wide range of extracellular enzymes to degrade the lignocellulosic substrates, including cellulase, laccases, peroxidases, xylanase, protease, etc. (Magnelli and Forchiassin 1999). In the present study, the mycelial cellulase and laccase activity at 40 day of spawn run (upon colonization of substrate) were determined to reveal possible associations of substrate degradation rate with substrate formulae (Table 2). Mn peroxidase was not determined in this study because it displayed a similar pattern to laccase (Zeng et al. 2013).
As shown in Table 2, the cellulase activity in mycelia of Formulae 2 and 4 were not significantly different from that in Formula 1, but Formulae 3, 5 and 6 had significantly higher cellulase activity than Formula 1, which indicated that both the inclusion of wheat bran and T. molitor feces significantly enhanced cellulase activity as the inclusion rate reached 20% (either separately or combined), and supplementation of 20% feces (Formula 5) demonstrated the highest value.
All supplemented formulae demonstrated significantly higher laccase activity than the Control, indicating that either supplementation of wheat bran or T. molitor feces could stimulate laccase excretion. And Formulae 4 (10% feces) and 6 (10% feces + 10% bran) had significantly higher laccase activity than Formulae 2 and 3 (10 and 20% bran), but not higher than Formula 5 (20% feces), indicating T. molitor feces was superior to wheat bran in stimulating laccase activity in Ganoderma. According to substrate inducing theory, such enzyme activity enhancing effect of T. molitor feces might be due to the presence of digested products of cellulose, chitin and other polymeric compounds.
The spawn run period reflects how the substrate suits the mycelial growth, in the present study the spawn run periods with all the 6 formulae of substrate were over 40 days as the mycelia were cultured under natural temperatures (18–23 °C). It can be seen from Table 3 that the spawn run period of Chaxingu was the shortest (40 days) in the substrate of Formulae 1 and 4, supplementation of 10–20% (Formulae 2, 3) wheat bran significantly extended the spawn run period by about 3 days as compared with Formula 1, and supplementation of 20% T. molitor feces or 10% wheat bran plus 10% feces (Formulae 5, 6) significantly extended the spawn run period by about 4 days; The extended spawn run period by supplementation of wheat bran or/and feces might be due to two reasons: (1) both wheat bran and T. molitor feces were not elastic as was sugarcane bagasse and cottonseed hulls, thus reducing the porosity of supplemented substrates with poorer oxygen transmission within the substrate; (2) supplementation of the ingredients led the mycelia to allocate more resources to excrete lignocellulosic enzymes (as evidenced by data in Table 2) and other enzymes such as protease, which facilitated final degradation of substrate but slowed vegetative mycelial growth in the first stage.
Fruitbody yield and biological efficiency
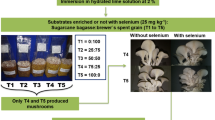
Primordia emerged successively in bags of all formulae of substrate one week after applying cropping management (as previously described measures, including cap removing, curtain opening and addition of air humidity) except seemingly slightly later on Formula 6. Due to the limited number (n = 30) of bags for each formula it was difficult to collect synchronical fruitbodies at a time, the separately collected representative fruiting bodies of each formula were shown in Fig. 1. The fruitbody yield and biological efficiency of each formula are shown in Table 3.
It can be seen from Table 3 that in the first flush, all the supplemented formulae had significant higher fruitbody yields than Formula 1, and no significant differences existed among the supplemented formulae. In the second flush the fruitbody yield of Formulae 3, 5 and 6 were significantly higher than Formulae 2 and 4 as well as Formula 1, indicating that as compared with the 10% supplementation level, supplementing 20% wheat bran or/and T. molitor feces could increase yield of successive flushes, and therefore, the total yield.
By looking at the total fruitbody yield of the six Formulae, it can be seen that the spent P. eryngii substrate supplemented with only 1% sucrose and 1% lime (Formula 1) could yield 404.17 g/kg fruitbody for 2 flushes, namely achieving a biological efficiency of 40.42%, which was satisfactorily obtained from a farm waste (Jeznabadi et al. 2016). Supplementation of 10–20% wheat bran (Formulae 2, 3) or 10% feces (Formula 4) significantly increased total fruitbody yield, so that the biological efficiency was raised to over 51.56%. Supplementation of 20% (Formula 5) or 10% feces plus 10% wheat bran (Formula 6) resulted in the highest biological efficiency of over 61.10% which was not only significantly higher than Formula 1 but also higher than the 10–20% wheat bran supplementation formula and the 10% feces supplementation formula. Therefore, T. molitor feces was superior to wheat bran to supplement the spent P. eryngii substrate for cultivation of A. chaxingu. It was shown previously that the nutritive value of T. molitor was superior to livestock meat (Rumpold and Schlüter 2013) and insects including T. molitor are far more efficient in transforming plant biomass into animal biomass than conventional livestock (Nakagaki and DeFoliart 1991).
Conclusion
Spent Pleurotus eryngii substrate was promising for cultivation of Agrocybe chaxingu under ambient temperatures. On the recycled substrate supplemented with only 1% sugar and 1% lime a biological efficiency of 40.42% was achieved. The formulae with an additional supplementation of 10% wheat bran or 10% Tenebrio molitor raised the biological efficiency to 52.50 and 51.56%, respectively. The formulae with an additional supplementation of 20% T. molitor feces or 10% T. molitor feces plus 10% wheat bran demonstrated the highest biological efficiency of 62.95 and 61.10%, respectively, significantly higher than that of the formula with supplementation of 20% wheat bran, therefore, T. molitor feces was an excellent supplement that was superior to wheat bran for Agrocybe chaxingu cultivation.
References
Bremner JM, Breitenbeck GA (1983) A simple method for determination of ammonium in semimicro-Kjeldahl analysis of soils and plant materials using a block digester. Commun Soil Sci PlantAnal 14:905–913. doi:10.1080/00103628309367418
Callac P, Guinberteau J, Ferandon C et al (2011) An Asian commercial strain of Agrocybe chaxingu and a European wild strain of Agrocybe cylindracea exhibiting morphological difference and high genetic divergence are interfertile. In: Proceedings of the Seventh International Conference on mushroom biology and mushroom products ICMBMP7, vol 1, pp 113–122
Choi KW (2004) Shelf cultivation of oyster mushroom with emphasis on substrate fermentation. In: Gush Rick (ed) Mushroom growers handbook. Handbook 1. Part II oyster mushroom. Aloha Medicinals Inc., Hawaii
Choi JH, Ogawa A, Abe N (2009) Chaxines B, C, D, and E from the edible mushroom Agrocybe chaxingu. Tetrahedron 30:1–4. doi:10.1016/j.tet.2009.09.064
Gan YK, Chen XJ, Su L et al (2008) Efects of aquatic extraction substance from Tenebrio molitor Linnaeus Feces on Mycelium growth of five edible fungi. J Anhui Agric Sci 36(26):11295–11296. doi:10.3969/j.issn.0517-6611.2008.26.064 (1132)
Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem 59(2):257–268
Goering HK, Van Soest PJ (1970) Forage fiber analysis (apparatus, reagents, procedures and some applications), agriculture handbook no. 379. ARS-USDA, Washington
Heinzkill M, Bech L, Halkier T, Schneider P, Anke T (1998) Characterization of Laccases and Peroxidases from Wood-Rotting Fungi (Family Coprinaceae). Appl Environ Microbiol 64(5):1601–1606
Holm J, Bjorck I, Drews A et al (1986) A rapid method for the analysis of starch. Starch 38:224–226. doi:10.1002/star.19860380704
Hyun JW, Kim CK, Pak SH et al (1996) Antitumor components of Agrocybe cylindracea. Arch Pharmacal Res 19(3):207–212
ISO 14235 (1998) Soil quality—determination of organic carbon by sulfochromic oxidation. ISO, Geneva
Jeznabadi EK, Jafarpour M, Eghbalsaied S (2016) King oyster mushroom production using various sources of agricultural wastes in Iran. Int J Recycl Org Waste Agric 5:17–24. doi:10.1007/s40093-015-0113-3
Lee KP, Rho MS (2014) Geometric analysis of nutrient balancing in the mealworm beetle, Tenebrio molitor L. (Coleoptera: Tenebrionidae). J Insect Physiol 71:37–45. doi:10.1016/j.jinsphys.2014.10.001
Lee BR, Lee YP, Kim DW et al (2010) Amelioration of Streptozotocin-Induced Diabetes by Agrocybe chaxingu Polysaccharide. Mol Cells 29:349–354. doi:10.1007/s10059-010-0044-9
Li C (2013) Optimization research on cultural formula of Pleurotus ostreatus using cultural residue in Flammulina venlutiper (Fr.) Sing industrial production. North Hortic 08:159–161
Magnelli P, Forchiassin F (1999) Regulation of the cellulase complex production by Saccobolus saccoboloides: induction and repression by carbohydrates. Mycologia 91:359–364. doi:10.2307/3761382
Mandeel Q, Al-Laith A, Mohamed S (2005) Cultivation of oyster mushrooms (Pleurotus spp.) on various lignocellulosic wastes. World J Microbiol Biotechnol 21:601–607. doi:10.1007/s11274-004-3494-4
Mohamed MF, Refaei EFS, Abdalla MMA (2016) Fruiting bodies yield of oyster mushroom (Pleurotus columbinus) as affected by different portions of compost in the substrate. Int J Recycl Org Waste Agric 5:281–288. doi:10.1007/s40093-016-0138-2
Nakagaki BJ, DeFoliart GR (1991) Comparison of diets for mass-rearing Acheta domesticus (Orthoptera: Gryllidae) as a novelty food, and comparison of food conversion efficiency with values reported for livestock. J Econ Entomol 84:891–896
Philippoussis A, Zervakis G, Diamantopoulou P (2001) Bioconversion of agricultural lignocellulosic wastes through the cultivation of the edible mushrooms Agrocybe aegerita, Volvariella volvacea and Pleurotus spp. World J Microbiol Biotechnol 17:191–200. doi:10.1023/A:1016685530312
Royse DJ (1992) Recycling of spent shiitake substrate for production of oyster mushroom, Pleurotus sajor-caju. Appl Microbiol Biotechnol 38(2):179–182
Rumpold BA, Schlüter OK (2013) Potential and challenges of insects as an innovative source for food and feed production. Innov Food Sci Emerg Technol 17:1–11. doi:10.1016/j.ifset.2012.11.005
Spiro R (1966) Analysis of sugar found in glycoprotein. Methods Enzymol 1966:3–26
Wang JH, Wang XM, Yang YH et al (2012) Analysis on the conventional nutrients in Tenebrio molitor manure and silkworm excrement. J Anhui Agric Sci 40(21):10924–10925
Zeng XL, Lin JF, Guo LQ et al (2013) Evaluation of Burma reed as substrate for production of Pleurotus eryngii. Indian J Microbiol 53(2):181–186. doi:10.1007/s12088-012-0320-9
Acknowledgements
We would like to thank the Guangdong Provincial Science and Technology Foundation, China (Grant No. 2016A020210139) for financial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Zeng, Xl., Han, F., Ye, Jl. et al. Recycling spent Pleurotus eryngii substrate supplemented with Tenebrio molitor feces for cultivation of Agrocybe chaxingu . Int J Recycl Org Waste Agricult 6, 275–280 (2017). https://doi.org/10.1007/s40093-017-0171-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40093-017-0171-9